Abstract
Background and objectives
Cytokine release syndrome in COVID‐19 is due to a pathological inflammatory response of raised cytokines. Removal of these cytokines by therapeutic plasma exchange (TPE) prior to end‐organ damage may improve clinical outcomes. This manuscript is intended to serve as a preliminary guidance document for application of TPE in patients with severe COVID‐19.
Material and methods
The available literature pertaining to the role of TPE for treatment of COVID‐19 patients was reviewed to guide optimal management. It included indication, contraindication, optimal timing of initiation and termination of TPE, vascular access and anticoagulants, numbers and mode of procedures, outcome measures and adverse events.
Results
Out of a total of 78 articles, only 65 were directly related to the topic. From these 65, only 32 were acceptable as primary source, while 33 were used as supporting references. TPE in critically ill COVID‐19 patients may be classified under ASFA category III grade 2B. The early initiation of TPE for 1–1·5 patient’s plasma volume with fresh frozen plasma, or 4–5% albumin or COVID‐19 convalescent plasma as replacement fluids before multiorgan failure, has better chances of recovery. The number of procedures can vary from three to nine depending on patient response.
Conclusion
TPE in COVID‐19 patients may help by removing toxic cytokines, viral particles and/or by correcting coagulopathy or restoring endothelial membrane. Severity score (SOFA & APACHE II) and cytokine levels (IL‐6, C‐reactive protein) can be used to execute TPE therapy and to monitor response in COVID‐19 patients.
Keywords: COVID‐19, cytokine release syndrome, therapeutic plasma exchange, preliminary guidance
Introduction
Severe acute respiratory syndrome coronavirus type 2 (SARS‐CoV‐2) was first reported to the World Health Organization (WHO) on 31 December 2019 and was subsequently declared as a pandemic on 11 March 2020. This causative SARS‐CoV‐2 virus is classified under Family Coronaviridae which is enveloped, spherical shaped virion with 80–220 nm diameter, containing positive‐sense single‐stranded RNA genome. The disease caused by this virus is collectively termed as coronavirus disease 2019 (COVID‐19), and the viral RNA genome has >90% nucleotide identity with pangolin and bat coronaviruses (CoV), but only 50% identity to the common cold CoV [1]. Although it may produce less frequent respiratory symptoms in infected patients, it can also cause acute respiratory distress syndrome (ARDS), multiorgan failure (MOF), significant coagulopathy and substantial mortality [2, 3].
As in severe sepsis, critically ill COVID‐19 patients often die from the host’s maladaptive response rather that the primary infection. Cytokine release syndrome (CRS) or cytokine storm, a pathological inflammatory response at local and systemic levels following infection, is thought to influence disease severity and mortality [4, 5]. CRS was previously described during the severe acute respiratory syndrome coronavirus type 1 (SARS‐CoV‐1) and Middle East respiratory syndrome coronavirus (MERS) epidemics, when significantly higher levels of serum cytokines were observed, which correlated with pulmonary pathology [6, 7, 8, 9, 10, 11]. During SARS‐CoV‐2 infection, similar phenomena were observed [12, 13] with increased levels of inflammatory cytokines activating T helper type 1 (Th1) and Th2 responses [5]. Although cytotoxic T cells generally assist in attenuating viral infections, they also accelerate progression of the systematic inflammatory response in COVID‐19 by increasing circulating CD14+ CD16+ monocytes and interleukin (IL)‐6 levels [13].
Critically ill COVID‐19 patients have elevated levels of multiple inflammatory cytokines, including IL‐1β, IL‐2, IL‐6, IL‑7, IL‐8, IL‑10, granulocyte colony‑stimulating factor, interferon γ‑induced protein 10, monocyte chemoattractant protein‐1, macrophage inflammatory protein‐1α, tumour necrosis factor‐α, and chemokines (e.g. C‐C motif chemokine ligand (CCL)‐2, CCL‐3, CCL‐5) [13]. Lung epithelial cells produce IL‐8, a neutrophil and T‐cell chemoattractant, which encourages pulmonary infiltration of inflammatory cells [14]. In COVID‐19, cytokine levels (e.g. IL‐2R, IL‐6) positively correlate with disease severity, progression, ARDS and mortality [15, 16].
Therapeutic plasma exchange (TPE), where patient plasma is replaced by an iso‐oncotic fluid (e.g. donor plasma, colloid [frequently 4–5% albumin], crystalloid), was proposed as a potential treatment for CRS. TPE was previously used in patients with septic shock and MOF [17, 18], and it was also applied as an optional treatment during SARS‐CoV‐1, H1N1 influenza and MERS epidemics. For example, three cycles of TPE on consecutive days were associated with improved outcomes in three critically ill paediatric H1N1 influenza patients with ARDS and hemodynamic instability [19].
Removing cytokines and blocking the CRS, as early as possible, before end‐organ damage or substantial endothelial damage has occurred, are currently thought to provide the best chance for therapeutic benefit [1]. To date, very few high‐quality studies have evaluated TPE in COVID‐19 [20, 21]. Considering the lack of standardized guidelines, the current manuscript can serve as a guidance document for clinicians and transfusion apheresis professionals regarding using TPE to treat COVID‐19 patients especially those with evidence of CRS.
Materials and methods
This document was prepared by a subgroup of the convalescent plasma working group of the International Society of Blood Transfusion (ISBT), consisting of physicians from different parts of the world with extensive expertise in clinical apheresis and critical care. The subgroup developed and addressed a series of questions related to this topic using current American Society for Apheresis (ASFA) guidelines and evidence from peer‐reviewed publications; the latter were identified using PubMed, Science Direct and Google Scholar. The search terms were “COVID‐19,” “SARS‐CoV‐2,” “Cytokine Storm,” “Cytokine Release syndrome,” “Apheresis,” “Plasma Exchange,” and “Therapeutic Plasma Exchange.”
All identified publications such as case series, case reports or original studies were included. The inclusion criteria of publications were directly related to COVID‐19 and TPE therapy, and they included all outcome of interest such as mortality, length of hospital or ICU stay, or requirement of mechanical ventilation after treatment. Literature search on other infectious diseases or previous viral outbreaks in which TPE had partial or proven beneficial effect was performed. Data collection table was designed according to the purpose of the study. It included (1) study characteristics (year and country of publication, type of publication, patient population, number of TPE performed, type of replacement fluid and anticoagulant used), (2) quality assessment and (3) outcome (mortality, detachment from mechanical ventilation, length of hospital and ICU stay). One author extracted data from all searched manuscripts to data collection table and assessed the quality of each study. Remaining group of authors evaluated the searched publications and validated the extracted data.
Discussion points were cross‐referenced based on available data to prepare the objective variables of this study. These variables of TPE demarcated from data extractions were indication and contraindication, optimal time to start and stop the procedure, choice of vascular access and anticoagulants, mode of therapy, outcome measure(s) to determine the number of procedures, and possible adverse events.
Results
The literature search identified 78 potential articles. Of these, only 65 were directly related to COVID‐19, CRS or TPE, and the remaining 13 were excluded because they were not directly related to the topic. Of the 65 selected manuscripts, 32 were considered acceptable as primary source material, while the remaining 33 were considered supporting references because they related to other septic diseases, reply letters to previous articles, and pre‐trial protocols (Fig. 1).
Fig. 1.
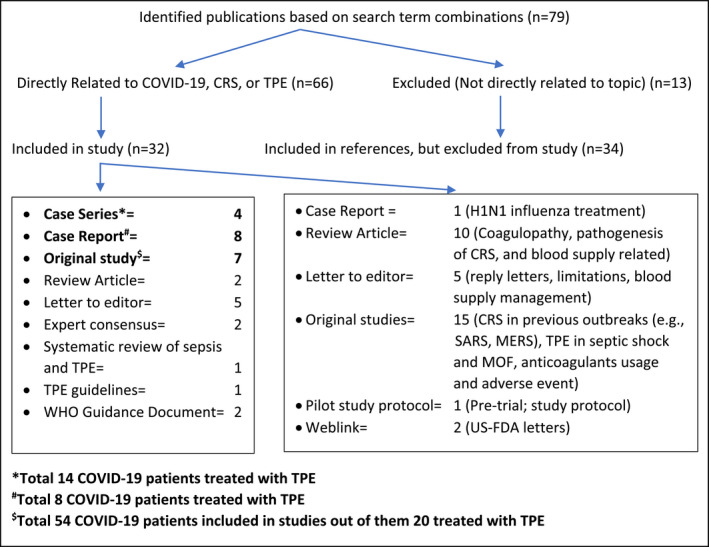
Literature search strategy. CRS, Cytokine Release Syndrome; TPE, Therapeutic Plasma Exchange; WHO, World Health Organization; SARS, Severe Acute Respiratory Syndrome; MERS, Middle East Respiratory Syndrome; MOF, Multiorgan Failure; US‐FDA, United States Food and Drug Administration.
From all, selected manuscripts were summarized:
American Society for Apheresis (ASFA) Guidelines: As per ASFA‐2019 guidelines [22], TPE for patients with infections and MOF due to various causes falls under Category III Grade 2B, meaning that the optimum role of apheresis therapy in treating these patients is not established, and that the current moderate quality of peer‐reviewed evidence only supports an overall weak recommendation for this approach. Therefore, using TPE for COVID‐19 patients with MOF may also be considered under this category.
Indications: Studies of TPE in septic patients with MOF, due to pathogens other than SARS‐CoV‐2, suggested that TPE can help to restore hemodynamic stability [23, 24]. It has been applied in a small case series of 3 severe to critically ill COVID‐19 patients when they begin to exhibit thrombocytopenia, elevated levels of C‐reactive protein and lactate dehydrogenase, and an increased neutrophil/lymphocyte ratio, along with deteriorating oxygenation that might require high flow nasal oxygen or mechanical ventilation and/or exhibiting initial signs of shock or MOF [12]. For example, an expert panel from the National Clinical Research Centre for Infectious Diseases in China suggests that blood purification therapy, artificial liver support system (ALSS), may be indicated for COVID‐19 patients when pro‐inflammatory cytokines (e.g. IL‐6) are more than 5 times the normal value or have doubled in 24 h along with >10% lung involvement by pulmonary imaging [24].
Contraindications: Although there are no absolute contraindications for TPE in COVID‐19, patients who may be at higher risk for complications include those who are coagulopathic (e.g. with disseminated intravascular coagulation or receiving anticoagulants), severely haemodynamically unstable (or unable to tolerate significant fluid shifts), unable to tolerate central line placement, allergic to donor plasma or albumin, severely hypocalcaemic, or who received angiotensin‐converting enzyme inhibitors within the last 24 h.
Optimal Time to Initiate Therapy: Owing to the short half‐life of pro‐inflammatory cytokines, early initiation of TPE was suggested for patients with severe COVID‐19, especially for those exhibiting symptoms of MOF and ARDS. However, waiting until specific markers of MOF and ARDS are present may limit efficacy [18]. Nonetheless, some case reports and case series reported that TPE at 2–20 days after PCR positivity for SARS‐CoV‐2 improved clinical parameters, including PaO2/FiO2, extubation rates, C‐reactive protein levels, neutrophil/lymphocyte ratios, and all‐cause mortality at 14 and 28 days [12, 25, 26, 27, 28, 29]. Various healthcare Scoring systems (e.g. Acute Physiology and Chronic Health Evaluation II (APACHE), Age, PaO2/FIO2, and Plateau Pressure Score (APPS), Sequential Organ Failure Assessment (SOFA), Paediatric Logistic Organ Dysfunction (PELOD), Organ Failure Index (OFI)) were devised to help predict patient outcomes and drive clinical decisions; they have been variably used in assessing COVID‐19 patients [30, 31, 32]. For example, in one study, eight patients with maximum SOFA scores were treated with TPE, and changes in the SOFA score correlated well with CRS patient outcomes, suggesting that changes in SOFA scores, and potentially other scoring systems, could help predict outcomes in COVID‐19 patients [33].
Mode of Therapy: TPE can be performed alone or in conjunction with other blood purification modalities, including column immunoadsorption, double volume plasma filtrations, continuous plasma filtration and adsorption, multi‐filtration systems, continuous veno‐venous haemofiltration, slow continuous ultrafiltration, artificial liver plasma exchange, adsorption, and perfusion [12, 34]. Additional treatment options (e.g. immunoglobulins [35], corticosteroids [36], IL‐6 receptor inhibitor tocilizumab [37], alpha adrenergic receptor antagonists [38]) have also been considered with variable success to date. As such, this article focuses mainly on the use of TPE in isolation.
Vascular access: Although vascular access for TPE can be peripheral or central, central venous access is preferred, especially for patients with severe disease admitted to an intensive care unit and receiving multiple procedures. Ultrasound‐guided insertion, preferably of a double lumen dialysis central venous femoral or jugular catheter, is recommended to reduce the risk of vascular damage and infection risk to medical staff by limiting the number of insertion attempts [34].
Anticoagulation: Due to its safety and efficacy, Acid Citrate Dextrose formula‐A (ACD‐A) is the recommended anticoagulant for TPE [39]. Citrate anticoagulation has also been suggested in septic patients with MOF due to its potential immunosuppressive role [40]; however, it can induce hypocalcaemia, as 1‐1·8 mg/kg/min of a citrate infusion reduces ionized calcium by 25–35% [41]. Because infused citrate is mainly metabolized by the liver and kidneys, hypocalcaemia occurs more frequently in patients with liver or renal dysfunction. To prevent severe hypocalcaemia during TPE, oral or intravenous calcium supplementation, or combined citrate‐heparin anticoagulation are recommended. Despite undesirable side‐effects (e.g. bleeding, thrombocytopenia, osteoporosis), heparin alone may be considered in selected patients with contraindications to citrate infusion, including those with hypoxemia, insufficient tissue perfusion, severe liver or renal dysfunction, or receiving long duration TPE or LDL apheresi s [42].
Replacement Fluid: Due to the haemodynamic fragility of COVID‐19 patients, the use of iso‐oncotic replacement solutions is recommended (e.g. fresh frozen plasma (FFP), normal saline, or 4–5% albumin; separately or in combination) [43]. Although albumin and saline avoid donor exposure risks, their excessive use can increase risks of bleeding or thrombosis by depleting coagulation proteins [44]. Due to the thrombotic diathesis in COVID‐19 [43], FFP may be preferred as it has been shown to replenish levels of antithrombin III, protein C, protein S, and tissue plasminogen inhibitor [44]. When all three types of replacement fluid are used together, it is preferable to end the TPE procedure with FFP to help restore pre‐treatment levels of fibrinogen and other coagulation factors. Also, COVID‐19 convalescent plasma (CCP) as a replacement fluid has been considered for critically ill patients because it can possibly add (neutralizing) anti‐SARS‐CoV‐2 antibodies in addition to restore the dysregulated coagulation [45, 46].
Exchange Volume: Exchanging 1–1·5 of the patient’s plasma volume is recommended, as ~65% of an intravascularly present toxic substance (i.e. an “ideal solute”) is removed in this way. Plasma volume can be calculated by the patient’s total blood volume (TBV) and haematocrit (Hct) using formula: TBV = (~70 ml/kg) × (1 – Hct/100) [47]. In one case series of three severe COVID‐19 patients, all 3 patients improved in oxygenation, CRP and IL6 levels following a single TPE procedure using 3L of FFP [12]; however, when FFP is scarce, a minimum of 2L is suggested [34].
Using Outcome Measures to Determine the Total Number of TPE Procedures: The expected outcome from TPE is improvement in those parameters that led to the initiation of TPE, including amelioration of CRS. In some studies, patient outcomes improved after only one TPE procedure [12, 27, 29]; however, others used three to nine procedures to yield the desired effects [25, 26, 27, 28, 29, 35, 48, 49].
Adverse events: In COVID‐19 patients, all commonly known adverse events (AEs) of TPE can occur, including allergic reactions, hypocalcaemia and hypomagnesemia due to citrate, hypotension, arrhythmias, sensations of cold with temporarily elevated temperature, and vasovagal reactions [22]. Most AEs are mild and transient, although severe and potentially life‐threatening symptoms can occur, especially in critically ill patients (e.g. shock, severe hypotension requiring pressor drugs, persistent arrhythmias, haemolysis) [50]. In a pilot study, acute kidney injury and pulmonary embolism occurred in 10% and 20% of critically ill COVID‐19 patients, respectively [51].In addition to pro‐inflammatory cytokines, TPE also removes other plasma proteins, such as coagulation factors and immunoglobulins. Although a short course of TPE is unlikely to produce immunodeficiency, there is evidence that TPE might, at least temporarily, negatively affect the adaptive immune system (especially T‐cell) homeostasis [50, 52]. Monitoring the patient’s haemostasis parameters especially if FFP and/or CCP are not used as replacement fluids can mitigate additional haemorrhagic and thrombotic risks. Finally, TPE influences coagulation parameters in patients receiving therapeutic anticoagulation; thus, anti‐Xa levels may decrease while partial thromboplastin and prothrombin times may increase, which might lead to mild risks of bleeding or thrombosis [53].
Discussion
In view of the global increase in the number of critically ill COVID‐19 patients, clinicians have tried many experimental therapies. ASFA guidelines suggest TPE as a supportive therapy in patients with sepsis and MOF. Therefore, based on its pathophysiology and disease course, using TPE for COVID‐19 patients may be considered; nonetheless, high‐level evidence is still needed. Four mechanisms were proposed supporting the role of TPE to help modify the course of COVID‐19 and ameliorate end‐organ damage. The first is the removal of toxic substances with suppression of CRS [22]; the second suggests that double filtration TPE removes 60–140 nm viral particles [54]; the third proposes that TPE can correct the coagulopathy in COVID‐19 patients when using FFP as a replacement fluid [55]; and the fourth hypothesizes that TPE provides factors that stabilize and restore endothelial membranes [1].
The current contribution, based on available peer‐reviewed publications, is intended to guide clinicians and transfusion apheresis professionals on using TPE in COVID‐19. Because high‐level evidence is still lacking, we strongly support monitoring these patients within the context of clinical studies, ideally prospective randomized controlled trials, whenever possible. Suggested initiation and target levels of severity scores and cytokines for these studies are outlined in Table 1, based on severity criteria for CRS and other previous case reports and case series [56]. Assessing all parameters may not be necessary to start or stop the TPE procedures; single or multiple combinations of these parameters can be used to assess eligibility for TPE and subsequent responses. At this time, we cannot endorse one parameter over another; however, we do encourage using these tools to help identify and report on COVID‐19 patients who would benefit most from TPE.
Table 1.
Critical levels and proposed target levels of various parameters for TPE in COVID‐19.
| Parameters | Levels to initiate TPE | Target levels after TPE |
|---|---|---|
| SOFA score [31] | ≥3 | ≤2 |
| APACHE II score [30] | ≥17 | <17 |
| PiO2/FiO2 [56] | <150 | ≥150 |
| Oxygen saturation [12] | ≤93% | ≥98% |
| Respiratory rate [12] | >30/Min | <20/min |
| Lymphocyte Count (1·1–3·2 × 109/L) [56] | ≤0·6 | >1·1 |
| Neutrophil–lymphocyte ratio (NLR) [66] | ≥3·3 | <3·3 |
| C‐reactive protein (10–50 mg/L) [56, 57] |
≥100 at presentation, or ≥50 and doubled in past 48 h |
<50 |
| Lactate dehydrogenase (100–190 U/L) [56] | ≥250 | <250 |
|
Ferritin (23–336 µg/ml) [56] |
≥600 at presentation ≥300 and doubled in past 24 h |
<300 |
| D‐dimer (<1 µg/ml) [56] | ≥1 | <1 |
| IL‐6 (1–7 pg/ml) [56] | ≥30 | <30 |
The number of procedures for COVID‐19 patients can be decided based on the reduction or maintenance of pro‐inflammatory cytokine levels [57], organ severity scores, and/or improvement in respiratory function [12] following TPE. For example, a greater than 2‐fold reduction in IL‐6 levels, as compared to pre‐treatment values, or normal IL‐6 levels for 3 consecutive days, or blood lactate levels below 2 mmol/L for 3 days, or CRP levels <50 mg/L [57]. Improved respiratory function can be assessed by a decrease or cessation of the need for supplementary oxygen or mechanical ventilation, and/or pulmonary imaging demonstrating reduction of pulmonary lesion area by >30% as compared to earlier imaging [34]. Others also suggested normalization of heart rate and/or body temperature as potential endpoints [21, 34]. The number of procedures can also be determined on a case‐by‐case basis using at least one parameter in Table 1 as a key indicator to guide therapy.
Multiple pros and cons should be considered regarding using TPE in COVID‐19 patients (Table 2). One critical consideration is the high cost, which limits using TPE as a primary treatment, particularly in low‐ and middle‐income countries (LMIC), especially when more affordable therapies (e.g. dexamethasone) might be readily available. Another concern is that the efficacy of TPE for CRS is questionable because of the short half‐life and rapid activity of cytokines [58]; thus, therapies blocking cytokine action (e.g. receptor antagonists) might be more effective than intermittent cytokine removal by TPE [59]. A third consideration is decontamination and local restrictions after use of apheresis equipment after apheresis procedures in highly contagious patients. Also, the personal safety of the apheresis operator is of importance [60].
Table 2.
Pros and Cons of TPE procedure in COVID‐19.
| Pros | Cons |
|---|---|
| Studies from other causes of CRS suggesting a potential role of TPE in sepsis | Current level of moderate–high quality of peer‐reviewed evidence only supports a weak recommendation for TPE in COVID‐19 |
| Likely effective in early stage of illness to reduce severity or prevent MODS progression | Expensive, time‐ and resource‐intensive procedure not readily available everywhere, especially in rural settings and LMIC. Patients must be triaged to determine who will most likely benefit |
| No absolute contraindications for usage, procedure can be ended successfully at any time, if needed | Adverse events of TPE (e.g. citrate toxicity, hypotension) may contribute to haemodynamic instability |
| Can be used as an adjunctive therapy with other drugs | Removing patient’s neutralizing antibodies or therapeutic drugs during TPE can possibly delay recovery or reduce therapeutic benefit |
| Can directly remove 60‐140nm viral particles |
This is only beneficial for removing intravascular free viral particles, although viraemia is minimal‐absent in COVID‐19 |
| FFP as a replacement fluid is helpful in coagulopathy. Pathogen‐inactivated FFP may provide additional safety. |
Using albumin or saline as a replacement fluid can increase risk of coagulopathy. The use of FFP alone may increase risk of complications, including transfusion reactions |
| Using CCP towards the end of the TPE procedure can provide patients with neutralizing antibodies | May affect FFP and CCP inventory, based on local supply and volume used. |
| Operators need appropriate training for personal protective equipment (PPE) donning and doffing. | |
| Instruments require decontamination if used for COVID patients or requirement of dedicated apheresis instrument for treatment of COVID‐19 patients. |
The choice of replacement fluid is also another important consideration for TPE. For example, albumin and/or saline alone can worsen the coagulopathy. However, using FFP alone is also challenging due to its geographically variable risk of transfusion‐transmitted infections and transfusion‐related acute lung injury (TRALI) dependent on the country’s TRALI mitigation strategies and a relatively limited, type‐specific blood supply, particularly because the pandemic has affected blood centres’ collection abilities worldwide [61, 62]. Solvent‐detergent‐treated plasma or pathogen‐inactivated plasma could overcome these challenges, but this method may not be available in all countries. However, using albumin at the beginning of TPE, followed by FFP towards the end of the procedure, can mitigate these issues. The exact volume of FFP to use can be determined based on the patient’s coagulation parameters. Although in limited supply, CCP, which contains neutralizing antibodies, can also be used as a replacement fluid, again preferably at the end of procedure, because it can supply both coagulation factors and virus‐neutralizing antibodies [63]. Another option is to use albumin and FFP in a 1:1 ratio, substituting CCP towards the end of last TPE procedure, if available.
Recently, the United States’ FDA provided emergency use authorization (EUA) for TPE therapy with the Spectra Optia Apheresis system, along with the Marker Therapeutics’ Depuro D2000 adsorption cartridge, for treating adult COVID‐19 patients admitted to an ICU with confirmed or imminent respiratory failure [64]. According to the manufacturers’ press release, the disposable column is intended to reduce inflammatory cytokines and other inflammatory mediators [65]. It has shown some initial promise in treating patients with ARDS, pneumonia, liver failure and sepsis; in addition, at least one clinical trial is underway (ClinicalTrials.gov Identifier: NCT04358003). Nonetheless, the clinical efficacy of this adsorption cartridge for treating COVID‐19 patients is still unknown.
Limitations
This guidance document has several limitations, the primary one being the lack of high‐level evidence based on prospective randomized controlled trials. Presently, no such trials of TPE in COVID‐19 have been published. In contrast, most of the cited publications were individual case reports, or small case series, or were derived from related, but non‐COVID‐19 sources. This preliminary guidance document also includes reference statements from other diseases or previous viral outbreaks in which the optimum role of apheresis therapy is not established, like SARS‐CoV‐1, H1N1 infection and MERS infection. It is worthwhile to note that this guidance provided herein comprised only 42 COVID‐19 patients in total, which is far from ideal (Fig. 1). Fortunately, this research field is very active, and additional studies are underway. This preliminary guidance should be reviewed and updated based on future accumulating evidence.
Conclusions
Several encouraging experimental therapies are emerging for COVID‐19, but there is currently no definitive cure. Based on general apheresis practice and results of TPE in patients with COVID‐19 compatible disease, it is reasonable to consider TPE as a potential option to treat critically ill COVID‐19 patients with CRS and it may help in reducing mortality. Therapy can be initiated, and outcome determined using the critical and target levels of multiple parameters described herein. Because the safety and efficacy of TPE are uncertain in haemodynamically unstable and critically ill COVID‐19 patients, the use of this therapy should be considered on an individual basis. Nonetheless, it would be best to evaluate the use of TPE alone, or in combination with other therapies, for COVID‐19 patients within the context of prospective, randomized controlled clinical trials. This approach may yield fruitful results by saving lives and paving the way for future consideration of TPE in similar diseases.
Conflict of interest
The authors have no conflict of interest.
Acknowledgements
We would like to acknowledge the all ISBT convalescent plasma working party members and special thanks to Vernon J Louw, Division of Clinical Haematology, Department of Medicine, University of Cape Town, South Africa; Adaeze Oreh, National Blood Transfusion Service, Department of Hospital Services, Federal Ministry of Health, Abuja, Nigeria; Evan M Bloch, Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, MD, USA; Dana Devine, Department of Pathology & Laboratory Medicine, University of British Columbia, Vancouver, BC, Canada; Jinho Shin, Medical Officer, World Health Organization, Manila, Phillippines; Vincenzo De Angelis, Italian National Blood Centre, Roam, Italy; Richard Schafer, Institute for Transfusion Medicine and Immunohematology, Goethe University Hospital, Frankfurt am Main, Germany; Ruchika Goel, Division of Hematology/Oncology, Simmons Cancer Institute at SIU School of Medicine and Mississippi Valley Regional Blood Center, Springfield, Illinois, USA; Arwa Al Riyami, Department of Hematology, Sultan Qaboos University Hospital, Muscat, Sultanate of Oman; and Silvano Wendel, Hospital Sirio Libanes, Sao Paulo, Brazil for reviewing the manuscript and give valuable comments.
References
- 1. Bar‐On YM, Flamholz A, Phillips R, et al. SARS‐CoV‐2 (COVID‐19) by the numbers. Elife 2020;9. 10.7554/elife.57309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chang JC. Sepsis and septic shock: endothelial molecular pathogenesis associated with vascular microthrombotic disease. Thromb J 2019;17:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chang JC. Acute respiratory distress syndrome as an organ phenotype of vascular microthrombotic disease: based on hemostatic theory and endothelial molecular pathogenesis. Clin Appl Thromb Hemost 2019;25:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Davidson S, Maini MK, Wack A. Disease‐promoting effects of type I interferons in viral, bacterial, and coinfections. J Interferon Cytokine Res 2015;35:252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `Cytokine Storm' in COVID‐19. J Infect 2020;80:607–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chien JY, Hsueh PR, Cheng WC, et al. Temporal changes in cytokine/chemokine profiles and pulmonary involvement in severe acute respiratory syndrome. Respirology 2006;11:715–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang CH, Liu CY, Wan YL, et al. Persistence of lung inflammation and lung cytokines with high‐resolution CT abnormalities during recovery from SARS. Respir Res 2005;6:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wong CK, Lam CW, Wu AK, et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol 2004;136:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Law HKW, Cheung CY, Ng HY, et al. Chemokine up‐regulation in SARS‐coronavirus–infected, monocyte‐derived human dendritic cells. Blood 2005;106:2366–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim ES, Choe PG, Park WB, et al. Clinical progression and cytokine profiles of middle east respiratory syndrome Coronavirus infection. J Korean Med Sci 2016;31:1717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Min CK, Cheon S, Ha NY, et al. Comparative and kinetic analysis of viral shedding and immunological responses in MERS patients representing a broad spectrum of disease severity. Sci Rep 2016;6:1–12. 10.1038/srep25359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang L, Zhai H, Ma S, et al. Efficacy of therapeutic plasma exchange in severe COVID‐19 patients. Br J Haematol 2020;190:e181–e183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yuki K, Fujiogi M, Koutsogiannaki S. COVID‐19 pathophysiology: A review. Clin Immunol 2020;215:108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yoshikawa T, Hill T, Li K, et al. Severe acute respiratory syndrome (SARS) coronavirus‐induced lung epithelial cytokines exacerbate SARS pathogenesis by modulating intrinsic functions of monocyte‐derived macrophages and dendritic cells. J Virol 2009;83:3039–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen L, Liu HG, Liu W, et al. Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi 2020;43:203–8. [DOI] [PubMed] [Google Scholar]
- 16. Parsons PE, Eisner MD, Thompson BT, et al. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med 2005;33:1–6. discussion 230–2. [DOI] [PubMed] [Google Scholar]
- 17. Knaup H, Stahl K, Schmidt BMW, et al. Early therapeutic plasma exchange in septic shock: a prospective open‐label nonrandomized pilot study focusing on safety, hemodynamics, vascular barrier function, and biologic markers. Crit Care 2018;22:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Philip Keith, Wells Adam H, Jeremy H, et al. The therapeutic efficacy of adjunct therapeutic plasma exchange for septic shock with multiple organ failure: a single center retrospective review. PREPRINT (Version 1). Research Square 2020. [DOI] [PMC free article] [PubMed]
- 19. Patel P, Nandwani V, Vanchiere J, et al. Use of therapeutic plasma exchange as a rescue therapy in 2009 pH1N1 influenza A–an associated respiratory failure and hemodynamic shock. Pediatr Crit Care Med 2011;12:e87–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Keith P, Day M, Perkins L, et al. A novel treatment approach to the novel coronavirus: an argument for the use of therapeutic plasma exchange for fulminant COVID‐19. Crit Care 2020;24:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Keith P, Day M, Choe C, et al. The successful use of therapeutic plasma exchange for severe COVID‐19 acute respiratory distress syndrome with multiple organ failure. SAGE Open Med Case Rep 2020;8:2050313X20933473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Padmanabhan A, Connelly‐Smith L, Aqui N, et al. Guidelines on the use of therapeutic apheresis in clinical practice – evidence‐based approach from the writing committee of the american society for apheresis: the eighth special issue. J Clin Apher 2019;34:171–354. [DOI] [PubMed] [Google Scholar]
- 23. Kawai Y, Cornell TT, Cooley EG, et al. Therapeutic plasma exchange may improve hemodynamics and organ failure among children with sepsis‐induced multiple organ dysfunction syndrome receiving extracorporeal life support. Pediatr Crit Care Med 2015;16:366–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.APA Editor(s): van der Veen S. Translation: expert consensus on the application of artificial liver blood purification system in the treatment of severe and critical COVID‐19. Infect Microbes Dis 2020;2:64–6. [Google Scholar]
- 25. Adeli SH, Asghari A, Tabarraii R, et al. Therapeutic plasma exchange as a rescue therapy in patients with coronavirus disease 2019: a case series. Pol Arch Intern Med 2020;130:455–8. [DOI] [PubMed] [Google Scholar]
- 26. Khamis F, Al‐Zakwani I, Al Hashmi S, et al. Therapeutic plasma exchange in adults with severe COVID‐19 infection. Int J Infect Dis 2020;99:214–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tian H, Sui Y, Tian S, et al. Case report: clinical treatment of the first critical patient with coronavirus disease (COVID‐19) in Liaocheng, Shandong Province. Front Med (Lausanne) 2020;7:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Torzewski J, Heigl F, Zimmermann O, et al. First‐in‐man: case report of selective C‐reactive protein apheresis in a patient with SARS‐CoV‐2 infection. Am J Case Rep 2020;21:e925020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lin JH, Chen YC, Lu CL, et al. Application of plasma exchange in association with higher dose CVVH in Cytokine Storm Complicating COVID‐19. J Formos Med Assoc 2020;119:1116–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zou X, Li S, Fang M, et al. Acute physiology and chronic health evaluation II score as a predictor of hospital mortality in patients of Coronavirus disease 2019. Crit Care Med 2020;48:e657‐e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu S, Yao N, Qiu Y, et al. Predictive performance of SOFA and qSOFA for in‐hospital mortality in severe novel coronavirus disease. Am J Emerg Med 2020;38(10):2074–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. WHO Clinical Management of COVID‐19 . Interim Guidance; 27th May 2020. COVID‐19: Clinical care (access on 01/09/2020). https://www.who.int/publications/i/item/clinical‐management‐of‐covid‐19 (Last accessed)
- 33. Kikuchi H, Maruyama H, Omori S, et al. The sequential organ failure assessment score as a useful predictor for estimating the prognosis of systemic inflammatory response syndrome patients being treated with extracorporeal blood purification. Ther Apher Dial 2003;7:456–60. [DOI] [PubMed] [Google Scholar]
- 34. Yang XH, Sun RH, Zhao MY, et al. Expert recommendations on blood purification treatment protocol for patients with severe COVID‐19☆. Chronic Dis Transl Med 2020;6:106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shi H, Zhou C, He P, et al. Successful treatment with plasma exchange followed by intravenous immunoglobulin in a critically ill patient with COVID‐19. Int J Antimicrob Agents 2020;56(2):105974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. WHO Living Guidance . Corticosteroids for COVID‐19. 2 September 2020 (accessed on 06 September 2020) https://www.who.int/publications/i/item/WHO‐2019‐nCoV‐Corticosteroids‐2020.1
- 37. Luo S, Yang L, Wang C, et al. Clinical observation of 6 severe COVID‐19 patients treated with plasma exchange or tocilizumab. Zhejiang Da Xue Xue Bao Yi Xue Ban 2020;49:227–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Konig MF, Powell M, Staedtke V, et al. Preventing cytokine storm syndrome in COVID‐19 using α‐1 adrenergic receptor antagonists. J Clin Invest 2020;130:3345–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee G, Arepally GM. Anticoagulation techniques in apheresis: from heparin to citrate and beyond. J Clin Apher 2012;27:117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Oudemans‐van Straaten HM, Bosman RJ, Koopmans M, et al. Citrate anticoagulation for continuous venovenous hemofiltration. Crit Care Med 2009;37:545–52. [DOI] [PubMed] [Google Scholar]
- 41. Bolan CD, Cecco SA, Wesley RA, et al. Controlled study of citrate effects and response to i.v. calcium administration during allogeneic peripheral blood progenitor cell donation. Transfusion 2002;42:935–46. [DOI] [PubMed] [Google Scholar]
- 42. Hirsh J, Bauer KA, Donati MB, et al. Parenteral anticoagulants: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th Edition). Chest 2008;133:141S–59S. [DOI] [PubMed] [Google Scholar]
- 43. Jhaveri KD, Meir LR, Flores Chang BS, et al. Thrombotic microangiopathy in a patient with COVID‐19. Kidney Int 2020;98:509–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nguyen TC, Han YY. Plasma exchange therapy for thrombotic microangiopathies. Organogenesis 2011;7:28–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID‐19 patients. Proc Natl Acad Sci USA 2020;117:9490–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically Ill patients with COVID‐19 with convalescent plasma. JAMA 2020;323(16):1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Neyrinck MM, Vrielink H. Certification JTFfEa: calculations in apheresis. J Clin Apher 2015;30:38–42. [DOI] [PubMed] [Google Scholar]
- 48. Liu J, Dong YQ, Yin J, et al. Critically ill patients with COVID‐19 with ECMO and artificial liver plasma exchange: A retrospective study. Medicine (Baltimore) 2020;99:e21012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dogan L, Kaya D, Sarikaya T, et al. Plasmapheresis treatment in COVID‐19‐related autoimmune meningoencephalitis: Case series. Brain Behav Immun 2020;87:155–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Szczeklik W, Wawrzycka K, Włudarczyk A, et al. Complications in patients treated with plasmapheresis in the intensive care unit. Anaesthesiol Intensive Ther 2013;45:7–13. [DOI] [PubMed] [Google Scholar]
- 51. Faqihi F, Alharthy A, Alodat M, et al. Therapeutic plasma exchange in adult critically ill patients with life‐threatening SARS‐CoV‐2 disease: A pilot study. J Crit Care 2020;60:328–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Venken K, Hellings N, Liblau R, et al. Disturbed regulatory T cell homeostasis in multiple sclerosis. Trends Mol Med 2010;16:58–68. [DOI] [PubMed] [Google Scholar]
- 53. Hodulik KL, Root AG, Ledbetter LS, et al. Effects of therapeutic plasma exchange on anticoagulants in patients receiving therapeutic anticoagulation: a systematic review. Transfusion 2019;59:1870–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Turgutkaya A, Yavaşoğlu İ, Bolaman Z. Application of plasmapheresis for Covid‐19 patients. Ther Apher Dial 2020; 1–2. 10.1111/1744-9987.13536 Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zachariah U, Nair SC, Goel A, et al. Targeting raised von Willebrand factor levels and macrophage activation in severe COVID‐19: Consider low volume plasma exchange and low dose steroid. Thromb Res 2020;192:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Moore JB, June CH. Cytokine release syndrome in severe COVID‐19. Science 2020;368:473–4. [DOI] [PubMed] [Google Scholar]
- 57. Kayser S, Kunze R, Sheriff A. Selective C‐reactive protein apheresis for Covid‐19 patients suffering from organ damage. Ther Apher Dial 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Whiteside TL. Cytokines and cytokine measurements in a clinical laboratory. Clin Diagn Lab Immunol 1994;1:257–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Daoud AM, Soliman KM, Ali HK. Potential limitations of plasmapheresis in treatment of COVID‐19 patients and how to overcome them? Ther Apher Dial 2020. 10.1111/1744-9987.13568 Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 60. Vossoughi S, Winters JL, Burgstaler EA, et al. The ABC's of disaster management: Managing apheresis operations during the SARS‐CoV‐2 pandemic. J Clin Apher 2020;35:243–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Shander A, Goobie SM, Warner MA, et al. Essential role of patient blood management in a pandemic: a call for action. Anesth Analg 2020;131(1):74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pagano MB, Hess JR, Tsang HC, et al. Prepare to adapt: blood supply and transfusion support during the first 2 weeks of the 2019 novel coronavirus (COVID‐19) pandemic affecting Washington State. Transfusion 2020;60:908–11. [DOI] [PubMed] [Google Scholar]
- 63. Kesici S, Yavuz S, Bayrakci B. Get rid of the bad first: Therapeutic plasma exchange with convalescent plasma for severe COVID‐19. Proc Natl Acad Sci USA 2020;117:12526–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. FDA U . TPE in COVID‐19 patient, Approval letter, 2020, accessed on 10 August 2020. 2020, https://www.fda.gov/media/136834/download
- 65. Terumo , Marker Therapeutic get authorization for blood purification device (accessed on September 3, 2020). https://www.medicaldevice‐network.com/news/terumo‐marker‐therapeutics‐blood‐purification‐device/
- 66. Yang AP, Liu JP, Tao WQ, et al. The diagnostic and predictive role of NLR, d‐NLR and PLR in COVID‐19 patients. Int Immunopharmacol 2020;84:106504. [DOI] [PMC free article] [PubMed] [Google Scholar]


