To the Editor:
Solid organ transplant (SOT) recipients represent a high-risk group for all SARS-CoV-2 infection-related adverse outcomes.1 Therefore, most European countries have established prioritization of SOT recipients in their vaccination programs. Suboptimal vaccine efficacy to the SARS-CoV-2 vaccines is anticipated; however, they have been excluded from vaccination trials. Overall response rates to other vaccines, such as influenza vaccine, have demonstrated high, though acceptable, variability.2 Herein we report preliminary results of the humoral immune response in 34 SOT recipients and 116 matched health-care workers (HCW) after vaccination with the BNT162b2 mRNA vaccine.
The first 34 SOT (10 kidney and 24 heart) recipients who were vaccinated in the country with BNT162b2 and 116 age- and sex-matched HCW were included. The study was approved by the Ethics committee of the “Onassis Cardiac Surgery Center” and all the participants had signed written informed consent. Postvaccination antibodies were tested by an anti-SARS-CoV-2-RBD IgG assay (Abbott SARS-CoV-2 IgG II Quant). It quantifies IgG antibodies against the receptor-binding domain (RBD) of the S1 subunit of the spike protein of SARS-CoV-2 by a chemiluminescent microparticle immune assay (CMIA). The linear range is between 21.0 and 40 000 AU/ml and according to the manufacturer, the clinical specificity is estimated at 99.55% and the clinical sensitivity at 98.81% in samples collected >=15 days following a positive PCR at a cut-off value of 50 AU/ml. Anti-SARS-CoV-2 RBD IgG assays have shown an excellent correlation with neutralizing antibodies.3
Antibodies were measured at a median of 10 (IQR: 9–10) days from the second vaccination dose. We evaluated the associations among demographic and clinical characteristics and positive antibody response using modified Poisson regression with a robust variance estimator.
A total of 34 SOT recipients were analyzed. There was a male predominance (79.4%). The median age was 60 (IQR: 49.1–68.4) years, and median time from transplantation was 11.1 (IQR: 7.3–15.8) years. Almost all (94%) SOT recipients received a calcineurin inhibitor-based immunosuppressive regimen, 44% an antimetabolite, 15% corticosteroids, and 62% (all the heart and a minority of kidney recipients) a mTOR inhibitor. Anti-SARS-CoV-2 RBD-IgG antibodies were detected in 20 of the 34 SOT recipients (58.8%). From the covariates assessed, antimetabolite-containing immunosuppression was the only factor that negatively influenced immune response: 33% in those receiving MPA versus 79% in those who did not (adjusted incidence ratio IRR 0.42, p = .027) ( Table 1). The study was underpowered for the assessment of immunogenicity by transplantation type. When SOT recipients were compared to HCW, antibody response rates (58.8% vs. 100%, p < .001), median (1370 vs. 11 710, p < .001), and geometric mean titers (948 vs. 11 300, p < .001) of anti-SARS-CoV-2 RBD IgG titers were all highly significant ( Figure 1).
TABLE 1.
Prevalence of anti-SARS-CoV-2 RBD IgG after second dose of BNT162b2 vaccine in organ transplant recipients (OTR). Samples with anti-SARS-CoV-2 RBD IgG concentration ≥50 AU/ml were considered positive
| Covariates | Prevalence of anti-SARS-CoV-2 RBD IgG |
|||
|---|---|---|---|---|
| N | n/N | IRR (95% CI)a | pb | |
| Total | 34 | 20 (58.8) | ||
| Age (years), n (%) | ||||
| ≤60 | 17 | 11 (64.7) | 1 | |
| >60 | 17 | 9 (52.9) | 0.82 (0.46–1.46) | .496 |
| Sex, n (%) | ||||
| Male | 27 | 16 (59.3) | 1 | |
| Female | 7 | 4 (57.1) | 0.96 (0.47–1.99) | .922 |
| Type of transplant, n (%) | ||||
| Heart | 24 | 18 (75.0) | 1 | |
| Kidney | 10 | 2 (20.0) | 0.27 (0.07–0.96) | .043 |
| Time since transplant (years), median (25th–75th) | ||||
| ≤11 | 17 | 9 (52.9) | 1 | |
| >11 | 17 | 11 (64.7) | 1.22 (0.69–2.18) | .496 |
| Antimetabolite maintenance immunosuppression, n (%)c | ||||
| No | 19 | 15 (79.0) | 1 | |
| Yes | 15 | 5 (33.3) | 0.42 (0.20–0.91) | .027 |
95% CI: 40.7–75.4.
We evaluated the associations among demographic and clinical characteristics and positive antibody response using modified Poisson regression with a robust variance estimator.
Includes mycophenolate mofetil and mycophenolate acid.
FIGURE 1.
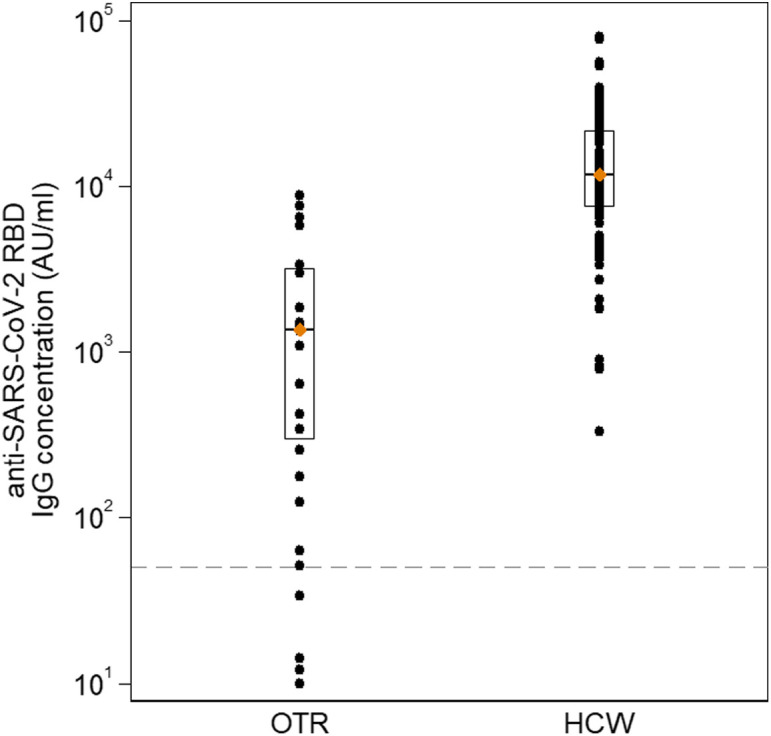
Anti-SARS-CoV-2 RBD IgG concentration (AU/ml) in organ transplant recipients (OTR) in comparison with health-care workers (HCW). Diamonds and endpoints of the rectangles represent median concentration and 25th–75th percentile, respectively. Dashed horizontal line indicates the assay limit of detection (LOD) 50 AU/ml [Color figure can be viewed at wileyonlinelibrary.com]
These preliminary results demonstrated a reduced humoral immune response to the BNT162b2 mRNA vaccine in SOT recipients compared to their healthy counterparts. Our study further confirms a recent study by Boyarsky et al,4 describing an anti-RBD IgG prevalence of 31% in 223 SOT recipients after the first vaccination dose. Our study included 34 SOT recipients who had completed their immunization with both vaccine doses, and the immunogenicity was assessed at a time where antibody titers are considered highest.5 We found low antibody response rates and low antibody titers in immunocompromised individuals (58.5%). The study limitations include: the small sample size, the single measurement of antibodies, sole use of the BNT162b2 vaccine, and assessment only of the humoral component and not the T-cell immune response. Our findings, however, may be significant. They indicate that a substantial proportion of SOT recipients are expected to develop antibody titers below the protective threshold, which may eventually wane faster. Subsequently, immunocompromised individuals may remain at infection risk even after complete vaccination. Furthermore, if confirmed by large-scale studies, alternative approaches to improve vaccine response may be indicated in patients receiving lifelong immunosuppression.
ACKNOWLEDGMENTS
This work was funded by the Onassis Cardiac Surgery Center; fund 704/13.01.2021. The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript and decision to submit the manuscript for publication. We acknowledge the following individuals for their assistance with this study, none of whom was compensated for his or her contributions: Aggeliki Gkouziouta, Heart Failure and Transplant Cardiologist, affiliated with Heart Failure and Transplant Units, Onassis Cardiac Surgery Center and Efstathios Xagas, Nephrologist, affiliated with the Clinic of Nephrology and Renal Transplantation NKUA, Medical School, Laiko General Hospital, both for data collection of SOT recipients; Joseph Papaparaskevas, Associate Professor, Department of Microbiology, NKUA, Medical School, Central Diagnostic Laboratories, affiliated with the Onassis Cardiac Surgery Center for sample analysis; Mina Psichogiou, Associate Professor of Internal Medicine and Infectious Diseases, affiliated with the First Department of Internal Medicine, Laiko General Hospital, Medical School, NKUA, for data collection of HCWs; Vana Sypsa, Associate Professor of Epidemiology and Medical Statistics, affiliated with the Department of Hygiene, Epidemiology and Medical Statistics, NKUA, Medical School, for statistical consultation.
FUNDING INFORMATION
Onassis Cardiac Surgery Center, Grant/Award Number: 704/13.01.2021
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
REFERENCES
- 1.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using Open SAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar D, Blumberg EA, Danziger-Isakov L, et al. Influenza vaccination in the organ transplant recipient: review and summary recommendations. Am J Transplant. 2011;11:2020–2030. doi: 10.1111/j.1600-6143.2011.03753.x. [DOI] [PubMed] [Google Scholar]
- 3.Perkmann T, Nagele-Perkmen W, Koller T,. et al. Anti-Spike protein assays to determine post vaccination antibody levels: a head to head comparison of five quantitative assays. MedRxiv. 2021. 10.1101/2021.03.05.21252977. [DOI] [PMC free article] [PubMed]
- 4.Boyarsky BJ, Werbel WA, Avery RK, et al. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA. Published online March 15, 2021. 2021. 2021. 10.1001/jama.2021.4385. [DOI] [PMC free article] [PubMed]
- 5.Walsh EE, Frenck RW, Falsey AR, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]


