Abstract
Introduction
Critically ill Covid‐19 pneumonia patients are likely to develop the sequence of acute pulmonary hypertension, right ventricular (RV) strain, and eventually RV failure due to known pathophysiology (endothelial inflammation plus thrombo‐embolism) that promotes increased pulmonary vascular resistance and pulmonary artery pressure. This study aimed to investigate the occurrence of acute pulmonary hypertension (aPH) as per established trans‐thoracic echocardiography (TTE) criteria in Covid‐19 patients receiving intensive care and to explore whether short‐term outcomes are affected by the presence of aPH.
Methods
Medical records were reviewed for patients treated in the intensive care units at a tertiary university hospital over a month. The presence of aPH on the TTE was noted, and plasma NTproBNP and troponin were measured as markers of cardiac failure and myocardial injury, respectively. Follow‐up data were collected 21 d after the performance of TTE.
Results
In total, 26 of 67 patients (39%) had an assessed systolic pulmonary artery pressure of > 35 mmHg (group aPH), meeting the TTE definition of aPH. NTproBNP levels (median [range]: 1430 [102‐30 300] vs. 470 [45‐29 600] ng L−1; P = .0007), troponin T levels (63 [22‐352] vs. 15 [5‐407] ng L−1; P = .0002), and the 21‐d mortality rate (46% vs. 7%; P < .001) were substantially higher in patients with aPH compared to patients not meeting aPH criteria.
Conclusion
TTE‐defined acute pulmonary hypertension was frequently observed in severely ill Covid‐19 patients. Furthermore, aPH was linked to biomarker‐defined myocardial injury and cardiac failure, as well as an almost sevenfold increase in 21‐d mortality.
Keywords: Covid‐19, echocardiography, intensive care, outcome, prevalence, pulmonary hypertension, tricuspid valve regurgitation
Editorial comment.
This large single centre cohort analysis study shows that the presence of acute pulmonary hypertension in ICU‐treated Covid‐19 patients is associated with signs of cardiac failure and a markedly increased mortality.
1. INTRODUCTION
The pathophysiologic mechanisms associated with severe Covid‐19 pneumonia promote the sequence of acute pulmonary hypertension (aPH), right ventricular strain (RVS) and right ventricular failure (RVF). First, the disease process produces generalized inflammation of the endothelium (‘endothelitis’), including the microvasculature of the pulmonary circulation. 1 , 2 Consequently, the production of the vasodilators nitric oxide and prostacyclin by the endothelium is reduced, promoting vasoconstriction of the pulmonary vessels. Second, Covid‐19 is associated with coagulopathy that causes microembolization and macroembolization in the pulmonary circulation, 3 further increasing the risk of aPH. Coagulopathy combined with the reduced antiplatelet action of nitric oxide and prostacyclin will prompt in situ thrombosis of microvessels in the pulmonary vasculature, 2 which rarely occurs in association with more typical ICU diseases. These factors set the stage for the development of aPH and associated problems.
Despite the occasional publication linking severe Covid‐19 infection to the development of acute core pulmonale, 4 there has been remarkably little information published regarding the sequence of aPH, RVS and RVF. For example, a recent comprehensive review article on the cardiovascular aspects of Covid‐19 failed to mention aPH as an important pathophysiologic issue. 5
Thus, the primary aim of the present retrospective observational study was to determine how frequently aPH occurs in severe Covid‐19 infection and to assess whether aPH is associated with a worse short‐term outcome (mortality). Secondary outcomes included left ventricular ejection fraction (LVEF), plasma D‐dimer (a proxy for the degree of coagulopathy), NTproBNP (a proxy for cardiac failure) and troponin T (a proxy for myocardial injury), as well as the composite outcome measure of death or still in need of mechanical ventilation at 3 weeks after TTE.
2. METHODS
This study was approved by the National Swedish Ethics Committee (Dnr 2020‐02250; Chairperson Gunilla Robertsson), which waived the need for patient consent, and an amendment (Dnr 2020‐03428). It was performed and reported under the STROBE guidelines for cohort studies. The study was registered at ClinicalTrials.gov (NCT04459364). A flowchart of the study process is provided in Figure 1.
FIGURE 1.
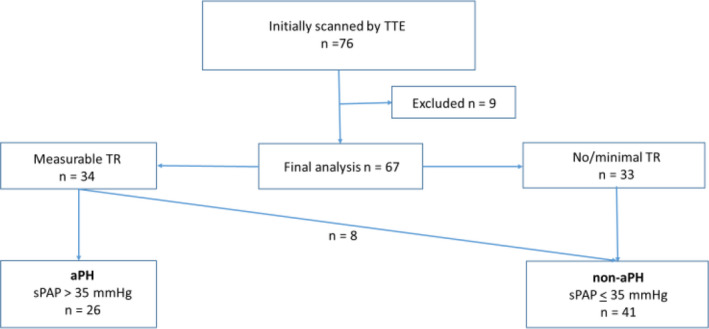
Study flowchart. Study cohort consists of patients with Covid‐19 pneumonia with pending or established respiratory failure admitted to ICU for ventilator support [Colour figure can be viewed at wileyonlinelibrary.com]
Eligible participants for this study were Covid‐19 patients admitted to ICUs at the Karolinska University Hospital, at the Solna hospital site, who underwent a TTE examination as part of standard blood tests and other investigations. The study period was part of the first Covid‐19 wave in Stockholm; the ICU care was undertaken at four different Covid‐19 ICUs at Karolinska‐Solna, two regular (total 30 beds), and two provisional (total 42 beds).
Inclusion criteria consisted of a positive test for Covid‐19 (polymerase chain reaction), admission to the ICU due to pending or manifest respiratory insufficiency from Covid‐19 pneumonia for those over 18 y of age, and TTE performed by a single investigator (JN). Exclusion criteria were known pre‐existing chronic pulmonary hypertension and a complex pre‐existing comorbidity that would make it difficult to interpret the results of the TTE adequately. Ongoing veno‐arterial extracorporeal membrane oxygenation was also an exclusion criterion due to the completely altered pulmonary circulation that occurs with this treatment modality.
Patient‐related information was extracted from each patient's electronic medical record file.
Per the study protocol, the outcome data for all included patients were analysed 21 days after the performance of the TTE. The outcomes were classified as (1) dead, (2) still on mechanical ventilation in the ICU, or (3) alive without the need for mechanical ventilation.
2.1. Risk factors
Based on previous publications and the data routinely recorded by the Swedish Intensive Care Registry (SIR), 6 the following parameters were considered risk factors in association with Covid‐19: age over 70 y, hypertension, cardiopulmonary disease, renal disease, obesity, diabetes mellitus, and immunosuppression.
2.2. Anticoagulation regimen
Per the hospital ICU care Covid‐19 protocol for the period of the study, all patients received prophylactic anticoagulation by low‐molecular‐weight heparin at 50‐75 IU kg−1 twice daily or intravenous heparin (to an APTT target of 70‐90 s) for those with verified thrombo‐embolism.
2.3. Trans‐thoracic echocardiography
All TTE examinations were performed by one experienced physician, certified in echocardiography, using a Vivid S70 or a Vivid IQ ultrasound scanner (GE Healthcare, Horten, Norway). All TTE examinations were analysed by JN and later verified by another experienced and certified echocardiographer (ME). Image analyses were performed offline using the EchoPAC workstation (GE EchoPAC sw only, Horten, Norway).
The evaluation included two‐dimensional echocardiography plus conventional and colour Doppler recordings per current recommendations. 7 , 8 , 9 , 10 RV evaluation was performed using multiple acoustic windows for image acquisition. The RV size was measured as a basal diameter of RV in the four‐chamber apical view at end‐diastole. Dilatation of the RV was also assessed in relation to LV size. The systolic RV function was assessed using tricuspid annular longitudinal excursion (TAPSE) recorded by M‐mode and by measuring s´ velocity (ie, a peak systolic velocity of the tricuspid annulus by Doppler tissue imaging), according to current guidelines. 8 Global RV function—radial and longitudinal—was also assessed visually.
Left ventricular (LV) end‐diastolic dimension was measured in the apical four‐chamber view, and LV systolic function was reported as ejection fraction (EF). 8 The severity of tricuspid regurgitation (TR) was quantified by an integrated approach using characteristics of colour flow and continuous wave Doppler recordings. The maximal systolic TR velocity from any available view provided an estimation of systolic acute pulmonary artery pressure (sPAP). 8 , 11 The presence or absence of aPH was estimated based on calculated sPAP via the RV and right atrial (RA) pressure gradient from the maximal TR velocity, adding the estimated RA pressure (from the inferior vena cava diameter and collapse). We used a definition of elevated sPAP > 35 mmHg based on sPAP estimation from TTE as previously described in Covid‐19 patients by Pagnesi et al 12 to determine the presence of aPH. For patients in whom the TR Doppler signal could not be measured or recorded, the sPAP could not be calculated. Patients without TR or patients with an estimated sPAP ≤ 35 mmHg and no RV dysfunction have a low echocardiographic probability of PAH and were classified as a non‐aPH group. 11 The estimation of RA pressure was performed in the subcostal view by measuring the diameter of the inferior vena cava and recording its respiratory changes. 8
2.4. Laboratory tests
Values for D‐dimer (mg L−1, fibrinogen equivalent units [FEU], fibrin degradation products; turbidimetry; decision limit, age dependent: 0.01 × age [years]) as a marker of deep venous thrombosis/pulmonary embolism, NTproBNP (ng L−1; prohormone of brain natriuretic peptide; electrochemiluminescence; reference interval: women, <222; men, <194) as a marker of cardiac failure, and troponin T (ng L−1; electrochemiluminescence; reference interval: <15) as a marker of myocardial injury were taken from the same day as the TTE, forming an additional part of the daily clinical routine. All laboratory analyses were performed at the Karolinska University Hospital Laboratory.
2.5. Statistics
Since this was a purely exploratory observational study, no formal power calculation was performed, mainly because no previous data exist regarding Covid‐19 and central hemodynamics.
2.5.1. Primary aim
The primary aim of the study was twofold. First, the goal of this exploratory observational study was to determine the presence of aPH based on described TTE criteria and to investigate if the presence of aPH affected mortality in ICU‐treated Covid‐19 patients. The follow‐up data were collected 21 d after the performance of TTE. Prior to the study, we decided to compare the outcome of dead vs. alive for aPH patients vs. non‐aPH patients. Thus, 30‐d mortality following the admission to ICU was also assessed.
2.5.2. Secondary aim
Another aim was to investigate the correlation between the various patient‐related factors (Table 1) with severe Covid‐19 disease. In particular, the relationship between aPH and the plasma levels of D‐dimer, NTproBNP, troponin T and LVEF was identified for exploration. A further 21‐d post‐TTE outcome analysis was also performed based on a worse (dead or still on mechanical ventilation) or a better (still in the ICU but not requiring mechanical ventilation, discharged to a regular ward or discharged home) outcome between patients with or without aPH as determined by TTE.
TABLE 1.
Patient data for total population and for patients with estimated sPAP ≤ 35 mmHg (group non‐aPH) and patients with an estimated sPAP > 35 mmHg (group aPH)
| Parameter |
Total population (n = 67) |
Non‐aPH (n = 41) |
aPH (n = 26) |
|---|---|---|---|
| Gender (male/female) | 63/4 | 38/3 | 25/1 |
| Age (years) | 58 (34‐79) | 56 (34‐74) | 60 (37‐79) |
| No risk factor (n%) | 24 (35.8%) | 15 (36.6%) | 9 (34.6%) |
| One or more risk factors (n%) | 43 (64.2%) | 26 (63.4%) | 17 (65.4%) |
| Days with symptoms prior to admission to hospital (range) | 10 (2‐28) | 10 (2‐28) | 10 (3‐28) |
| Hospital admission to ICU admission (range) | 2 (0‐10) | 1 (0‐10) | 2 (0‐7) |
| Blood type (missing n = 12) | (9) | (3) | |
| A | 30 | 19 | 11 |
| B | 6 | 4 | 2 |
| AB | 4 | 3 | 1 |
| O | 15 | 6 | 9 |
| RhD‐positive | 51 | 29 | 22 |
| RhD‐negative | 4 | 3 | 1 |
| Cardiovascular drugs at TTE (n) | |||
| Vasopressor (norepinephrine IV, yes/no) | 41/26 | 24/17 | 17/9 |
| Inotrope (milrinone IV, yes/no) | 4/63 | 4/37 | 0/26 |
| CT for PE (n) | |||
| Positive for PE (any degree) | 11 | 4 | 7 |
| Negative for PE | 14 | 8 | 6 |
| CT not performed | 42 | 29 | 13 |
| CRRT anytime during the ICU stay (n) | 21 | 9 | 12 |
| Tricuspid regurgitation at TTE (n) | |||
| No or minimal TR | 33 | 33 | — |
| TR allowing assessment of sPAP | 34 | 8 | 26 |
| Plasma D‐dimer (mg L−1) |
3.4 (0.61 above detection limit of 35) |
3.2 (0.61‐31.4) | 3.7 (0.64‐>35) |
| Plasma NTproBNP (ng L−1) | 671 (45‐30 300) | 470 (45‐24 600) | 1430 (102‐30 300)*** |
| Plasma Troponin T (ng L−1) | 26 (22‐407) | 15 (5‐407) | 63 (22‐352)*** |
Median (range) when appropriate. ***represents a statistical difference between non‐aPH vs. aPH with a P value < .001.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Since a Gaussian distribution could not be expected, the data were presented and analysed using a non‐parametric approach. Data are presented as median and range. Chi‐square analysis with Yates' correction (outcome) or a Mann–Whitney U test (D‐dimer, NTproBNP, troponin, and LVEF) was used for comparison between groups; P values < 0.05 were considered statistically significant.
3. RESULTS
A total of 76 patients were retrospectively enrolled in the study from 10 April to 19 May 2020. Nine patients were excluded for various reasons (ie, incorrect diagnosis of Covid‐19 [n = 2], ongoing veno‐arterial ECMO [n = 4], never admitted to ICU [n = 2], or TTE performed by another echocardiographer [n = 1]). The characteristics of the remaining 67 patients are displayed in Table 1. Data related to group non‐aPH and group aPH are also shown in Table 1.
The mode of ventilatory support and ventilatory settings are summarized in Table 2.
TABLE 2.
Type of ventilator support and settings
| Parameter |
Total population (n = 67) |
Non‐aPH (n = 41) |
aPH (n = 26) |
|---|---|---|---|
| Spontaneous/CPAP | 5 | 3 | 2 |
| NIV/APRV | 6 | 5 | 1 |
| Pressure support | 33 | 23 | 10 |
| Pressure control | 22 | 10 | 12 |
| Pressure‐regulated volume control | 1 | 0 | 1 |
| FiO2 | 0.5 (0.27‐1.0) | 0.45 (0.27‐1.0) | 0.65 (0.3‐1.0) |
| CPAP/PEEP (cm H2O) | 10 (0‐16) [1] | 10 (0‐16) [1] | 10 (5‐16) |
| Support/driving pressure (cm H2O) | 11 (0‐32) [11] | 13 (0‐28) [6] | 15 (5‐32) [5] |
Abbreviations: [ ], missing values; APRV, airway pressure release ventilation; CPAP, continuous positive airway pressure; FiO2, fraction of inspired oxygen; NIV, non‐invasive ventilation.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
TTE parameters for the aPH and non‐aPH groups are shown in Table 3.
TABLE 3.
Echocardiographic parameters for groups aPH and non‐aPH
| Variable | Non‐aPH (n = 41) | aPH (n = 26) |
|---|---|---|
| Tricuspid valve insufficiency (yes/no) | 8/33 [n = 41] | 26/0 [n = 26] |
| TR velocity (m/s) | 2.5 (2.2‐2.6) [n = 8] | 3.2 (2.6‐3.9) [n = 26] |
| Estimated sPAP (mmHg) | 32 (22‐35) [n = 8] | 50 (37‐76) [n = 26] |
| PAAT (ms) | 100 (65‐146) [n = 36] | 84 (50‐130) [n = 21] |
| ≤90 ms (n) | 9 | 13 |
| IVC diameter (mm) | 19 (9‐37) [n = 40] | 23 (14‐29) [n = 26] |
| Estimated RAP (mmHg) | 8 (0‐15) [n = 40] | 11.5 (3‐15) [n = 26] |
| RV size and function | ||
| RV dilatation (yes/no) | 13/25 [n = 38] | 14/12 [n = 26] |
|
RV basal diameter in 4‐chamber view (mm) |
38 (29‐51) [n = 37] | 42.5 (30‐53) [n = 26] |
| TAPSE (mm) | 21 (14‐30) [n = 38] | 23.5 (15‐31) [n = 26] |
| RV s´ (m/s) | 0.15 (0.08‐0.22) [n = 29] | 0.17 (0.13‐0.22) [n = 22] |
| RV global systolic function (normal/impaired) | 27/13 [n = 40] | 17/9 [n = 26] |
| RV radial function (normal/impaired) | 26/14 [n = 40] | 14/12 [n = 26] |
| LV size and function | ||
| LVEDD (mm) | 50 (34‐60) [n = 38] | 49 (4052) [n = 26] |
| LVEF (%) | 55 (40‐80) [n = 41] | 58 (50‐75) [n = 26] |
| LVEF (normal/impaired) | 31/10 [n = 41] | 22/4 [n = 26] |
Data presented as median (range) or n, as appropriate.
Abbreviations: IVC, inferior vena cava; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; PAAT, pulmonary artery acceleration time; RAP, right atrial pressure; RV, right ventricle; s´, peak systolic Doppler tissue velocity recorded in RV close to tricuspid annulus; sPAP, systolic pulmonary artery pressure; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
The time between ICU admittance and the TTE was 8 (1‐28) days.
3.1. Primary aim
In total, 26 patients (39%) displayed a sPAP value of > 35 mmHg and were designated as having aPH; 33 patients either had no TR or such a small TR that the assessment of sPAP was not possible. Eight patients had a TR that allowed the estimation of sPAP but did not demonstrate sPAP values exceeding 35 mmHg. Thus, the non‐aPH group consisted of 41 patients in total (Table 1).
At the 21‐d follow‐up, a greater proportion of patients (n = 12) in the aPH group had died compared to patients (n = 3) in the non‐aPH group (46% vs. 7%, respectively; P < .001; see Figure 2). Also, 30‐d mortality following admission to ICU care follow‐up showed a higher mortality rate in the aPH group (n = 11) as compared to non‐aPH group (n = 3; 42% vs. 7%, respectively; P < .002). The patient in group aPH who differed between the 21‐d follow‐up and the 30‐d ICU mortality died on ICU day 31.
FIGURE 2.
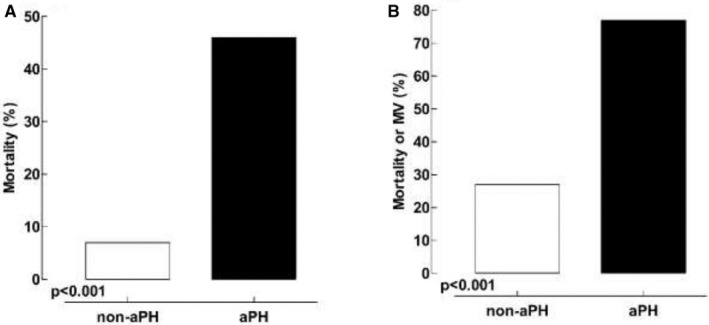
Twenty‐one‐day outcome following the performance of the TTE. Panel A, primary outcome (mortality) in patients diagnosed with aPH vs. patients belonging to the non‐aPH group. Panel B, secondary outcome (mortality or still requiring mechanical ventilation [MV]) in patients diagnosed with aPH vs. patients belonging to the non‐aPH group
3.2. Secondary aims
Patients with aPH displayed higher NTproBNP and troponin T plasma levels compared to patients in the non‐aPH group (1430 [102‐30 300] vs. 470 [45‐24 600] ng L−1, P = .0007 and 63 [22‐352] vs. 15 [5‐407] ng L−1, P = .0002, respectively), as noted in Table 1 and Figure 3. There was no statistical difference between the groups regarding D‐dimer (P = .4319) or LVEF (P = .3299) values (Table 1, Figure 3).
FIGURE 3.
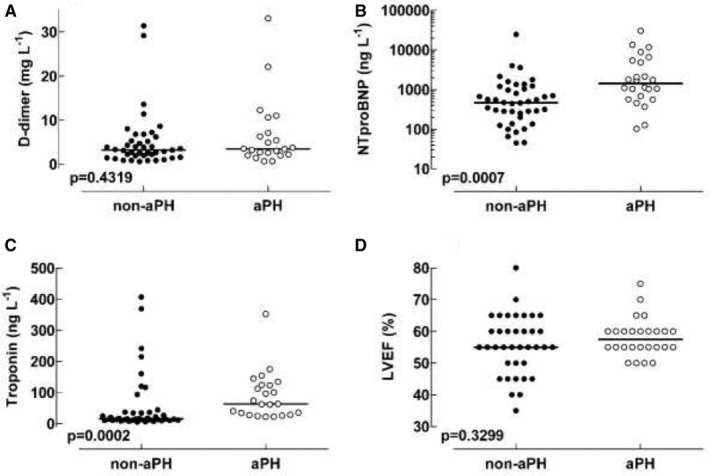
Values for A, D‐dimer, B, NTproBNP, C, Troponin T, and D, LVEF. Note the y‐axis in panel B is logarithmic
More patients in the aPH group belonged to the combined outcome of being dead or still on mechanical ventilation at 21‐day post‐TTE (n = 20) compared to patients in the non‐aPH group (n = 11; P < .001), as shown in Figure 2.
4. DISCUSSION
The main finding of this study was that approximately 40% of Covid‐19 patients treated in an ICU were diagnosed with aPH as per established echocardiographic criteria. Furthermore, patients with aPH also had higher NTproBNP plasma values (a biomarker of cardiac failure) and displayed a significantly worse 21‐d outcome (46% mortality) compared to patients without aPH (7% mortality).
Although data are available regarding the presence and degree of aPH in regular ICU patients with acute lung injury (ALI) or acute respiratory distress syndrome (ARDS), 13 , 14 limited data have been reported specifically for patients with Covid‐19 pneumonia. Moreover, it has been speculated that aPH, with associated negative effects on the right ventricle, may be both underdiagnosed and underestimated as a reason for the deterioration and death of patients with regular ALI/ARDS. In fact, previous studies have shown that the presence of aPH is an independent risk factor for poor outcomes in ICU care. 13 , 14
Typical ALI/ARDS is known to be associated with pulmonary vascular inflammation and pulmonary microembolism. 15 , 16 However, the different facets of Covid‐19 pathophysiology appear especially prone to jeopardize pulmonary circulation. First, the endothelium of pulmonary microvessels suffers from local inflammation known as endothelitis, which occurs partly in response to an activated complement system, 1 , 2 resulting in the reduced local production of vasodilating substances (eg, nitric oxide and prostacyclin). This yields a spastic component of increased pulmonary vascular resistance, resulting in elevated pulmonary artery pressure. Furthermore, the disease is associated with hypercoagulability that causes both microembolization and macroembolization of the pulmonary vasculature. 3 This situation is further compounded by an unusual tendency for in situ thrombosis of small pulmonary vessels, 2 which does not typically occur in regular ALI/ARDS. The occlusion of pulmonary vessels by embolization and in situ thrombosis will add a fixed component of increased pulmonary vascular resistance, thereby augmenting the increase of pulmonary artery hypertension. Thus, the combination of a spastic with a fixed component affecting pulmonary vascular resistance and pulmonary artery pressure will set the stage for the deleterious sequence of aPH, RVS, and RVF. Support for this line of reasoning includes the recent publication of fatal cases of Covid‐19 due to the development of acute cor pulmonale. 4
4.1. Primary aim
The results of this study show that aPH, when diagnosed by TTE criteria as an sPAP > 35 mmHg, 12 , 13 is common in Covid‐19 ICU patients. Furthermore, patients who demonstrated aPH on TTE had a worse short‐term outcome (mortality rate of 46%) than patients without aPH (mortality rate of 7%, see Figure 2).
Our findings are in line with recently published data by Pagnesi et al, who reported on hospitalized, pre‐ICU patients with Covid‐19. 12 Their study used similar TTE observations to define aPH and found the presence of aPH and RVS to be 12% and 14.5%, respectively. Furthermore, ICU admission or in‐hospital mortality was substantially increased in patients with aPH compared to patients not diagnosed with aPH (41.7% vs. 8.5%), a finding that corroborates our observation of an almost 7 times higher mortality rate in ICU‐treated Covid‐19 patients diagnosed with aPH.
In a prospective study, Bossier et al reported a 22% incidence of aPH in a regular ALI/ARDS population; the presence of aPH (as determined by pulmonary artery catheterization) was associated with a 60% 28‐d mortality, in contrast to 36% in patients without aPH. 14 In comparison, the incidence of aPH in our cohort of severe Covid‐19 was twice as high (46%) but with a similar mortality rate (46%). However, the mortality rate of Covid‐19 patients without aPH was found to be substantially lower (7%). Thus, it can be speculated that if aPH can be prevented or successfully treated (eg, via thrombo‐prophylaxis, active thrombolysis, and selective pulmonary vasodilatation) in severe Covid‐19 infection, then mortality may be lower than in regular ALI/ARDS.
Since the finalization of the original manuscript, d'Alto et al have reported on overall mortality (26%) in severe Covid‐19 infection in relation to TTE findings 17 and found increased sPAP, reduced TAPSE, and reduced P/F ratio (arterial partial pressure of oxygen [PaO2]/fraction of inspired O2 [FIO2] ratio) in non‐survivors. Since our study is primarily focused on the presence of aPH and not overall mortality, it is difficult to make an adequate comparison with the d'Alto study. A further discrepancy is that d'Alto et al mainly studied non‐intubated patients (83%), whereas only 18% of our patients received nasal oxygen or non‐invasive ventilation. Thus, the d'Alto study is, in this aspect, more akin to research by Pagnesi et al 12 However, both studies identify that an sPAP above 35 mmHg appears to be associated with a poorer outcome, even though we did not observe any significant decrease in TAPSE in the aPH group in our study (Table 3).
4.2. Secondary observations
A striking overrepresentation of males was observed in the ICU cohort, which reflects findings from previous publications. Regarding age, the presence of one or more risk factors, and days of symptoms prior to hospital admission, our data did not diverge from the SIR results. 6
There was no difference between the aPH and non‐aPH groups with regard to biomarkers for thrombo‐embolism (D‐dimer) or LVEF. The lack of difference between D‐dimer values among the two groups is somewhat surprising since it could be assumed that patients in the aPH group would have a greater problem with thrombo‐embolization than the non‐aPH group, thereby at least partly explaining the difference in sPAP. This lack of difference could be due to the following finding: both groups had a similar thrombo‐embolism burden, but the blood clots in the non‐aPH group were fixed in the peripheral veins, whereas a certain amount of blood clots had detached and embolized the pulmonary circulation in the aPH group, thereby producing increased sPAP. Alternatively, perhaps the aPH patients had more severe ‘endothelitis’ of the pulmonary micro‐vessels, thereby creating a more pronounced spastic component of pulmonary vascular resistance than the non‐aPH group.
In contrast, the biomarkers of cardiac failure (NTproBNP 18 and myocardial injury (troponin T) 19 were higher in the aPH group as compared to non‐aPH patients. Our observation that few patients were judged to need inotropic support, paired with a well‐preserved LVEF on TTE (Table 1), indicated that NTproBNP and troponin release likely stems from a strained or failing right ventricle.
4.3. Study limitations
Determining the presence of aPH by echocardiography poses certain challenges. To handle this issue, we decided to use only TTE evaluations performed by a single operator to minimize interoperator variability. The decision to allow a single echocardiographer may have introduced a certain degree of selection bias. However, the decision to refer a patient for TTE was made by the physician responsible for the patient's care and not by the echocardiographer. Since our results are in line with the findings of previous publications in this specific context, 12 , 17 we believe that the effect of any such bias is appropriately limited. However, our findings would benefit from further validation in a less selected population.
The diagnosis of aPH by TTE may be challenging and inaccurate in individual patients, especially when sPAP is calculated as a sum of the pressure gradient across the tricuspid valve from TR velocity and estimated RA pressure from inferior vena cava dimension and respiratory changes. Therefore, right heart catheterization is a recommended reference method. 11 However, in the Covid‐19 pandemic situation, these invasive procedures were not feasible, while non‐invasive TTE was easily available and repeatable. Furthermore, we opted to adhere to the TTE definitions from Pagnesi and colleagues, 12 who used an estimated sPAP > 35 mmHg as the cut‐off value for the diagnosis of aPH. We defined the presence of aPH from calculated sPAP and have included TR velocity results in Table 3.
The extreme workload in the ICUs and the lack of research nurses precluded any extra blood sampling for study purposes; therefore, no specific arterial blood gas sampling was performed during the TTE. Since blood gas parameters (eg, pronounced hypoxia, hypercarbia, and acidosis) may affect the pulmonary artery pressure, this needs to be considered when interpreting the study results.
Due to existing circumstances, the timing of TTE could not be standardized, which resulted in a variable time point with regard to ICU admission. Even if standardization had been possible, it is not clear to which event the TTE should be timed (eg, days from the start of symptoms, time from hospital admission, or time from ICU admission). However, the presence of aPH at any time during the ICU stay (early, intermediate, or late) appeared to translate to a worse short‐term outcome.
In conclusion, when using an accepted echocardiographic definition of elevated systolic pulmonary arterial pressure (ie, estimated sPAP > 35 mmHg), approximately 40% of Covid‐19 patients admitted to ICU care at our hospital met the criteria for aPH. The presence of aPH was also associated with high NTproBNP values (indicating cardiac failure) and troponin T values (indicating myocardial injury), as well as a sevenfold increase of 21‐d mortality compared to patients without aPH.
CONFLICTS OF INTEREST
JN, AL, and ME have no competing interests to declare. CF, and PAL act as advisors to ATTGENO AB, a startup pharmaceutical drug development company (Sweden). CF is also a minor stock owner in ATTGENO AB and directs Claes Frostell Research & Consulting AB (Sweden). PAL is a minor stock owner in ATTGENO AB and directs AnestInvest AB (Sweden). CA and PA are the founders and major owners of ATTGENO AB.
AUTHOR CONTRIBUTIONS
JN performed all TTE, TTE data handling and analysis and contribution to and review of the manuscript. AL supported JN during the performance of TTE and reviewed the manuscript. CF contribution to study design and ethics application. Data retrieval and manuscript text and review. CA contributed to study design and ethics application and data retrieval and manuscript review. PA contributed to study design and ethics application and data retrieval and manuscript review. ME performed TTE data handling and analysis and contribution to and review of manuscript. PAL. performed the primary investigation and contribution to study design and ethics application, data retrieval, data analysis, and manuscript text and review.
ACKNOWLEDGEMENTS
The authors would like to thank Senior Professor Göran Hedenstierna for providing a critical review of the manuscript and Senior Professor Staffan Eksborg for assistance with Mann–Whitney U statistics and figure production. We also want to thank Professor Edide Weitzberg for help with data retrieval. This study was supported by the Swedish Research Council (2015‐02880) and an unrestricted educational grant from Attgeno AB.
Norderfeldt J, Liliequist A, Frostell C, et al. Acute pulmonary hypertension and short‐term outcomes in severe Covid‐19 patients needing intensive care. Acta Anaesthesiol Scand. 2021;65:761–769. 10.1111/aas.13819
Christofer Adding and Per Agvald also represent Attgeno AB, Stockholm, Sweden
REFERENCES
- 1. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endothelitis in COVID‐19. Lancet. 2020;395(10234):1417‐1418. 10.1016/S0140-6736(20)30937-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID‐19 infection: a report of five cases. Transl Res. 2020;220:1‐13. 10.1016/j.trsl.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res. 2020;191:145‐147. 10.1016/j.thromres.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Creel‐Bulos C, Hockstein M, Amin N, et al. Acute cor pulmonale in critically Ill patients with Covid‐19. N Engl J Med. 2020;382(21):e70. 10.1056/NEJMc2010459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guzik TJ, Mohiddin SA, Dimarco A, et al. COVID‐19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020;116(10):1666‐1687. 10.1093/cvr/cvaa106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Swedish Intensive Care Registry . https://www.icuregswe.org/data‐‐resultat/covid‐19‐i‐svensk‐intensivvard/
- 7. Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American society of echocardiography endorsed by the European association of echocardiography, a registered branch of the European society of cardiology, and the Canadian society of echocardiography. J Am Soc Echocardiogr. 2010;23(7):685‐713. [DOI] [PubMed] [Google Scholar]
- 8. Lang RM, Badano LP, Mor‐Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. 2015;28(1):1‐39.e14. [DOI] [PubMed] [Google Scholar]
- 9. Cordina RL, Playford D, Lang I, Celermajer DS. State‐of‐the‐art review: echocardiography in pulmonary hypertension. Heart Lung Circ. 2019;28(9):1351‐1364. 10.1016/j.hlc.2019.03.003 [DOI] [PubMed] [Google Scholar]
- 10. Fisher MR, Forfia PR, Chamera E, et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med. 2009;179(7):615‐621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European society of cardiology (ESC) and the European respiratory society (ERS): endorsed by: association for European paediatric and congenital cardiology (AEPC), international society for heart and lung transplantation (ISHLT). Eur Heart J. 2016;37(1):67‐119. [DOI] [PubMed] [Google Scholar]
- 12. Pagnesi M, Baldetti L, Beneduce A, et al. Pulmonary hypertension and right ventricular involvement in hospitalised patients with COVID‐19. Heart. 2020;106(17):1324‐1331. 10.1136/heartjnl-2020-317355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stamm JA, McVerry BJ, Mathier MA, et al. Doppler‐defined pulmonary hypertension in medical intensive care unit patients: Retrospective investigation of risk factors and impact on mortality. Pulm Circ. 2011;1(1):95‐102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boissier F, Katsahian S, Razazi K, et al. Prevalence and prognosis of cor pulmonale during protective ventilation for acute respiratory distress syndrome. Intensive Care Med. 2013;39(10):1725‐1733. 10.1007/s00134-013-2941-9 [DOI] [PubMed] [Google Scholar]
- 15. Tomashefski JF Jr, Davies P, Boggis C, Greene R, Zapol WM, Reid LM. The pulmonary vascular lesions of the adult respiratory distress syndrome. Am J Pathol. 1983;112(1):112‐126. [PMC free article] [PubMed] [Google Scholar]
- 16. Hill NS, Roberts K, Preston I. Pulmonary vasculopathy in acute respiratory distress syndrome: something new, something old. Am J Respir Crit Care Med. 2010;182(9):1093‐1094. 10.1164/rccm.201007-1116ED [DOI] [PubMed] [Google Scholar]
- 17. D'Alto M, Marra AM, Severino S, et al. Right ventricular‐arterial uncoupling independently predicts survival in COVID‐19 ARDS. Crit Care. 2020;24(1):670. 10.1186/s13054-020-03385-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roberts E, Ludman AJ, Dworzynski K, et al. The diagnostic accuracy of the natriuretic peptides in heart failure: systematic review and diagnostic meta‐analysis in the acute care setting. BMJ. 2015;350:h910. 10.1136/bmj.h910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McCarthy CP, Raber I, Chapman AR, et al. Myocardial injury in the era of high‐sensitivity cardiac troponin assays: a practical approach for clinicians. JAMA Cardiol. 2019;4(10):1034‐1042. 10.1001/jamacardio.2019.2724 [DOI] [PubMed] [Google Scholar]


