Abstract
COVID-19 is associated with increased morbidity and mortality in transplant recipients. There are no efficacy data available regarding these patients with any of the available SARS-CoV-2 vaccines. We analyzed the humoral response following full vaccination with the BNT162b2 (Pfizer-BioNTech) in 136 kidney transplant recipients, and compared it to 25 controls. In order to exclude prior exposure to the virus, only participants with negative serology to SARS-CoV-2 nucleocapsid protein were included. All controls developed a positive response to spike protein, while only 51 of 136 transplant recipients (37.5%) had positive serology (p < .001). Mean IgG anti-spike level was higher in the controls (31.05 [41.8] vs. 200.5 [65.1] AU/mL, study vs. control, respectively, p < .001). Variables associated with null humoral response were older age (odds ratio 1.66 [95% confidence interval 1.17–2.69]), high-dose corticosteroids in the last 12 months (1.3 [1.09–1.86]), maintenance with triple immunosuppression (1.43 [1.06–2.15]), and regimen that includes mycophenolate (1.47 [1.26–2.27]). There was a similar rate of side effects between controls and recipients, and no correlation was found between the presence of symptoms and seroconversion. Our findings suggest that most kidney transplant recipients remain at high risk for COVID-19 despite vaccination. Further studies regarding possible measures to increase recipient’s response to vaccination are required.
KEYWORDS: clinical research/practice, immunosuppressant, kidney transplantation/nephrology, vaccine
Abbreviations: BMI, body mass index; CI, confidence interval; CNIs, calcineurin inhibitors; COVID-19, coronavirus disease 2019; eGFR, estimated glomerular filtration rate; IQR, interquartile range; MMF, mycophenolate mofetil or mycophenolate sodium; mTORs, mammalian target of rapamycin (mTOR) inhibitors; OR, odds ratio; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation; SPK, simultaneous pancreas and kidney transplantation
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and the resulting disease, coronavirus disease 2019 (COVID-19), have spread to millions of people worldwide. COVID-19 in solid organ transplant recipients is associated with increased morbidity and mortality due to comorbidities and immunosuppression state.1 , 2
Based on data from other vaccinations, the immune response of solid organ recipients to vaccination may be blunted,3, 4, 5 and an adequate vaccine response cannot be assumed.
Transplant recipients were not enrolled in phase 3 studies of SARS-CoV-2 vaccines,6 , 7 and currently no efficacy, immunogenicity, or safety data are available for this vulnerable population with any of the approved COVID-19 vaccines. Recently, in a study of 436 solid organ transplant recipients, the majority of participants did not mount appreciable immune response following a single dose of mRNA SARS-CoV-2 vaccine.8
The present study was aimed, for the first time to the best of our knowledge, to quantify the humoral response following full vaccination with the BNT162b2 (Pfizer-BioNTech) SARS-CoV-2 vaccine, in a cohort of kidney transplant recipients, by determining the level of antibodies directed against the S (spike) antigen, comparing it to controls, and exploring the factors that may be associated with it. In order to eliminate prior exposure to the virus and evaluate the influence of the vaccine itself, only participants with negative serology to SARS-CoV-2 nucleocapsid protein were included.
2. METHODS
2.1. Study design
The study includes two cohorts: A study group, composed of adult kidney transplant recipients who are routinely followed in our transplant center clinic and were in a routine visit during the study period. The control group, composed of 25 health-care workers (none of them receiving immunosuppressive treatment) from our institution. All the participants provided written informed consent.
The patients were included if they have completed the full vaccination and self-reported of no positive polymerase chain reaction (PCR) to SARS-CoV-2 before. All participants had been vaccinated with the BNT162b2 SARS-CoV-2 (Pfizer-BioNTech) vaccine, with the recommended dosing interval of 21 days between the two doses. The blood samples were collected between 10 and 20 days after the second vaccine dose injection.
Freshly collected blood in clot activator and gel tube was centrifuged at 4500 rpm for 10 min. The sera were separated and stored at 4°C for analysis.
LIAISON SARS-CoV-2 S1/S2 IgG chemiluminescent assay (DiaSorin S.p.A.) was used according to the manufacture instructions, to detect IgG antibodies directed against a recombinant S protein (S1/S2).9 Samples displaying <12.0 AU/mL were considered negative, those ranging between 12.0 and 15.0 AU/mL are equivocal, and those >15 AU/mL were considered as positive. For the purpose of the analysis, participants with equivocal response (a total of two participants from the study group) were considered as negative.
In addition, in order to explore prior exposure to SARS-CoV-2, every participant had a test to detect IgG antibodies directed against the SARS-CoV-2 nucleocapsid protein, performed with an Architect i2000SR analyzer (Abbot Diagnostics) and Abbott chemistry according to the manufacture instructions. A cutoff of 1.4 index (S/C) was used.10
Exclusion criteria included a history of prior positive PCR test for SARS-CoV-2; detectable IgG antibodies for the SARS-CoV-2 nucleocapsid protein; and individuals who were not fully vaccinated or had less than 10 days following the second vaccine dose.
Every participant was asked to report the side effects after each dose of the vaccine (during 7 days following every dose). The side effects were divided to local (pain, redness, swelling, and regional lymphadenopathy) and systemic side effects (fever, chills, headache, fatigue, myalgia, arthralgia, nausea and vomiting, diarrhea) and ranked on a scale (0–4).
In our center the induction immunosuppression therapy consists of antithymocyte globulin or basiliximab, according to patients’ risk of rejection, in addition to methylprednisolone intravenously. We use a maintenance regimen consisting of triple immunosuppression therapy including calcineurin inhibitors (CNIs; tacrolimus or cyclosporin), mycophenolate mofetil or mycophenolate sodium (MMF), and low-dose prednisone (5 mg/day). This is consistent with the 2009 Kidney Disease: Improving Global Outcomes (KDIGO)11 guideline for the Care of Kidney Transplant Recipients. According to the patient’s risk stratification for rejection, side effects or other considerations, the maintenance regimen may be intensified or reduced, including changing doses or suspending specific agent, adding or switching to mTOR inhibitors (everolimus or sirolimus) or azathioprine.
Clinical and epidemiological data were obtained from the medical charts. We used the records on the maintenance immunosuppression, as well as the baseline recorded laboratory tests that were routinely taken during the last clinic visit prior the first dose of vaccine and processed in the hospital central laboratory. Triple immunosuppression was defined as any combination of three different medications (including prednisone, CNIs, MMF, mTOR inhibitors, or azathioprine). Treatment with high-dose corticosteroids was defined as a pulse of methylprednisolone (≥125 mg), or prednisone ≥40 mg/day. Low-dose prednisone was defined as 5 mg/day.
Estimated glomerular filtration rate (eGFR) was calculated using MDRD formula12 and adjusted to body surface area (Mosteller calculation). Body mass index (BMI) was defined as dry weight in kilograms divided by height in square meters.
The study was approved by the local ethical institutional review board.
2.2. Statistical analysis
Continuous variables were first tested for normal distribution using the Kolmogorov–Smirnov test and Q–Q plots, and were summarized and displayed as mean (standard deviation, SD) for normally distributed variables, and as median (IQR, interquartile range) for nonnormally distributed variables.
Categorical variables were displayed as number of patients and the percentage in each group. For all categorical variables, the chi-square statistic was used to assess the statistical significance between groups. Continuous variables were compared by using a t-test if normally distributed or by Kruskal–Wallis/Mann–Whitney test if nonnormally distributed.
Correlation between two continuous parameters was calculated by Spearman analysis. In order to identify which variables are affected by multicollinearity and the strength of the correlation, we calculated variance inflation factors (VIF) and reported VIF above 3. We fitted binary logistic regression models for the risk of negative serology test including the significant variables that were found in univariate analysis.
p < .05 was considered statistically significant for all analyses. IBM SPSS Statistics for Windows, version 22 (IBM Corp.) was used for all statistical analyses.
3. RESULTS
The control group was composed of 25 participants. One hundred thirty-nine kidney transplant recipients were recruited to the study group, three of them were excluded from further analysis due to positive IgG antibodies to SARS-CoV-2 nucleocapsid protein (all had a positive level of anti-spike antibodies). All participants in both cohorts were Caucasians. Kidney transplant recipients were significantly older than the controls (age ranges 30–78 and 22–81 years, respectively), while female sex was more prevalent in the control group. However, mean period of time after administration of both vaccine doses was similar in both groups ( Table 1).
TABLE 1.
Characteristics of kidney transplant recipients and control group who received the Pfizer BNT162b2 vaccine
| Factor | Kidney transplant recipients (N = 136) | Control group (N = 25) | p value |
|---|---|---|---|
| Age, years (mean, SD) | 58.6 (12.7) | 52.7 (11.5) | .028 |
| Sex, female (%) | 25 (18.3) | 17 (68) | <.001 |
| Days after first vaccine dose (median, IQR) | 36.5 (8.3) | 37.8 (4.6) | .29 |
| Days after second vaccine dose, (median, IQR) | 16.5 (6.2) | 16.8 (2.9) | .43 |
Abbreviations: IQR, interquartile range; SD, standard deviation.
The majority of recipients (125, 90%) were after kidney transplant, nine simultaneous kidney and pancreas transplant, two kidney after liver transplant, and three after simultaneous kidney and liver transplant. Ten recipients had at least two transplantations. Median time after first transplantation was 39.2 months (IQR 18.4–61.9 months, range 1.3–404 months), and after last transplantation 38.0 months (IQR 18.4–61.0, range 1.3–313). Twenty-two patients were transplanted in the last 12 months prior to vaccination, five of them in the last 3 months. Kidney donation was from living donor in 61.7% (living related 39 [28.6%] and living unrelated 45 [33%]). Four recipients received desensitization treatment with rituximab in the last 12 months. Eighty-six recipients (64%) received induction with basiliximab and 47 recipients (34.5%) received induction with antithymocyte globulin, 18 of them within the last 24 months prior to SARS-CoV-2 vaccination. One patient had an acute cellular rejection in the last year (5 days posttransplant, treated for the rejection with antithymocyte globulin and high-dose corticosteroids).
CNIs were used as the backbone of the immunosuppressive regiment in 90.4% of the patients (tacrolimus in 121 and cyclosporine in two patients). Prednisone was used in 121 (88.9%) patients and MMF in 104 (76.4%). Most recipients (107, 78.6%) were treated with a combination of three immunosuppressive medications, most of them with prednisone, tacrolimus, and MMF (81, 59.6%). Two recipients had a reduction in the maintenance immunosuppression protocol in the 30 days prior to the vaccine (suspending MMF treatment).
Thirty-six patients (26.5%) were transplanted preemptively (without need for dialysis prior to kidney transplantation). Comorbidities included ischemic heart disease in 48 recipients (35%), hypertension in 105 (77%), diabetes mellitus in 59 (43%) (16 of them had new-onset diabetes after transplantation), and 6 had active malignancies other than nonmelanomatous skin cancer (one renal cell carcinoma, one posttransplant lymphoproliferative disease (PTLD), two prostate carcinoma and two Kaposi’s sarcoma). Twenty-six recipients (19%) had a BMI >30 kg/m2.
3.1. Side effects and adverse reactions after the vaccine administration
Unfortunately, one kidney transplant recipient, who had undetectable antibody levels after full vaccination, expired due to severe PCR-proven COVID-19, 9 weeks after the second dose of vaccine. Currently, 12 weeks following the vaccine, another kidney recipient with undetectable antibody levels is hospitalized with moderate PCR-proven COVID-19. There were no additional cases of PCR-confirmed SARS-CoV-2 infections in the time period of 1 week after the second dose of vaccine until now (April 12, 2021), nor were biopsy-proven acute rejections, new neurological diagnoses (Guillain-Barre syndrome, Bell’s palsy or other neuropathy), or severe allergic reactions in our cohorts.
The most common side effect after the vaccine administration was local pain, in 84 of 161 (52.2%) participants. Systemic symptoms developed in 31 (19.2%) of participants. There was a similar rate of symptoms between seropositive and seronegative individuals, as well as between controls and recipients. No correlation was found between the presence of side effects and seropositivity.
3.2. Humoral response of kidney transplant recipients compared to controls
All participants in the control group had a positive antibody response to spike protein, while only 51 of 136 transplant recipients (37.5%) had positive serology (p < .001).
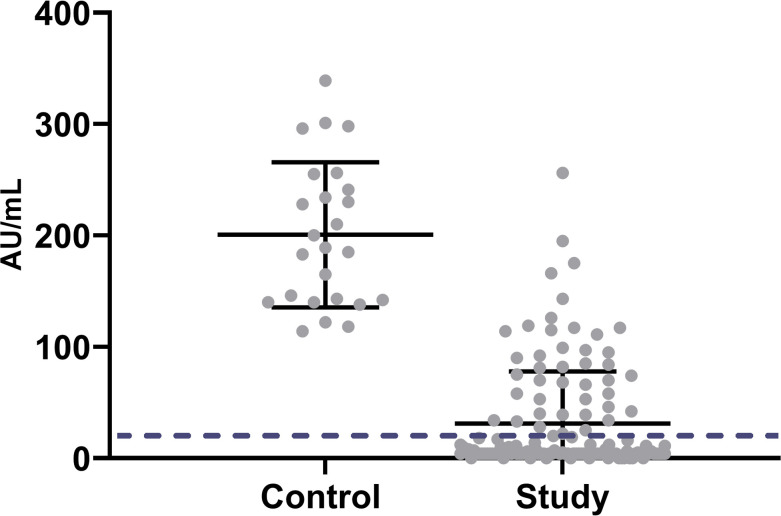
The mean IgG anti-spike level in the kidney transplant group (median = 5.9 (IQR 3.8–42.0 AU/mL) was lower than in the control group (median = 189.0 [IQR 141.10–248] AU/mL). A Mann–Whitney test indicated that this difference was statistically significant (u = 69.5, p < .001) ( Figure 1). In addition, even in seropositive recipients, mean antibody levels were significantly lower compared to controls (median = 71.8 [IQR 37.6–111.7] vs. 189.0 [IQR 141.10–248] AU/mL), study and control groups, respectively; p < .001).
FIGURE 1.
Scatterplot of IgG anti S of both groups. Solid lines represent mean and SD. Dashed line represents cutoff of 15 AU/mL (defined as positive serology)
3.3. Risk factors for negative serology
Table 2 shows clinical and laboratory data of transplant recipients with positive and negative antibody response. Participants with a positive anti-spike serology were significantly younger, had a shorter period of time on maintenance dialysis before transplantation, and had a higher prevalence of living donors. The main difference in immunosuppression between the two groups was a lower rate of treatment with MMF and a lower rate of triple maintenance immunosuppression in seropositive patients. Patients with a positive postvaccination antibody levels had a significantly higher eGFR in addition to a higher mean hemoglobin and lymphocyte count. Longer period of time since transplantation was significantly associated with positive response to the vaccination.
TABLE 2.
Comparison of recipients with negative vs positive serology
| Variable | Negative | Positive | p value |
|---|---|---|---|
| Number | 85 | 51 | |
| Agea | 60.9 (12.2) | 54.55 (12.8) | .005 |
| Sex, female (%) | 32 (37.6) | 17 (33.3) | .71 |
| BMI | 26.71 (4.2) | 27.40 (3.9) | .35 |
| Time on dialysis before transplantation, months | 28.34 (35.5) | 12.47 (19.1) | .001 |
| Time post first transplantation, monthsb | 56.6 (67.9) | 87.7 (95.6) | .05 |
| Time post last transplantation, monthsc | 49.1 (54.8) | 68.9 (69.4) | .076 |
| First transplant (%) | 76 (89.4) | 45 (88.2) | 1.0 |
| Etiology for kidney failure | |||
| Diabetes/nephrosclerosis | 37 | 13 | .61 |
| Glomerulonephritis | 18 | 14 | |
| Polycystic kidney | 14 | 6 | |
| Other | 16 | 18 | |
| Donor type, living (%) | 47 (55.2) | 37 (72.5) | .03 |
| SPK, (%) | 6 (7.0) | 3 (5.8) | 1 |
| Hypertension, (%) | 70 (82.3) | 39 (76) | .50 |
| Diabetes mellitus, (%) | 39 (45.9) | 20 (3.2) | .47 |
| High-dose steroids last 12 months, (%) | 25 (29.4) | 7 (13.7) | .038 |
| Antithymocyte globulin last 12 months, (%) | 9 (10.5) | 1 (1.9) | .08 |
| Rituximab last 12 months | 3 (3.5) | 1 (1.9) | 1.0 |
| Low-dose prednisone, (%) | 79 (92.9) | 42 (82.3) | .08 |
| CNIs, (%) | 77 (90.5) | 46 (90.1) | 1.0 |
| Mean tacrolimus level, ng/mL | 7.3 (2.7) | 6.9 (2.1) | .41 |
| mTORs, (%) | 5 (5.8) | 5 (9.8) | .50 |
| MMF, % | 72 (84.7) | 32 (62.7) | .006 |
| Median (IQR) dose of mycophenolate sodium, mg/day | 360 (360–720) | 360 (360–720) | .15 |
| Triple maintenance immunosuppression, % | 73 (85.9) | 34 (66.6) | .044 |
| Hemoglobin, g/dL | 13.41 (1.9) | 14.03 (1.5) | .07 |
| White blood cell count, 10e3/µL | 8.37 (2.6) | 8.54 (2.5) | .70 |
| Neutrophil count, 10e3/µL | 5.53 (2.1) | 5.24 (1.6) | .46 |
| Lymphocyte count, 10e3/µL | 1.83 (0.9) | 2.27 (0.8) | .013 |
| Serum creatinine, mg/dL | 1.42 (0.6) | 1.14 (0.32) | .002 |
| eGFR, ml/min/m2 | 59.4 (21.7) | 72.6 (20.5) | .001 |
| Serum albumin | 39.8 (11.4) | 40.1 (13.0) | .89 |
| Days after first dose | 36.7 (4.6) | 36.1 (4.9) | .85 |
| Days after second dose | 16.8 (5.3) | 16.1 (5.9) | .91 |
Abbreviations: BMI, body mass index; CNIs, calcineurin inhibitors (tacrolimus or cyclosporin); eGFR, estimated glomerular filtration rate; MMF, mycophenolate mofetil or mycophenolate sodium; mTORs, mammalian target of rapamycin (mTOR) inhibitors; SPK, simultaneous pancreas and kidney transplantation.
Data presented as mean (SD) unless otherwise stated.
Age range 24.7–81.4 versus 22.5–77.1 years for recipients with negative vs positive serology.
Range 1.5–310 versus 4.8 versus 409 months post last transplantation for recipients with negative versus positive serology.
Range 1.4–313 versus 4.4 versus 265 months post last transplantation for recipients with negative vs positive serology.
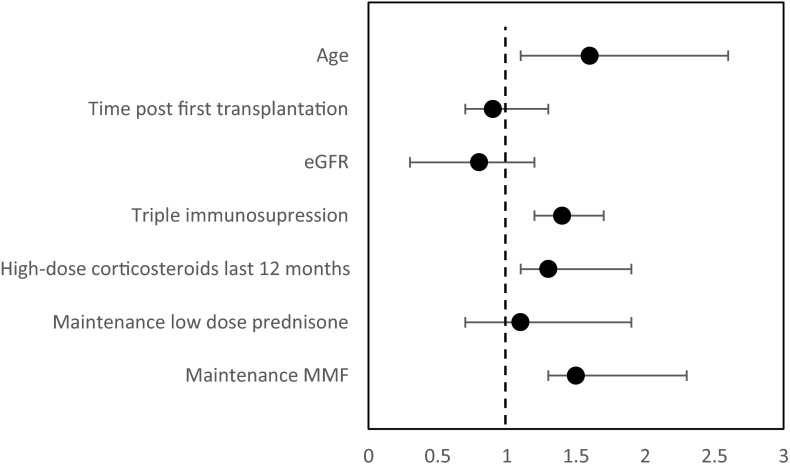
When multivariate analysis was performed, variables associated with negative humoral response were older age (odds ratio 1.66 [95% confidence interval 1.17–2.69], p = .026), high-dose corticosteroids in the last 12 months (1.3 [1.09–1.86], p = .048], maintenance with triple immunosuppressive medications (1.43 [1.06–2.15], p = .038], and a regimen that includes MMF (1.47 [1.26–2.27], p = .049] ( Figure 2). A moderate multicollinearity (VIF = 9.3) for MMF and triple immunosuppression regimen was found.
FIGURE 2.
Multivariate analysis for the risk of negative serology (odds ratio) in the transplant recipients’ group. Note: dashed line represents OR = 1
In the control group, we found a trend of inverse correlation between age and antibody levels (correlation coefficient = –0.27, p= .084); however, it did not reach statistical significance.
4. DISCUSSION
Organ transplant recipients exhibit worse outcome from SARS-CoV-2 infection, due to comorbidities and immunosuppression.13 , 14 Accordingly, an effective and safe vaccination against SARS-CoV-2 is critical in this population. Data regarding the safety of mRNA SARS-CoV-2 vaccines in solid organ transplant recipients are scarce since there were no transplant recipients in the phase 3 trials of Moderna7 or Pfizer.6
In the current study we report, for the first time to best of our knowledge, the serologic response to full mRNA SARS-CoV-2 vaccine among kidney transplant recipients, who did not have a prior exposure to the virus (by including only participants with undetectable level of IgG against SARS-CoV-2 nucleocapsid protein).
mRNA SARS-CoV-2 vaccines, similar to other common vaccines, are noted to cause mild to moderate side effects, including local pain, headaches, and fatigue, in immunocompetent individuals.15 A recent publication describing 187 transplant recipients who were vaccinated with Pfizer and Moderna vaccines did not show any unexpected short-term local and systemic side effects following the vaccine administration.16 In our cohorts, in accordance with that, most common side effect was mild local pain, without major adverse events. We could not find any correlation of the vaccine side effects or their severity to humoral response, or any difference in the side effects between controls and kidney transplant recipients.
Despite a theoretical concern that vaccination may trigger organ rejection,17 , 18 numerous trials of some common vaccines have shown no casual association between the two.19, 20, 21, 22, 23 Our study confirms the safety of mRNA SARS-CoV-2 vaccine (BNT162b2, Pfizer-BioNTech) in kidney transplant recipients, as no biopsy-proven rejection was documented during the follow-up period.
Our most impressive finding was that only 37.5% of kidney transplant recipients mount an appreciable anti-spike antibody response, in contrast to healthy controls who had a robust and universal humoral response, similar to recent studies of immunocompetent individuals.24 This reduced rate of response among transplant recipients to vaccination was described in previous studies exploring other common vaccines, including pneumococcal vaccination,25 , 26 hepatitis B virus,5 , 27 and influenza.28 Recently, Boyarsky et al8 found poor immune response following a single dose of mRNA SARS CoV-2 vaccine in solid organ transplant recipients, with a rate of only 31 out of 219 (14.1%) kidney recipients had a detectable IgG anti-spike. The clinical significance of this finding should be further evaluated. In our 3 months follow-up, two patients in seronegative group developed severe COVID-19 infection, while no infection was documented in seropositive group, but our follow-up was not long enough to evaluate clinical outcomes.
In addition, we demonstrated a reduced level of antibodies even in seropositive kidney transplant recipients. The clinical significance of this finding should be further evaluated. Although studies in SARS-CoV-2-infected individuals demonstrated a correlation between neutralizing antibody level and COVID-19 severity,29 there is no well-established protective antibody threshold. Further studies are needed in order to determine whether patients with a positive albeit low antibody level possess a higher risk of SARS-CoV-2 infection, as well as the duration of the response.
The most significant predictors of failure to mount a humoral response in our cohort of kidney transplant recipients were advanced age, need for high-dose corticosteroids during the last (prevaccination) year, maintenance with three immunosuppressive medications, and a regimen that includes MMF.
Some association between advanced age and positive but lower antibody response was found in immunocompetent patients after COVID-19,30 as well as after mRNA SARS-CoV-2 vaccination.31 , 32 Our finding indicates that even in immunosuppressed patients age may be an independent predictor of poor immunological response. In our control group, there was an inverse trend of age and antibody level; however, it was not statistically significant.
The influence of different immunosuppressive regimens on vaccination response was explored in previous years. A number of studies evaluated response rates to influenza and pneumococcal vaccines in patients receiving glucocorticoids. While immune response was preserved, although mildly reduced, in most patients on chronic low dose of glucocorticoids,33, 34, 35 the response was inadequate in patients on high dose.36 Our findings in regard to mRNA SARS CoV-2 vaccine demonstrate concordant results with significant reduction of the humoral immune response during the first year following high-dose steroids treatment.
Similarly, MMF has a well-known suppressive effect on the immune system, including the inhibition of antibody production.37 , 38 Our finding of a significant decrease in vaccination response in kidney recipients on MMF treatment is in line with previous reports, which demonstrated a dose-related correlation of MMF with decreased influenza and cholera vaccine responsiveness in renal transplant subjects.28 , 39, 40, 41, 42
We could not find an independent association between vaccine humoral immune response and treatment with antithymocyte globulin during the prevaccination year. Although the small number of participants who received this treatment preclude us from a valid conclusion, these findings are in agreement with result of influenza vaccination studies, which failed to show a significant relation between induction immunosuppression and vaccine immunogenicity.43, 44, 45
Our study demonstrated that the serological response to vaccination was related to the net burden of immunosuppression: the greater the degree of immunosuppression, the less likely the patient will respond to immunization (OR 1.43 for triple vs double immunosuppressive regimens). This finding is not only clinically obvious, but also supported by previous studies that found an increased risk to poor seroprotection46 and increased infection rates47 with triple maintenance immunosuppression regimen.
Our main finding of such a low immunization rate after administration of full dose of BNT162b2 SARS-CoV-2 vaccine (Pfizer-BioNTech) in kidney transplant recipients raises an urgent question regarding possible measures to increase recipient’s response to vaccination.
Optimal timing may be important in maximizing the response to immunization following solid organ transplantation. It is common to wait at least 3 and often up to 12 months after transplantation before giving vaccines, once maintenance immunosuppression levels have been attained,48 , 49 in order to maximize the likelihood of developing a protective immune response. In our study, patients shortly after transplantation demonstrated reduced immune response rate, but this correlation was not an independent predictor when high-dose steroids were included into analysis. This finding emphasizes the need for an individual approach to transplanted patients’ immunization; while specific timing may vary based on individual circumstance, we should try to vaccinate when the effect of any immunosuppressive agents is at its nadir.48 , 49
Strengths of this study include its novelty. It is the first published data about full SARS-CoV-2 vaccine in kidney transplant recipients. The exclusion of participants with IgG antibodies to nucleocapsid protein eliminates the possibility of humoral response to the former virus exposure and validates our results.
Limitations of the study include a relatively small sample size, with a nonmatched control group. Short follow-up period and absence of assessing the cellular immune response preclude us to address full spectrum of vaccine immunogenicity or clinical correlation of viral protection. Further follow-up for clinical outcomes and further studies including patients with prior SARS-CoV-2 infection, assessing the humoral response in this important setting, are needed.
Despite that, our real-life data of significantly reduced level of immune response to a full dose of the SARS-CoV-2 vaccine in kidney transplant recipients warrant prompt consideration and further studies about possible ways to improve vaccine immunogenicity in this population.
5. CONCLUSIONS
Our finding of poor humoral response to the BNT162b2 SARS-CoV-2 vaccine in transplant recipients suggests that such patients may remain at high risk for COVID-19 despite appropriate vaccination. Based on these findings, we strongly suggest that all transplant recipients should be counseled to continue the practice of protective COVID-19 measures including wearing masks, hand hygiene, and social distancing.
Acknowledgments
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Caillard S, Anglicheau D, Matignon M, et al. An initial report from the French SOT COVID Registry suggests high mortality due to COVID-19 in recipients of kidney transplants. Kidney Int. 2020;98(6):1549. doi: 10.1016/j.kint.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caillard S, Chavarot N, Francois H, et al. Is COVID-19 infection more severe in kidney transplant recipients? Am J Transplant. 2021;21(3):1295–1303. doi: 10.1111/ajt.16424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eckerle I, Rosenberger KD, Zwahlen M, et al. Serologic vaccination response after solid organ transplantation: a systematic review. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0056974. e56974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gangappa S, Kokko KE, Carlson LM, et al. Immune responsiveness and protective immunity after transplantation. Transpl Int. 2008;21(4):293–303. doi: 10.1111/j.1432-2277.2007.00631.x. [DOI] [PubMed] [Google Scholar]
- 5.Serrano B, Bayas JM, Bruni L, Díez C. Solid organ transplantation and response to vaccination. Vaccine. 2007;25(42):7331. doi: 10.1016/j.vaccine.2007.08.031. [DOI] [PubMed] [Google Scholar]
- 6.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccines. N Engl J Med. 2021;384(5):403. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyarsky BJ, Werbel WA, Avery RK, et al. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA. 2021;15:e214385. doi: 10.1001/jama.2021.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.https://www.diasorin.com/sites/default/files/allegati_prodotti/liaisonr_sars-cov-2_s1s2_igg_m0870004366-d_lr.pdf. Accessed April 14, 2021.
- 10.Perkmann T, Perkmann-Nagele N, Breyer MK, et al. Side-by-side comparison of three fully automated SARS-CoV-2 antibody assays with a focus on specificity. Clin Chem. 2020;66(11):1405–1413. doi: 10.1093/clinchem/hvaa198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9:S1–S155. doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 13.Cravedi P, Mothi SS, Azzi Y, et al. COVID-19 and kidney transplantation: results from the TANGO International Transplant Consortium. Am J Transplant. 2020;20(11):3140–3148. doi: 10.1111/ajt.16185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azzi Y, Parides M, Alani O, et al. COVID-19 infection in kidney transplant recipients at the epicenter of pandemics. Kidney Int. 2020;98(6):1559–1567. doi: 10.1016/j.kint.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gee J, Marquez P, Su J, et al. First month of COVID-19 vaccine safety monitoring-United States, December 14, 2020-January 13, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(8):283–288. doi: 10.15585/mmwr.mm7008e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyarsky BJ, Ou MT, Greenberg RS, et al. Safety of the first dose of SARS-CoV-2 vaccination in solid organ transplant recipients. Transplantation. 2021. 10.1097/TP.0000000000003654. [published online ahead of print 2021]. Epub ahead of print. PMID: 33560728. [DOI] [PMC free article] [PubMed]
- 17.Avery RK, Michaels M. Update on immunizations in solid organ transplant recipients: what clinicians need to know. Am J Transplant. 2008;8(1):9. doi: 10.1111/j.1600-6143.2007.02051.x. [DOI] [PubMed] [Google Scholar]
- 18.Blumberg EA, Fitzpatrick J, Stutman PC, et al. Safety of influenza vaccine in heart transplant recipients. J Heart Lung Transplant. 1998;17(11):1075. [PubMed] [Google Scholar]
- 19.Croce E, Hatz C, Jonker EF, et al. Safety of live vaccinations on immunosuppressive therapy in patients with immune-mediated inflammatory diseases, solid organ transplantation or after bone-marrow transplantation - A systematic review of randomized trials, observational studies and case reports. Vaccine. 2017;35(9):1216–1226. doi: 10.1016/j.vaccine.2017.01.048. [DOI] [PubMed] [Google Scholar]
- 20.Candon S, Thervet E, Lebon P, et al. Humoral and cellular immune responses after influenza vaccination in kidney transplant recipients. Am J Transplant. 2009;9(10):2346. doi: 10.1111/j.1600-6143.2009.02787.x. [DOI] [PubMed] [Google Scholar]
- 21.Mulley WR, Dendle C, Ling JEH, et al. Does vaccination in solid-organ transplant recipients result in adverse immunologic sequelae? A systematic review and meta-analysis. J Heart Lung Transplant. 2018;37(7):844. doi: 10.1016/j.healun.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Hurst FP, Lee JJ, Jindal RM, et al. Outcomes associated with influenza vaccination in the first year after kidney transplantation. Clin J Am Soc Nephrol. 2011;6(5):1192. doi: 10.2215/CJN.05430610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scharpé J, Evenepoel P, Maes B, et al. Influenza vaccination is efficacious and safe in renal transplant recipients. Am J Transplant. 2008;8(2):332. doi: 10.1111/j.1600-6143.2007.02066.x. [DOI] [PubMed] [Google Scholar]
- 24.Walsh EE, Frenck RW, Falsey AR, et al. Safety and immunogenicity of two RNA-based COVID-19 vaccine candidates. N Engl J Med. 2020;383(25):2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dengler TJ, Strnad N, Bühring I, et al. Differential immune response to influenza and pneumococcal vaccination in immunosuppressed patients after heart transplantation. Transplant. 1998;66(10):1340. doi: 10.1097/00007890-199811270-00014. [DOI] [PubMed] [Google Scholar]
- 26.Kumar D, Welsh B, Siegal D, et al. Immunogenicity of pneumococcal vaccine in renal transplant recipients–three year follow-up of a randomized trial. Am J Transplant. 2007;7(3):633. doi: 10.1111/j.1600-6143.2007.01668.x. [DOI] [PubMed] [Google Scholar]
- 27.Loinaz C, de Juanes JR, Gonzalez EM, et al. Hepatitis B vaccination results in 140 liver transplant recipients. Hepatogastroenterol. 1997;44(13):235. [PubMed] [Google Scholar]
- 28.Madan RP, Tan M, Fernandez-Sesma A, et al. A prospective, comparative study of the immune response to inactivated influenza vaccine in pediatric liver transplant recipients and their healthy siblings. Clin Infect Dis. 2008;46(5):712–718. doi: 10.1086/527391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Legros V, Denolly S, Vogrig M, et al. A longitudinal study of SARS-CoV-2-infected patients reveals a high correlation between neutralizing antibodies and COVID-19 severity. Cell Mol Immunol. 2021;18:318–327. doi: 10.1038/s41423-020-00588-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein SL, Pekosz A, Park HS, et al. Sex, age and hospitalization drive antibody response in a COVID-19 convalescent plasma donor population. J Clin Invest. 2020;130(11):6141–6150. doi: 10.1172/JCI142004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campillo NE, Jimenez M, Canelles M. COVID-19 vaccine race: analysis of age-dependent immune responses against SARS-CoV-2 indicates that more than just one strategy may be needed. Curr Med Chem. 2020. 10.2174/0929867327666201027153123. [published online ahead of print 2020]. Epub ahead of print. PMID: 33109026. [DOI] [PubMed]
- 32.Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SAR-CoV-2 mRNA vaccine in older adults. N Engl J Med. 2020;383(25):2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kubiet MA, Gonzalez-Rothi RJ, Cottey R, et al. Serum antibody response to influenza vaccine in pulmonary patients receiving corticosteroids. Chest. 1996;110(2):367–370. doi: 10.1378/chest.110.2.367. [DOI] [PubMed] [Google Scholar]
- 34.Herron A, Dettleff G, Hixon B, et al. Influenza vaccination in patients with rheumatic diseases. JAMA. 1979;242(1):53–56. [PubMed] [Google Scholar]
- 35.Spika JS, Fish AJ, Lum GM, et al. Serum antibody response to pneumococcal vaccine in children with nephrotic syndrome. Pediatrics. 1982;69(2):219–223. [PubMed] [Google Scholar]
- 36.Friedman MA, Winthrop KL. Vaccines and disease-modifying antirheumatic drugs: practical implications for the rheumatologist. Rheum Dis Clin North Am. 2017;43(1):1–13. doi: 10.1016/j.rdc.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Smith KGC, Isbel NM, Catton MG, et al. Suppression of the humoral immune response by mycophenolate mofetil. Nephrol Dial Transplant. 1998;13(1):160–164. doi: 10.1093/ndt/13.1.160. [DOI] [PubMed] [Google Scholar]
- 38.Rentenaar RJ, van Diepen JFN, MeijerImmune RT, et al. Immune Responsiveness in renal transplant recipients: mycophenolic acid severely depresses humoral immunity in vivo. Kidney Int. 2002;62(1):319–328. doi: 10.1046/j.1523-1755.2002.00425.x. [DOI] [PubMed] [Google Scholar]
- 39.Nailescu C, Xu X, Zhou H, et al. Influenza vaccine after pediatric kidney transplant: a Midwest Pediatric Nephrology Consortium study. Pediatr Nephrol. 2011;26(3):459–467. doi: 10.1007/s00467-010-1729-1. [DOI] [PubMed] [Google Scholar]
- 40.Cowan M, Chon WJ, Desai A, et al. Impact of immunosuppression on recall immune responses to influenza vaccination in stable renal transplant recipients. Transplant. 2014;97(8):846–853. doi: 10.1097/01.TP.0000438024.10375.2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jonker EFF, Uijlings MAC, Visser LG, et al. Comparison of the immunogenicity of Dukoral® oral cholera vaccine between renal transplant recipients on either a calcineurin inhibitor or mycophenolate - A controlled trial. Vaccine. 2019;37(23):3133–3139. doi: 10.1016/j.vaccine.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 42.Khedmat H, Karbasi-Afshar R, Izadi M, et al. Response of transplant recipients to influenza vaccination based on type of immunosuppression: a meta-analysis. Saudi J Kidney Dis Transpl. 2015;26:877–883. doi: 10.4103/1319-2442.164556. [DOI] [PubMed] [Google Scholar]
- 43.Orcurto A, Pascual M, Hoschler K, et al. Impact of anti-T-cell therapy in the immunogenicity of seasonal influenza vaccine in kidney transplant recipients. Transplant. 2012;94(6):630–636. doi: 10.1097/TP.0b013e31825f7f82. [DOI] [PubMed] [Google Scholar]
- 44.Hequet D, Pascual M, Lartey S, et al. Humoral, T-cell and B-cell immune responses to seasonal influenza vaccine in solid organ transplant recipients receiving anti-T cell therapies. Vaccine. 2016;34:3576–3583. doi: 10.1016/j.vaccine.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 45.Kumar D, Blumberg EA, Danziger-Isakov L, et al. Influenza vaccination in the organ transplant recipient: review and summary recommendations. Am J Transplant. 2011;11(10):2020–2030. doi: 10.1111/j.1600-6143.2011.03753.x. [DOI] [PubMed] [Google Scholar]
- 46.Fairhead T, Hendren E, Tinckam K, et al. Poor seroprotection but allosensitization after adjuvanted pandemic influenza H1N1 vaccine in kidney transplant recipients. Transpl Infect Dis. 2012;14(6):575–583. doi: 10.1111/tid.12006. [DOI] [PubMed] [Google Scholar]
- 47.Bowman JS, Angstadt JD, Waymack JP, et al. A comparison of triple therapy with double therapy immunosuppression in cadaveric renal transplantation. Transplant. 1992;53(3):556–559. doi: 10.1097/00007890-199203000-00012. [DOI] [PubMed] [Google Scholar]
- 48.Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58(3) doi: 10.1093/cid/cit816. e44. [DOI] [PubMed] [Google Scholar]
- 49.Danziger-Isakov L, Kumar D, AST ID Community of Practice Vaccination of solid organ transplant candidates and recipients: Guidelines from the American society of transplantation infectious diseases community of practice. Clin Transplant. 2019;33 doi: 10.1111/ctr.13563. e13563. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.