Abstract
The novel coronavirus severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and coronavirus disease 2019 (COVID‐19) are new global problems. The understanding of the host immune response in COVID‐19 and its implications in the development of therapeutic agents are new challenges. Here, we evaluated the development of immunoglobulin G (IgG) and neutralizing (Nt) antibodies in symptomatic hospitalized COVID‐19 patients. We followed up 117 COVID‐19 confirmed patients from a reference health center for COVID‐19 during the epidemic in Santiago de Chile. One and two sequential blood samples from 117 to 68 cases were, respectively, obtained to evaluate the immune response. Immunofluorescence and neutralization assays in Vero E6 cells with a Chilean SARS‐CoV‐2 strain were performed. Out of the 68 patients, 44% were women and 56% men, and the most frequent comorbidities were hypertension (47.7%) and diabetes (27.4%). The most frequent symptoms or signs related to COVID‐19 were dyspnea, cough, fever, myalgia, and headache. In all the study population, 76.1% and 60.7% of patients were positive for IgG and Nt antibodies in the first blood sample. All patients except one were positive for IgG and Nt antibodies in the second sample. IgG and Nt antibodies positivity increased significantly according to the disease evolution periods. Higher Nt antibody titers were observed in the first sample in patients under 60 years of age. Obese and diabetic patients had no increase in Nt antibodies, unlike normal weight and diabetes‐free patients. Both hypertensive and normotensive patients showed a significant increase in Nt antibodies. These results show an early and robust immune response against SARS‐CoV‐2 infection during severe COVID‐19.
Keywords: COVID‐19 patients, IgG antibodies, neutralizing antibodies, SARS‐CoV‐2
1. INTRODUCTION
The World Health Organization (WHO) listed coronavirus disease 2019 (COVID‐19) and defined it as a pandemic on March 11, 2020. As of November 26, 2020, 60,776,978 cases and 1,428,228 deaths have been reported worldwide and in Chile 544,092 and 15,138, respectively. It has been declared the most important pandemic of the last 100 years (https://coronavirus.jhu.edu/map.html, accessed November 26, 2020). It has been estimated that COVID‐19 has an R 0 × 2.5 and a median incubation of 5 days, with some symptoms similar to a flu‐like disease. 1 The infecting period has been reported to begin 2 days before the onset of symptoms and extends for 8 days after the onset of the disease. 2 , 3 The infecting period of subjects with severe forms of COVID‐19 or those with some immune system compromise has not yet been established with certainty. An important feature of COVID‐19 is that a larger number of cases are oligoasymptomatic or asymptomatic, making it difficult to detect total patients and control transmission.
Antibody production is early, within 7 days of onset of symptoms, 40% of patients may have immunoglobulin G (IgG)‐class antibodies; at 2 weeks, virtually all patients produce them. 4 The immune system first produces IgM‐type antibodies, with faster and less potent activity against severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Later IgGs are produced, more potent and specific to the viral infection. IgM antibodies are produced from Days 5 to 7 after the onset of symptoms in COVID‐19, and it is closely followed by the production of IgG. In most cases studied IgM levels disappear within a few weeks, while IgG levels persist for at least three months. 5 Recently, a longitudinal study that evaluated changes in antibody levels in people recovered from COVID‐19, concluded that they did not decline after four months of diagnosis. 4
Neutralizing (Nt) antibodies are key to the elimination of viruses that cause various infections and are recognized for their critical role in the protective immune response against invasive viral infections. Their activity is triggered against proteins located on the surface of viruses, which they join to block the infection. In the face of this new SARS‐CoV‐2 infection, there is a lack of experience if these antibodies are sufficient to induce effective and lasting immunity. 5 , 6 , 7
Studies conducted so far in SARS‐CoV‐2 indicate that Nt appear about 2 weeks after infection begins, and that their maximum activity would occur between 4 and 6 weeks. Nevertheless, it has not been confirmed whether all patients generate Nt antibodies, if their titers are related to the severity of the infection, or whether their neutralization levels are always sufficient to induce protection, as their concentrations are variable and not detected in all patients. There are still questions regarding the function of Nt antibodies and their role in controlling infection and symptoms of the disease, or whether after recovering from the disease and generated the antibodies it is possible to re‐infect with SARS‐CoV‐2. 5 , 6 , 8 , 10 , 11
We aimed to evaluate the presence of IgG and Nt in symptomatic hospitalized COVID‐19 patients, the subsequent development of both antibodies when they are recovered, and to identify associated clinical issues.
2. MATERIALS AND METHODS
2.1. Study design and participants
We recruited and prospectively followed up 128 patients with a confirmed diagnosis of COVID‐19 who were hospitalized at Hospital Barros Luco Trudeau (HBL) from May to August 6, 2020. HBL is a tertiary public hospital in Santiago de Chile, which was an exclusive health center for COVID‐19 during the epidemic.
The inclusion criteria were: (1) patients older than 18 years of age and (2) patients hospitalized, and (3) RT‐PCR test positive for COVID‐19. We excluded patients with high‐risk multi‐organic failure (diagnoses under clinical criteria) and those with a lapse of more than 5 days from hospital admission. We also excluded patients who received convalescent plasma during hospitalization for the final analysis.
2.2. Ethical aspect
The protocol was approved by the Scientific‐Ethics Committee South Health Service of Metropolitan Region, Chile, through the document dated May 6, 2020.
Study participants were unable to directly sign informed consent, given the strict isolation measures indicated at the root of the pandemic; only the patient's proxy could be contacted via telephone and proxy signed documents. In those patients who were not in critical condition, they were asked for authorization to obtain data and blood sample, as the documents were not allowed to enter those units either.
2.3. Data collection and follow‐up
At the moment of recruitment, a trained internal medicine resident using a semi‐structured questionnaire collected the following information: (1) age in the year, (2) date of RT‐PCR results, (3) symptom onset date, (4) COVID‐19 symptoms (fever, cough, sore throat, myalgia, headache, diarrhea, dyspnea, hyposmia, others), (4) history of chronic diseases (diabetes, hypertension, chronic kidney disease, obesity, cancer, and other chronic or immunosuppressive diseases), and (5) hospitalization status (basic hospitalization or intermediate care unit (non‐ICU), intensive care unit (ICU), hospital discharge).
To evaluate the immune response against SARS‐CoV‐2, we obtained two blood samples from each infected patient separated by at least 18 days, first‐second and third‐fourth weeks of follow‐up, spanning 15–25 days after symptom onset date by a certified technical professional.
Hospitalization status was registered as non‐ICU hospitalization, ICU, and hospital discharge. By the time of the second blood sample, the patient clinical course was evaluated as no changes or aggravated (when the patient remained hospitalized in the same unit or worsening from a non‐ICU hospitalization to ICU), and favorable evolution (when the patient left the ICU to a non‐ICU hospitalization or hospital discharge).
2.4. Isolation of plasma and peripheral blood mononuclear cells (PBMCs) from whole blood
Blood draw collection (3–4 ml) was performed using EDTA or heparin tubes. PBMC isolation was performed using a density gradient separation medium (Histopaque; Sigma‐Aldrich) according to the manufacturer's instructions. Plasma and PBMC were collected and stored separately at –70° C. Blood samples were analyzed at the Virology Reference Laboratory of the Public Health Institute of Chile, they were transported at 5°C–8°C daily.
2.5. Neutralization assay
Vero E6 cells were infected with a SARS‐CoV‐2 strain obtained from our laboratory by viral isolation in tissue cultures (33782CL‐SARS‐CoV‐2 strain). Neutralization assays were carried out by the reduction of cytopathic effect (CPE) in Vero E6 cells (ATCC CRL‐1586). The titer of Nt antibodies was defined as the highest serum dilution that can neutralize virus infection, at which the CPE was absent compared with the virus control wells (cells with CPE). Briefly, the Vero E6 cells (4 × 104 cells/well) were seeded in 96‐well plates. For the neutralization assay, 100 μl 33782CL‐SARS‐CoV‐2 (at a dose of 100 TCID50) were incubated with serial dilutions of heat‐inactivated sera samples (dilutions of 1:10, 20, 40, 80, 160, 320, 640, and 1280) from patients and human normal serum as a negative control for 1 h at 37°C. Then, a mixture of samples and virus were added to the 96‐well Vero E6 cells). Cytopathic effect on Vero E6 cells was analyzed 7 days after infection. Neutralization was defined as absence of CPE compared to virus controls (Figure 1A,B). For each test, human normal and a Nt COVID‐19 patient serum were used as a negative and positive control, respectively. The negative control was serum from a blood donor negative for SARS‐CoV‐2 IgM and IgG by the OnSite™ COVID‐19 IgG/IgM Rapid Test (CTK Biotech, Ref. R0180C). The positive control was a serum from a recovered COVID‐19 patient, who agreed to donate blood for plasmapheresis.
Figure 1.
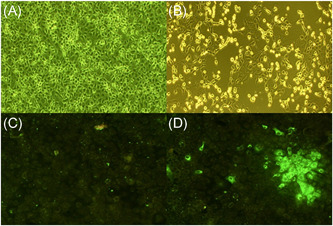
Neutralization and immunofluorescence assays to detect antibodies against SARS‐CoV‐2. (A) Negative and (B) positive result for the cytopathic effect of SARS‐CoV‐2 by neutralization in Vero E 6 cells. (C) Negative and (D) positive result by immunofluorescence assay for detection of IgG antibodies against SARS‐CoV‐2. IgG, immunoglobulin G; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
2.6. IgG detection by immunofluorescence assay
For immunofluorescence test, Vero E6 cells were cultured on an eight‐well plate (Lab‐Tek, Thermo Fisher Scientific; cat 155411; 5 × 104 cells/well) with 0.3 ml minimum essential medium + 2% fetal bovine serum + 2× penicillin–streptomycin at 37°C in a 5% carbon dioxide atmosphere. Supernatants of isolated virus (33782CL‐SARS‐CoV‐2 strain, 10 µl/well = 50 TCID50) were inoculated into Vero E6 cells. Cell cultures were observed for ECP during 24–48 h postinfection. Then, cells were washed with phosphate‐buffered saline (PBS) and fixed with cold acetone for 10 min. For IgG detection, heat‐inactivated patient sera (56°C, 30 min) were diluted in PBS sample buffer (dilutions of 1:10, 20, 40, 80, 160, 320, 640, and 1280) and 30 μl of the dilution was applied per incubation well in cover slides (Figure 1C,D). After 1 h at 37°C, cover slides were washed three times for 5 min with PBS. Secondary detection was done using a 1:400 dilution of a goat‐anti human Fab IgG‐FITC (Millipore; AQ112). After 30 min at room temperature, slides were washed two times for 5 min with PBS and rinsed with water. Slides were mounted using Prolong Diamond anti FADE (Thermo Fisher Scientific; p36970).
2.7. Statistical analysis
We describe the study population by age groups and according to the time of the first and second blood samples. Fisher's exact t‐test, analysis of variance, and the Student t test were used to compare the categorical and numerical variables between different groups. p values less than .05 were considered significant. The analysis was done in STATA (College Station, Texas) and GraphPad Prism 8.0 software (GraphPad Software).
3. RESULTS
One hundred twenty‐eight hospitalized COVID‐19 patients were enrolled between May 10 and August 6, 2020; in whom 11 patients were excluded because they had received convalescent plasma. Of the 117 (91.4%) remaining patients, 18 patients died during the follow‐up, four were transferred to another hospital outside the study hospital system, and 27 did not attend to phone calls or untraceable home address, or refused to take the second blood sample. Therefore, 68 (52.3%) patients had the second blood sample for antibodies determination.
Out of the 117 patients, 48 patients were women (41%), and 69 were men (59%), with a median age of 56 years similar in both groups (p = .847). Out of the 68 patients, 44% were women and 56% men, with no age differences.
3.1. Characteristics of the study population
Table 1 describes the study population regarding disease evolution, history of chronic diseases, and the frequency of symptoms or signs related to COVID‐19 stratified by age groups. Hospitalized COVID‐19 patients had diabetes and hypertension in 27.4% and 47.7% of cases, respectively. The prevalence of these chronic diseases was even significantly higher among adults aged 65 and older (50% p = .005 for diabetes; and 78.1% p < .001 for hypertension). Obesity (body mass index > 30) was present in 13.7% of the study population; nevertheless, this prevalence tended to be higher among patients younger than 40 years of age (30%, p = .007). In contrast, chronic kidney disease and cancer prevalences were lower in the study population, with no difference between age groups (Table 1). The most frequent symptoms or signs related to COVID‐19 were dyspnea (82.3% of the cases), cough (64.1%), fever (47%), myalgia (34.9%), and headache (21.4%). There was no significant difference in signs or symptoms between age groups, although fever and cough tended to be more frequent in adults younger than 40 years; meanwhile, headache in adults among 40–60 years of age (Table 1). Additionally, 6.8% of the patients presented a compromised general state at the time of recruitment.
Table 1.
Characterization of the study population and history of chronic diseases and frequency of symptoms and signs related to COVID‐19 by groups of agea in 117 SARS‐CoV‐2 infected patients
| Age groups | |||||
|---|---|---|---|---|---|
| All | <40 years | 40–60 years | ≥65 years | ||
| n = 117 | n = 20 | n = 65 | n = 32 | p valueb | |
| Gender | |||||
| Female | 48 (41) | 10 (50) | 24 (36.9) | 14 (43.8) | .559 |
| Male | 69 (59) | 10 (50) | 41 (63.1) | 18 (56.3) | |
| Days from onset of symptoms, and the first blood sample, mean (min‐max) | 10.5 (1–27) | 10.3 (4–17) | 11 (1–27) | 9.8 (3–20) | .216 |
| Days from the first to the second blood sample, mean (min‐max)c | 18.6 (6–43) | 18.3 (7–42) | 19 (7–43) | 17.5 (1–29) | .498 |
| History of chronic diseases | |||||
| Diabetes | 32 (27.4) | 3 (15) | 13 (20) | 16 (50) | .005 |
| Hypertension | 53 (47.7) | 3 (15) | 25 (38.5) | 25 (78.1) | <.001 |
| Chronic kidney disease | 5 (4.3) | 1 (5) | 2 (3.1) | 2 (6.3) | .575 |
| Cancer | 2 (1.7) | 1 (5) | 0 (0) | 1 (3.1) | .195 |
| Obesity | 16 (13.7) | 6 (30) | 6 (9.2) | 4 (12.5) | .07 |
| COVID‐19 signs and symptoms | |||||
| Fever | 55 (47) | 10 (50) | 34 (52.3) | 11 (34.4) | .256 |
| Cough | 75 (64.1) | 17 (85) | 39 (60) | 19 (59.4) | .093 |
| Sore throat | 11 (9.4) | 3 (15) | 6 (9.2) | 2 (6.3) | .564 |
| Dyspnea | 101 (82.3) | 16 (80) | 58 (89.2) | 27 (84.4) | .467 |
| Myalgia | 42 (34.9) | 6 (30) | 26 (40) | 10 (31.3) | .649 |
| Headache | 25 (21.4) | 3 (15) | 19 (29.2) | 3 (9.4) | .058 |
| Diarrhea | 12 (10.3) | 3 (15) | 7 (10.8) | 2 (6.3) | .587 |
| Hyposmia | 6 (5.1) | 1 (5) | 5 (7.7) | 0 (0) | .248 |
Note: Patients with different days of evolution of their disease were enrolled. Thus, the mean days from onset of symptoms and the first blood sample was 11.5.
Absolute frequencies and percentages in parentheses.
Two‐side Fisher exact test.
This information was available only for 68 patients.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
3.2. Detection of IgG and Nt against SARS‐CoV‐2 in the first blood sample
We recruited patients at different stages in the evolution of their disease. Thus, the mean time between the date of onset of sign or symptoms and the first blood sample date was 11 days with a standard deviation (SD) of 5 days, similarly in men and women. Henceforth, we present antibody results according to three disease evolution periods: 1–7 days of evolution of the disease (32 cases, 27.4%), 8–14 days (62, 53%), and 15 or more days of evolution (23, 19.7%).
In the all study population (n = 117), 76.1% (89) of patients tested were positive for IgG in the first blood sample. IgG seropositivity was 79.7% in men and versus 70.8% in women, with no significant differences (p = .280). The distribution of seropositivity was higher as the days passed as the onset of symptoms (p trend = 0.013) (Figure 2).
Figure 2.
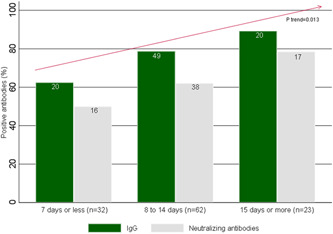
COVID‐19 patients with IgG and neutralizing antibodies by disease evolution periods from the onset of signs or symptoms and first blood sample date (n = 117). P trend: χ 2 statistic for the trend. COVID‐19, coronavirus disease 2019; IgG, immunoglobulin G
Regarding Nt, of 89 IgG‐positive patients 79.8% (71) were Nt positive in the first blood sample, with no difference between men and women (68.4% vs. 54%, p = .253). Similar to IgG, the Nt positivity showed a significant upward gradient according to the disease evolution periods. Thus, it was 50% in the first, 62.1% in the second, and 78.6% in the third disease evolution period (p trend = 0.013) (Figure 2).
3.3. Development and levels of Nt and IgG antibodies against SARS‐CoV‐2 in the second blood sample
Of the total number of patients studied (n = 117), paired plasma samples were obtained in 68 infected patients (53.1%), 38 were men (55.9%) and 30 (44.1%) were women. Table 2 shows that on average the difference in days between the first and the second sample was 18.6 days. Of these patients, 37 (54.4%) were non‐ICU hospitalized, and 31 (45.6%) were at the ICU at the moment of the first sample, while by the second blood sample, 28 (41.2) hospital discharged (Table 2). A significant increase in IgG and Nt antibody positivity rates were observed between the first and second samples, from 77.9% to 98.5% for IgG (p = .0002) and from 77.3% to 100% for Nt antibodies (p < .001) (Table 2).
Table 2.
IgG and neutralizing antibodies positivity, and COVID‐19 clinical course in patients with the first and second blood sample (n = 68)
| First blood sample | Second blood sample | |
|---|---|---|
| Days from onset of symptoms and blood sample, mean (min‐max) | 11.1 (1–27) | 29.7 (13–57) |
| Hospitalization status | ||
| Non‐ICU hospitalization | 37 (54.4) | 29 (42.7) |
| Intensive care unit | 31 (45.6) | 11 (16.2) |
| Hospital discharge | ‐ | 28 (41.2) |
| IgGa | 53 (77.9) | 67 (98.5) |
| Ntb | 41 (77.3) | 67 (100) |
Note: Absolute frequencies and percentages in parenthesis.
Abbreviations: COVID‐19, coronavirus disease 2019; ICU, intensive care unit; IgG, immunoglobulin G.
Paired χ 2 test second versus first blood sample: p = .0002.
Paired χ 2 test second versus first blood sample: p < .001.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Regarding the clinical evolution, 47 patients (69.1%) had a favorable evolution (hospital or ICU discharge), and 21 (30.9%) had no changes or aggravation (Table 3). In these patients, a significant increase in the average IgG and Nt antibody titers were observed between the first and the second sample, respectively. In the first samples were obtained average IgG titers of 1/71.2 ± 1/173.5, with a range from negative to 1/1280. Analogously, these samples showed average Nt titers of 1/49.6 ± 1/114.3, with a range from negative to 1/640 (Figure 3 and Table 3). Second samples showed an average IgG titer of 1/487.9 ± 1/443.1, with a range from negative to 1/1280. Additionally, these samples showed an average Nt titer of 1/159.1 ± 1/147.5, with a range from negative to 1/640.
Table 3.
Neutralizing and IgG antibody titer in the study population and stratified by age groups, clinical course, days from symptoms onset and chronic disease
| Population strata | First blood sample | Second blood sample | p valuea |
|---|---|---|---|
| All (n = 68) | |||
| Nt titer mean (SD) | 49.6 (114.3) | 159.1 (147.5) | <.001 |
| IgG titer mean (SD) | 71.2 (173.5) | 487.9 (443.1) | <.001 |
| Age group | |||
| Younger than 60 years (n = 24) | 76.3 (176.1) | 125.8 (131.8) | .101 |
| 60 years and older (n = 44) 1 | 35.0 (56.1) | 177.3 (153.8) | <.001 |
| Clinical evolution | |||
| Favorable evolution (n = 47) | 50.0 (130.2) | 169.1 (162.7) | <.001 |
| No changes or aggravated (n = 21) | 48.6 (69.1) | 136.7 (105.9) | .004 |
| Time from symptoms and second blood sample date | |||
| Less than 30 days | 48.6 (69.1) | 136.7 (105.9) | .004 |
| 30 day or more | 50 (130.2) | 169.1 (162.7) | <.001 |
| History of chronic diseases | |||
| Obesity (n = 12) | 111.7 (186.7) | 145.8 (178.8) | .647 |
| Non‐obesity (n = 56) 2 | 36.3 (89) | 162 (141.6) | <.001 |
| Diabetes (n = 13) | 80 (174.1) | 224.6 (215.7) | .017 |
| Non‐diabetes (n = 55) | 42.4 (95.9) | 146.6 (110.1) | <.001 |
| Hypertension (n = 28) 3 | 67.2 (131) | 223.6 (182.4) | <.001 |
| Non‐hypertension (n = 40) | 37.3 (101) | 114 (96.3) | <.001 |
Two‐side paired Student t test for second versus first plasma sample.
Abbreviation: IgG, immunoglobulin G.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Figure 3.
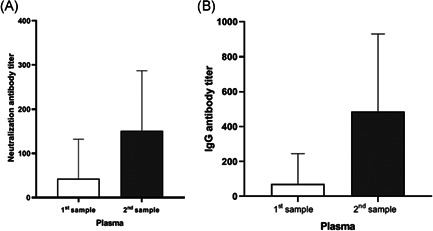
Neutralizing and IgG antibody titers of plasma samples against SARS‐CoV‐2 in 68 infected patients. (A) Neutralization and (B) IgG titers were determined by the reduction of CPE and immunofluorescence assays in Vero E6 cells. Each bar represents the average of antibody titer. CPE, cytopathic effect; IgG, immunoglobulin G; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
According to age groups, higher Nt titers were observed in the first sample in patients under 60 years of age, but without a significant increase in them in the second sample (Table 3). On the other hand, in the age of 60 and over (44 patients), there was a significant increase in Nt from 1/35.0 ± 56.1 to 1/177.3 ± 153.8. As for the classification of the clinical status of patients at the time of taking the second sample, we observed that of the 47 patients discharged (69.1% of the 68), Nt had a significant ascent between the first and second samples, from 1/50.0 ± 130.2 to 1/169.1 ± 162.7, a similar situation for those who had no change. There was also a significant increase of titers in those patients whose second sample was taken 30 days and more since the onset of symptoms.
As for the history of chronic diseases, only obese (n = 12) and diabetic patients (n = 13) had no significant increase in the Nt titers (from 1/111.7 to 1/145.8, p = .647), unlike normal‐weight (n = 56) and diabetes‐free patients (n = 55); in the first, this increase was from 1/36.3 ± 9 to 1/162 ± 141.6, in the second, this increase was from 1/42.4 ± 95.9 to 1/146.6 ± 110.1. In both hypertensive and normotensive patients was a significant increase in the Nt (Table 3).
When comparing the increments of Nt titers between age groups and chronic diseases status, patients aged 60 years or older had a statistically significant greater increase than younger patients (142.2 vs. 49.5, p = .0105). Non‐obese and hypertensive patients had significantly higher Nt increments than those obese (125.7 vs. 34.1, p = .036) and non‐hypertensive patients (56.4 vs. 76.7, p = .0105), respectively. In contrast, the Nt titer increments in diabetic and nondiabetic patients were similar (144.6 vs. 101.2, p = .192).
Figure 4 shows the dynamics of Nt antibodies for each of the 68 patients with whom the two plasma samples were available. In 44 cases (64.7%) there was a significant increase in Nt between the first (average 15.5 ± 24.6) and the second sample (194.1 ± 147.2) (Figure 4). Among these, there were 26 patients who in the first sample did not show Nt and then in the second sample were positive. In 20 cases (29.4%) the titers remained the same or similar in both samples (94.5 ± 143.6 and 103.5 ± 135.3) and in only 3 patients (4.4%) showed a significant decrease in Nt between the first (280 ± 317.5) and the second (70 ± 79.4) sample. Only one patient was negative for Nt and IgG antibodies against SARS‐CoV‐2 in both samples. This case was an 85‐year‐old woman with antecedents of hypertension, type 2 diabetes mellitus and asthma, all in treatment. She was admitted to the hospital in May 12th with a history of one week of dyspnea of minimal efforts, no fever, cough or expectoration. The diagnosis was confirmed by positive RT‐PCR at admission, and an X‐ray Computed Tomography was performed that showed multifocal pneumonia. The first sample was taken on 14 May, that is 9 days after the onset of symptoms. She did not require treatment in intensive care, and she was discharged from the hospital in May 16th. At the time of the second sample on May 28th, she was asymptomatic.
Figure 4.
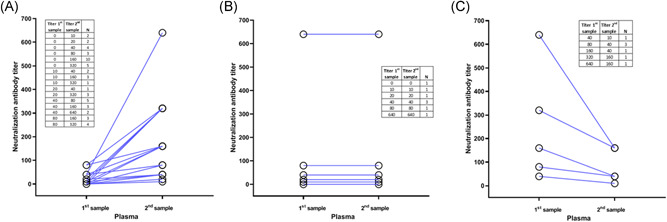
Comparison of neutralization antibody titers for individual patients. Neutralization titers were determined by the reduction of cytopathic effect (CPE) in Vero E6 cells. Each line links two samples from each patient. (A) Patients with increase of neutralizing (Nt) titers between first and second samples, (B) patients with the same Nt titers between first and second samples, (C) patients with a decrease of Nt titers between first and second samples
4. DISCUSSION
Little is currently known about the relationship between the specific immune response against SARS‐CoV‐2 and COVID‐19 severity. The specific immune response against the virus in SARS‐CoV‐2 infected individuals is controlled by the coordinated action of the three fundamental branches of adaptive immunity: the Nt antibody response and CD4+ and CD8+ T lymphocytes. 12 In this study we reported the immune response of IgG and Nt in 117 patients hospitalized with COVID‐19 whom we tracked and thus obtained a second sample, which we were able to confirm in 68 of them. The response of IgG and Nt was detected in some patients from 2 to 3 days of onset symptoms. In the 68 patients with the second sample the response of IgG and Nt was detected in all of them, with the exception of a case that remained seronegative until 23 days post‐onset of symptoms (period in which the second sample was taken). These data show a robust immune response that develops early during severe SARS‐CoV‐2 infection. This finding confirms what is previously detected in other serological studies. 2 , 5 , 8 , 9 , 13 , 14 , 15 , 16 However, in our knowledge, this is the first time that the very early presence of Nt antibodies has been reported in patients with COVID‐19, as early as 2 days after the onset of symptoms. This finding is interesting because it highlights the rapid development in severe patients of the immune response against SARS‐CoV‐2.
The application of these serological trials is an important contribution to making informed decisions about the immune system's ability to eliminate the virus and for the possible application of convalescent plasma therapies. 9 , 16 , 17 In addition, knowledge of the dynamic of the humoral response against SARS‐CoV‐2 is important for the correct use of rapid serological trials for diagnostic purposes, considering both the temporal dynamics of RT‐PCR trials and the time from the onset of symptoms in suspicious patients. Knowledge of the immune response is also key to understanding COVID‐19 pathogenesis and evaluating the development of SARS‐CoV‐2 vaccines.
The development of high Nt antibody titers and the presence of these antibodies in virtually all COVID‐19 patients may be an important factor in attenuating SARS‐CoV‐2 pathogenesis. Likewise, the presence of Nt can be key to protection against re‐infection. 18 The development of new studies that can corroborate the protective role of Nt is currently needed, and urgent; and assessing their long‐term presence by tracking recovered patients.
The limitations of this study are the following: first, the involved patients were selected by convenient sampling in a reference hospital in the metropolitan region, instead of random sampling, so it is not representative of the cases with COVID‐19; second, we could follow up on 58.1% of them for the second sample due to 23.1% of patients being lost and 15.4% dead; third, data were reported by patients or by the relatives in charge of them, so these could be somewhat inaccurate, for example, the time from the onset of symptoms.
The great importance of more knowledge about the immune response of patients to SARS‐CoV‐2 infection is such that it will be necessary to perform longitudinal studies about the dynamics of Nt and the other branches of the adaptive immunity to SARS‐CoV‐2. As well, it will be of great interest to advance possible hypothesis of the Nt response in obese, diabetic, and older patients. Although the number of patients with obesity and diabetes in whom we were able to get the second sample to determine the Nt titers were few, in both cases levels of Nt remained low without increasing in the second sample. Obesity may be a clinical predictor for adverse outcomes; the adipose tissue may play an important risk factor in severity of the disease. Individuals with obesity have higher leptin, lower levels of adiponectin and higher concentrations of pro‐inflammatory cytokines. 19 , 20 Many studies have shown that nutrient dysregulation in obese individuals impairs the immune response to infections.
CONFLICT OF INTERESTS
The authors declare that there is no conflict of interests.
AUTHOR CONTRIBUTIONS
MaríaTeresa Valenzuela, and Cinthya Urquidi participated in the study design, writing, critical review of the content and approved the final version of the manuscript. Nicolás Rodriguez contributed to selection, study and clinical follow‐up of patients, critical review of the content and approved the final version of the manuscript. Luis Castillo participated in the study design, selection, study and clinical follow‐up of patients, writing, critical review of the content and approved the final version of the manuscript. Jorge Fernandez contributed to the study design, experimental assays, critical review of the content and approved the final version of the manuscript. Eugenio Ramirez contributed to the study design, experimental assays, writing, critical review of the content and approved the final version of the manuscript.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/jmv.26939
ACKNOWLEDGMENT
We are grateful to Agencia Nacional de Investigación y Desarrollo de Chile (ANID), which supported this study (Grant COVID0557).
Teresa Valenzuela M, Urquidi C, Rodriguez N, Castillo L, Fernández J, Ramírez E.. Development of neutralizing antibody responses against SARS‐CoV‐2 in COVID‐19 patients J Med Virol. 2021;93:4334–4341. 10.1002/jmv.26939
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID‐19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172(9):577‐582. 10.7326/M20-0504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID‐2019. Nature. 2020;581(7809):465‐469. 10.1038/s41586-020-2196-x [DOI] [PubMed] [Google Scholar]
- 3. Cheng H‐Y, Jian S‐W, Liu D‐P, Ng T‐C, Huang W‐T, Lin H‐H. Contact tracing assessment of COVID‐19 transmission dynamics in Taiwan and risk at different exposure periods before and after symptom onset. JAMA Intern Med. 2020;180(9):1156. 10.1001/jamainternmed.2020.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gudbjartsson DF, Norddahl GL, Melsted P, et al. Humoral immune response to SARS‐CoV‐2 in Iceland. N Engl J Med. 2020:NEJMoa2026116. 10.1056/NEJMoa2026116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Suthar MS, Zimmerman MG, Kauffman RC, et al. Rapid generation of neutralizing antibody responses in COVID‐19 patients. Cell Rep Med. 2020;1(3):100040. 10.1016/j.xcrm.2020.100040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee Y‐L, Liao C‐H, Liu P‐Y, et al. Dynamics of anti‐SARS‐Cov‐2 IgM and IgG antibodies among COVID‐19 patients. J Infect. 2020;81(2):e55‐e58. 10.1016/j.jinf.2020.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang F‐S, Zhang C. What to do next to control the 2019‐nCoV epidemic? Lancet. 2020;395(10222):391‐393. 10.1016/S0140-6736(20)30300-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Long QX, Liu BZ, Deng HJ, et al. Antibody responses to SARS‐CoV‐2 in patients with COVID‐19. Nat Med. 2020;26(6):845‐848. 10.1038/s41591-020-0897-1 [DOI] [PubMed] [Google Scholar]
- 9. Bloch EM, Shoham S, Casadevall A, et al. Deployment of convalescent plasma for the prevention and treatment of COVID‐19. J Clin Invest. 2020;130(6):2757‐2765. 10.1172/JCI138745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang X, Guo X, Xin Q, et al. Neutralizing antibody responses to severe acute respiratory syndrome coronavirus 2 in coronavirus disease 2019 inpatients and convalescent patients. Clin Infect Dis. 2020:Jun 4 ciaa721. 10.1093/cid/ciaa721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ni L, Ye F, Cheng M‐L, et al. Detection of SARS‐CoV‐2‐specific humoral and cellular immunity in COVID‐19 convalescent individuals. Immunity. 2020;52(6):971‐977.e3. 10.1016/j.immuni.2020.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moderbacher CR, Ramirez SI, Dan JM, et al. Antigen‐specific adaptive immunity to SARS‐CoV‐2 in acute COVID‐19 and associations with age and disease severity. Cell. 2020;183:1‐17. 10.1016/j.cell.2020.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS‐CoV‐2 in patients with novel coronavirus disease 2019. Clin Infect Dis. 2020;71(16):2027‐2034. 10.1093/cid/ciaa344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Okba NMA, Müller MA, Li W, et al. Severe acute respiratory syndrome coronavirus 2−specific antibody responses in coronavirus disease 2019 patients. Emerg Infect Dis. 2020;26(7):1478‐1488. 10.3201/eid2607.200841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. To KK, Tsang OT, Leung WS, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS‐CoV‐2: an observational cohort study. Lancet Infect Dis. 2020;20(5):565‐574. 10.1016/S1473-3099(20)30196-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu F, Liu M, Wang A, et al. Evaluating the association of clinical characteristics with neutralizing antibody levels in patients who have recovered from mild COVID‐19 in Shanghai, China. JAMA Intern Med. 2020;180(10):1356‐1362. 10.1001/jamainternmed.2020.4616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically Ill patients with COVID‐19 with convalescent plasma. JAMA. 2020;323(16):1582‐1589. 10.1001/jama.2020.4783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Addetia A, Crawford KHD, Dingens A, et al. Neutralizing antibodies correlate with protection from SARS‐CoV‐2 in humans during a fishery vessel outbreak with a high attack rate. J Clin Microbiol. 2020;58:e02107‐e02120. 10.1128/JCM.02107-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Popkin BM, Du S, Green WD, et al. Individuals with obesity and COVID‐19: a global perspective on the epidemiology and biological relationships. Obesity Reviews. 2020;21:e13128. 10.1111/obr.13128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Földi M, Farkas N, Kiss S, et al. Obesity is a risk factor for developing critical condition in COVID‐19 patients: a systematic review and meta‐analysis. Obesity Reviews. 2020;21:e13095. 10.1111/obr.13095 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


