Abstract
Currently, there is no widely acceptable and proven effective treatment for coronavirus disease 2019 (COVID‐19). Colchicine has been shown to offer a benefit in reducing the inflammation in several inflammatory diseases. This study aims to analyze the efficacy of colchicine administration and outcomes of COVID‐19. We systematically searched the PubMed and Europe PMC database using specific keywords related to our aims until January 29, 2021. All articles published on COVID‐19 and colchicine treatment were retrieved. The quality of the study was assessed using the Newcastle–Ottawa Scale (NOS) tool for observational studies and Revised Cochrane risk‐of‐bias tool for randomized trials (RoB 2) for clinical trial studies. Statistical analysis was done using Review Manager 5.4 software. A total of eight studies with 5778 COVID‐19 patients were included in this meta‐analysis. This meta‐analysis showed that the administration of colchicine was associated with improvement of outcomes of COVID‐19 [OR 0.43 (95% CI 0.34–0.55), p < 0.00001, I 2 = 0%, fixed‐effect modelling] and its subgroup which comprised of reduction from severe COVID‐19 [OR 0.44 (95% CI 0.31–0.63), p < 0.00001, I 2 = 0%, fixed‐effect modelling] and reduction of mortality rate from COVID‐19 [OR 0.43 (95% CI 0.32–0.58), p < 0.00001, I 2 = 0%, fixed‐effect modelling]. Our study suggests the routine use of colchicine for treatment modalities of COVID‐19 patients. More randomized clinical trial studies are still needed to confirm the results from this study.
Keywords: colchicine, coronavirus disease 2019, immune therapy, treatment
1. INTRODUCTION
In December 2019, the first cases of an acute respiratory illness (now known as the coronavirus disease 2019 or COVID‐19) were first reported in Wuhan, China. As of December 22, 2020, a total of about 75.1 million cases and 1 680 794 deaths have been identified across the world. 1 This disease can have a wide variety of clinical manifestations, from mild respiratory symptoms such as fever, cough, and anosmia to severe‐life threatening conditions such as respiratory distress, arrhythmia, sepsis, shock, and loss of consciousness. 2 The pathophysiology of severe COVID‐19 involves hyperinflammatory response and excessive production of pro‐inflammatory cytokines such as interleukin‐1β (IL‐1β), interleukin‐6 (IL‐6), and tumour necrosis‐α (TNF‐α), which is commonly called a cytokine storm. 3 , 4 Meta‐analysis studies have identified several comorbidities that were associated with severe outcomes and mortality from COVID‐19, such as hypertension, diabetes, thyroid disease, dyslipidaemia, dementia, cardiovascular disease, and pulmonary disease. 5 , 6 , 7 , 8 , 9 Therefore, efforts have been made to reduce the severity and mortality of COVID‐19, including searching for a potential therapy. So far, there are no medications which have been proven effective for COVID‐19. Previously suggested agents like hydroxychloroquine, lopinavir‐ritonavir, remdesivir, and tocilizumab did not show promising results to the outcomes of COVID‐19. 10 Based on the aforementioned pathophysiologic process of COVID‐19, several experts have recommended the use of colchicine as treatment for COVID‐19. Colchicine is a drug that is commonly used to treat and prevent the acute gout attacks, other crystal arthropathies, Familial Mediterranean Fever (FMF), and systemic vasculitis such as Behcet disease. Colchicine has anti‐inflammatory properties which may be beneficial in alleviating the cytokine storm through its action on NLRP3 and inhibition of IL‐1β, IL‐6, and IL‐18 activation. 11 However, the evidence regarding the association between colchicine use and COVID‐19 is still unclear. This article aims to explore the potential association between treatment with colchicine and outcomes of COVID‐19.
2. MATERIALS AND METHODS
2.1. Eligibility criteria
Studies were included in this review if they met the following inclusion criteria: representation for clinical questions (P, positive/confirmed cases of COVID‐19; I, a group who receive colchicine as their medications; C, a group of patients who did not receive colchicine as their medication and only receive standard of care treatment or receive other medications besides colchicine; O, COVID‐19 outcomes which comprised of severe COVID‐19 and mortality from COVID‐19), type of study was a randomized control trial, cohort, clinical trial, case‐cohort, and cross‐over design, and if the full‐text article was available. The following types of articles were excluded: articles other than original research (e.g., review articles, letters, or commentaries); case reports; articles not in the English language; articles on research in paediatric populations (17 years of age or younger); and articles on research in pregnant women.
2.2. Search strategy and study selection
A systematic search of the literature was conducted on PubMed and Europe PMC using the keywords “colchicine” AND “coronavirus disease 2019” OR “COVID‐19”, between 2019 and the present time (January 29, 2021) with language restricted to English only. The title, abstract, and full text of all articles identified that matched the search criteria were assessed, and those reporting the rate of colchicine treatment in COVID‐19 patients with a clinically validated definition of “severe disease” and “mortality” were included in this meta‐analysis. The references of all identified studies were also analyzed (forward and backward citation tracking) to identify other potentially eligible articles. The study was carried out per the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines.
2.3. Data extraction and quality assessment
Data extraction was performed independently by two authors, we used standardized forms that include author, year, study design, number of participants, age, gender, colchicine dosage, time to colchicine administration, and proportion of patients in each groups of treatment.
The outcome of interest was outcomes of COVID‐19 which comprised of severe COVID‐19 and mortality from COVID‐19. Severe COVID‐19 was defined as patients who had any of the following features at the time of, or after, admission: (1) respiratory distress (≥30 breaths per min); (2) oxygen saturation at rest ≤93%; (3) ratio of the partial pressure of arterial oxygen (PaO2) to a fractional concentration of oxygen inspired air (fiO2) ≤300 mmHg; or (4) critical complication (respiratory failure, septic shock, and or multiple organ dysfunction/failure). The mortality outcome from COVID‐19 was defined as the number of patients who were dead because of COVID‐19 infection.
Two investigators independently evaluated the quality of the included cohort and case‐control studies using the Newcastle–Ottawa Scale (NOS). 12 The selection, comparability, and exposure of each study were broadly assessed and studies were assigned a score from zero to nine. Studies with scores ≥7 were considered of good quality. They also independently evaluated the quality of the included clinical trial studies using the Revised Cochrane risk‐of‐bias tool for randomized trials (RoB 2). 13
2.4. Statistical analysis
A meta‐analysis was performed using Review Manager 5.4 (Cochrane Collaboration) software. We used the Generic Inverse Variance formula with fixed‐effects models to calculate each outcome's risk. The heterogeneity was assessed by using the I 2 statistic with a value of <25%, 26–50%, and >50% were considered as low, moderate, and high degrees of heterogeneity, respectively. The effect estimate was reported as odds ratio (OR) along with its 95% confidence intervals (CIs) for dichotomous variables, respectively. The p‐value was two‐tailed, and the statistical significance was set at ≤0.05. We performed Begg's funnel‐plot analysis to qualitatively assess the risk of publication bias.
3. RESULTS
3.1. Study selection and characteristics
A total of 886 records were obtained through systematic electronic searches. After the removal of duplicates, 732 records remained. A total of 714 records were excluded after screening the titles/abstracts because they did not match our inclusion and exclusion criteria. After evaluating 18 full texts for eligibility, five full‐text articles were excluded because they did not have a control/comparison group, three full‐text articles were excluded because they do not have the outcome of interest (severe COVID‐19 or mortality), two full‐text article were excluded because the articles were not in English, and finally, eight studies 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 with a total of 5778 COVID‐19 patients and 2668 patients who receive colchicine treatment were included in the meta‐analysis (Figure 1). Of a total of eight included studies, three studies were a randomized clinical trial study, two studies were retrospective cohort, two studies were prospective cohort, and the remaining one study was a case‐control study. The essential characteristics of the included studies are summarized in Table 1.
FIGURE 1.
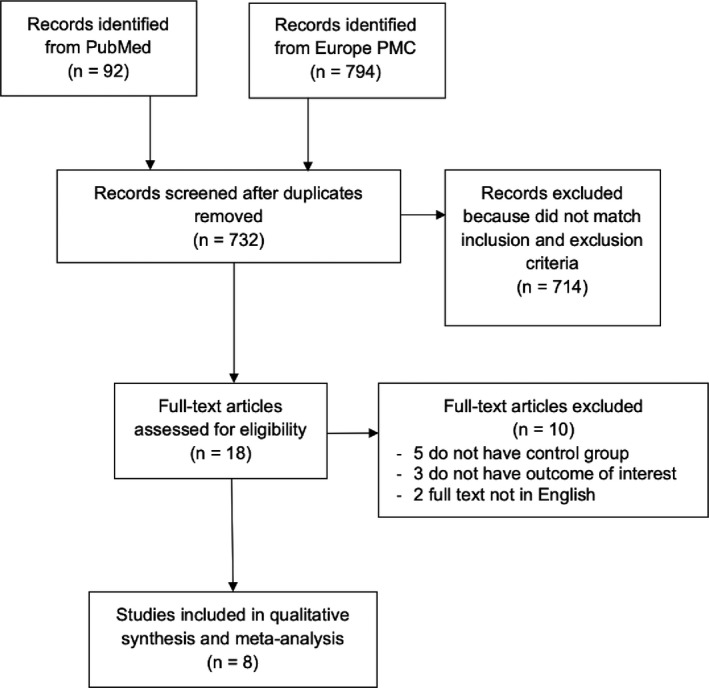
Prisma diagram flowchart
TABLE 1.
Characteristics of included studies
| Study | Sample size | Design | Overall age mean ±SD |
Male n (%) |
Colchicine dose | Time of colchicine administration |
Colchicine vs SOC n (%) |
Concurrent drug administration |
Adverse events of colchicine n (%) |
|---|---|---|---|---|---|---|---|---|---|
| Brunetti L et al. 14 2020 | 303 | Retrospective cohort | 62.9 ± 11.8 | 280 (65.2%) |
Loading dose: 1.2 mg Maintenance dose: 0.6 mg every 12 h for 30 days |
Dependent on the individual clinician (early before the progression of respiratory failure, typically within 72 h) | 41 (13.5%) vs 262 (86.5%) | Hydroxychloroquine (HCQ), azithromycin, tocilizumab, remdesivir, and supportive treatment | Not described |
| Deftereos SG et al. 15 2020 | 105 | Open‐label, randomized clinical trial | 64.6 ± 16.2 | 61 (58.1%) |
Loading dose: 1.5 mg Maintenance dose: 0.5 mg every 12 h until hospital discharge or maximum 21 days |
Median (IQR): 3 days (0–6 days) | 55 (52.3%) vs 50 (47.7%) | Hydroxychloroquine (HCQ), azithromycin, lopinavir/ritonavir, tocilizumab, and supportive treatment |
|
| Lopes MIF et al. 16 2020 (pre‐print) | 35 | Double‐blind, randomized clinical trial | 51.5 ± 22.2 | 14 (40%) | 0.5 mg every 8 h for 5 days then 0.5 mg every 12 h for the next 5 days | At the start of the trial | 17 (48.5%) vs 18 (51.5%) | Hydroxychloroquine (HCQ), azithromycin, heparin, corticosteroids, and supportive treatment |
|
| Pinzon MA et al. 17 2020 (pre‐print) | 301 | Prospective cohort | 56.8 ± 17.3 | 178 (59.1%) | 0.5 mg every 12 h for 7–14 days | At the start of hospital admission | 145 (48.2%) vs 156 (51.8%) | Hydroxychloroquine (HCQ), azithromycin, lopinavir/ritonavir, tocilizumab, corticosteroids, antibiotics, and supportive treatments | Not described |
| Rodriguez‐Nava G et al. 18 2020 | 87 | Retrospective cohort | 67 ± 12.5 | 56 (64.4%) | N/A | N/A | N/A | Hydroxychloroquine, azithromycin, tocilizumab, corticosteroids, and supportive treatment | Not described |
| Sandhu T et al. 19 2020 | 197 | Case‐control | 66.4 ± 13.3 | 114 (57.8%) | 0.6 mg every 12 h for 3 days and then 0.6 mg every 24 h for a total of 12 days | At the start of hospital admission | 53 (26.9%) vs 144 (73.1%) | Hydroxychloroquine (HCQ), oseltamivir, heparin, enoxaparin, DOAC, corticosteroids | Not described |
| Scarsi M et al. 20 2020 | 262 | Prospective cohort | 69.3 ± 9.6 | 167 (63.7%) | 1 mg every 24 h until hospital discharge | At the start of hospital admission | 122 (46.5%) vs 140 (53.5%) | Hydroxychloroquine (HCQ), corticosteroids, and supportive treatment | Not described |
| Tardif JC et al. 21 2020 (pre‐print) | 4488 | Double‐blind, randomized clinical trial | 54.4 ± 9.7 | 997 (44.6%) | 0.5 mg every 12 h for the first 3 days then once daily for 27 days thereafter | At the start of the trial | 2235 (49.7%) vs 2253 (50.3%) | Only supportive treatment |
|
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
3.2. Quality of study assessment
Studies with various study designs including cohort and case‐control were included in this review and assessed accordingly with the appropriate scale or tool. Newcastle–Ottawa Scales (NOS) were used to assess the cohort and case‐control studies (Table 2). All included studies were rated “good”. For clinical trial studies, the Revised Cochrane risk‐of‐bias tool for randomized trials (RoB 2) was used and all of the included trials showed a low risk of bias (Table 3). In conclusion, all studies were seemed fit to be included in the meta‐analysis.
TABLE 2.
Newcastle–Ottawa quality assessment of observational studies
| First author, year | Study design | Selection | Comparability | Outcome | Total score | Result |
|---|---|---|---|---|---|---|
| Brunetti L et al. 14 2020 | Cohort | **** | ** | *** | 9 | Good |
| Pinzon MA et al. 17 2020 | Cohort | *** | ** | *** | 8 | Good |
| Rodriguez‐Nava G et al. 18 2020 | Cohort | *** | ** | ** | 7 | Good |
| Sandhu T et al. 19 2020 | Case‐control | *** | ** | *** | 8 | Good |
| Scarsi M et al. 20 2020 | Cohort | *** | ** | *** | 8 | Good |
Asterisks indicate the star rating according to the Newcastle‐Ottawa scale. Good quality: *** or **** in selection domain AND * or ** in comparability domain AND ** or **** in outcome/exposure domain Fair quality: ** in selection domain AND * or ** in comparability domain AND ** or *** in outcome/exposure domain Poor quality: 0 or * in selection domain OR 0 stars in comparability domain OR 0 or * stars in outcome/exposure domain.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
TABLE 3.
Risk‐of‐bias assessment for clinical trial studies using RoB‐2 tool
| Study ID | Randomization process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported result | Overall |
|---|---|---|---|---|---|---|
| Deftereos SG et al. 15 2020 |

|

|

|

|

|

|
| Lopez MIF et al. 16 2020 |

|

|

|

|

|

|
| Tardif JC et al. 21 2020 |

|

|

|

|

|

|
 Low risk;
Low risk;  Some concerns;
Some concerns;  High risk.
High risk.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
3.3. Colchicine treatment and in‐hospital outcome
Our pooled analysis showed that administration of colchicine was associated with improvement in outcomes of COVID‐19 [OR 0.43 (95% CI 0.34–0.55), p < 0.00001, I 2 = 0%, fixed‐effect modelling] and its subgroup which comprised of reduction from severe COVID‐19, with no relevant heterogeneity [OR 0.44 (95% CI 0.31–0.63), p < 0.00001, I 2 = 0%, fixed‐effect modelling] and reduction of mortality rate from COVID‐19, with no relevant heterogeneity [OR 0.43 (95% CI 0.32–0.58), p < 0.00001, I 2 = 0%, fixed‐effect modelling] (Figure 2).
FIGURE 2.
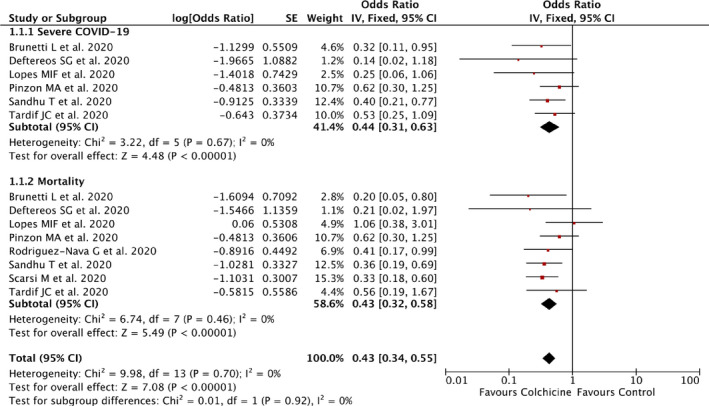
Forrest plot that demonstrates the association of colchicine with composite poor outcome of COVID‐19, severe COVID‐19, and mortality
3.4. Subgroup analysis
Subgroup analysis for randomized clinical trial studies showed a higher OR for COVID‐19 outcomes [OR 0.51 (95% CI 0.32–0.82), p = 0.005, I 2 = 0%, fixed‐effect modelling] compared to observational studies [OR 0.41 (95% CI 0.32–0.54), p < 0.00001, I 2 = 0%, fixed‐effect modelling], but both still showed statistically significant results.
3.5. Publication bias
The funnel‐plot analysis showed a qualitatively symmetrical inverted funnel‐plot for the association between treatment with colchicine and outcomes of COVID‐19 (Figure 3), showing no indication of publication bias.
FIGURE 3.
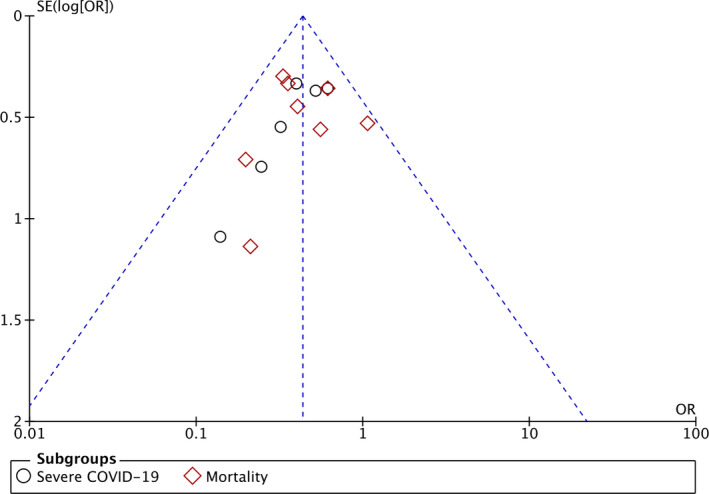
Funnel plot analysis for the association of colchicine with composite poor outcome of COVID‐19
4. DISCUSSION
Based on our pooled analysis of available data, colchicine treatments may be beneficial in improving the outcomes of COVID‐19 which comprised of reduction in COVID‐19 severity and reduction in the mortality rate from COVID‐19. Most of the included studies showed that the beneficial effects of colchicine treatment in COVID‐19 were seen the most when given early in the course of the disease (within the 3–6 days from symptoms onset or at the start of hospital admissions). One of the included clinical trial studies 21 also showed that colchicine treatment can be used in outpatient settings to prevent hospitalization, reducing the severity and mortality from the disease. These benefits of colchicine treatments in the outpatient settings were also supported by findings from case‐series study which demonstrated that oral colchicine treatment lead into the defervescence within 72 h in all COVID‐19 patients. Only one out of nine patients was admitted into the hospitals after administration of oral colchicine because of persistent dyspnea. 22 , 23 Several reasons can be proposed to explain the beneficial effects of colchicine treatment in COVID‐19 patients. First, colchicine can irreversibly intercalate into free α/β dimers that incorporate into and block microtubule extension. Microtubules itself is important to facilitate the movement of adhesion molecules onto cell surfaces during inflammation, including the migratory process of neutrophil towards the inflammatory cells. Colchicine can also interfere with neutrophil‐endothelial interactions by altering the number and/or distribution of selectins on endothelial cells and neutrophils and decreases E‐selectin‐mediated adhesiveness of the cytokine‐stimulated vascular endothelium to neutrophils at nano‐α‐prophylaxis. Moreover, colchicine may disrupt the microtubule structure and reduces neutrophil elasticity and relaxation, thus preventing neutrophil extravasation from the blood vessels to the site of inflammation. 24 Neutrophil itself is important in the inflammatory disease because it serves as the primary cells in innate immune response. In case of COVID‐19, neutrophil also plays an important role in the development of cytokine storm and that elevated levels of neutrophil or neutrophil‐lymphocytes ratio (NLR) has been associated with severe and mortality outcomes from COVID‐19. 25 Therefore, the inhibitory effects of colchicine on neutrophil functions, such as adhesiveness, motility, and chemotaxis can prevent the incidence of cytokine storm and reduce the mortality rate from COVID‐19. Second, the action of colchicine on tubulin ligands may also be beneficial in inhibiting the replication of viruses which depend on the microtubule network. Through inhibition of microtubule polymerization, colchicine has been reported to cause a significant decrease in virus replication in flaviviruses, such as dengue and Zika virus, hepatitis virus, and respiratory syncytial virus (RSV). 24 Coronaviruses, including SARS‐CoV‐2, are also dependent on microtubule‐associated transport for their replication process. The infection of cells by coronaviruses which leads to viral entry involves the interaction of the cytoplasmic tail of the spike protein with cytoskeletal proteins (i.e., tubulin). 24 , 26 Based on these reasons, the inhibition of viral entry and replication through administration of colchicine can help in reducing the risk of severe disease and mortality. Finally, colchicine can also inhibit the activation of the NLRP3 inflammasome and reducing the pro‐inflammatory cytokines production. Interruption of inflammasome activation will reduce IL‐1β production, which in turn prevents the induction of IL‐6 and TNF‐α and the recruitment of additional neutrophils and macrophages. 27 Elevated levels of cytokines may cause extensive lung consolidation which has been observed in the case of severe COVID‐19. 28 Thus, colchicine action in reducing the pro‐inflammatory cytokines can alter the severity and mortality outcomes of COVID‐19.
This study has limitations. First, the result of our meta‐analysis was largely based on observational studies and only includes three clinical trial studies because of the limited number of published clinical trial studies. Large clinical trial studies which evaluate the efficacy of colchicine treatment in COVID‐19 such as RECOVERY and COLCOVID trials are still underway and do not have results yet. Second, we include some pre‐print studies to minimize the risk of publication bias; however, the authors have made exhaustive efforts to ensure that only sound studies were included and we expect that most of those studies currently available in pre‐print form will eventually be published and that we will identify them through ongoing electronic literature surveillances. We hope that this study can give further insight into the treatment in COVID‐19 patients.
5. CONCLUSION
Colchicine administration can reduce the severity and mortality rate in COVID‐19 patients. Hence, physicians may consider adding/giving colchicine as a treatment for patients with COVID‐19 to help in preventing the severe outcome and mortality of the disease. More randomized clinical trial of colchicine is still needed to give a better assessment of colchicine efficacy. Finally, colchicine shall be regarded as an important agent in future treatment models for COVID‐19.
CONFLICT OF INTEREST
None.
ACKNOWLEDGEMENTS
None.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in PubMed at https://pubmed.ncbi.nlm.nih.gov/, reference number 14–21.
REFERENCES
- 1. World Health Organization . Coronavirus disease (COVID‐19): situation report. https://www.who.int/publications/m/item/weekly‐epidemiological‐update
- 2. Hariyanto TI, Rizki NA, Kurniawan A. Anosmia/Hyposmia is a good predictor of coronavirus disease 2019 (COVID‐19) infection: a meta‐analysis. Int Arch Otorhinolaryngol. 2021;25:e170‐e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ivan Hariyanto T, Kurniawan A. Tocilizumab administration is associated with the reduction in biomarkers of coronavirus disease 2019 infection. J Med Virol. 2021;93:1832‐1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hariyanto TI, Japar KV, Kwenandar F, et al. Inflammatory and hematologic markers as predictors of severe outcomes in COVID‐19 infection: a systematic review and meta‐analysis. Am J Emerg Med. 2020;41:110‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hariyanto TI, Kurniawan A. Thyroid disease is associated with severe coronavirus disease 2019 (COVID‐19) infection. Diabetes Metab Syndr. 2020;14(5):1429‐1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hariyanto TI, Kurniawan A. Anemia is associated with severe coronavirus disease 2019 (COVID‐19) infection. Transfus Apher Sci. 2020;59:102926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hariyanto TI, Putri C, Arisa J, Situmeang RFV, Kurniawan A. Dementia and outcomes from coronavirus disease 2019 (COVID‐19) pneumonia: a systematic review and meta‐analysis. Arch Gerontol Geriatr. 2020;93:104299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hariyanto TI, Putri C, Situmeang RFV, Kurniawan A. Dementia is a predictor for mortality outcome from coronavirus disease 2019 (COVID‐19) infection. Eur Arch Psychiatry Clin Neurosci. 2020:1‐3. 10.1007/s00406-020-01205-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hariyanto TI, Kurniawan A. Statin therapy did not improve the in‐hospital outcome of coronavirus disease 2019 (COVID‐19) infection. Diabetes Metab Syndr. 2020;14(6):1613‐1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hariyanto TI, Kristine E, Jillian Hardi C, Kurniawan A. Efficacy of Lopinavir/Ritonavir compared with standard care for treatment of coronavirus disease 2019 (COVID‐19): a systematic review. Infect Disord Drug Targets. 2020. 10.2174/1871526520666201029125725 [DOI] [PubMed] [Google Scholar]
- 11. Vitiello A, Ferrara F, Pelliccia C, Granata G, La Porta R. Cytokine storm and colchicine potential role in fighting SARS‐CoV‐2 pneumonia. Ital J Med. 2020;14(2):88‐94. [Google Scholar]
- 12. Margulis AV, Pladevall M, Riera‐Guardia N, et al. Quality assessment of observational studies in a drug‐safety systematic review, comparison of two tools: the Newcastle‐Ottawa Scale and the RTI item bank. Clin Epidemiol. 2014;6:359‐368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 14. Brunetti L, Diawara O, Tsai A, et al. Colchicine to weather the cytokine storm in hospitalized patients with COVID‐19. J Clin Med. 2020;9(9):2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Deftereos SG, Giannopoulos G, Vrachatis DA, et al. Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: the GRECCO‐19 randomized clinical trial. JAMA Netw Open. 2020;3(6):e2013136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lopes MIF, Bonjorno LP, Giannini MC, et al. Beneficial effects of colchicine for moderate to severe COVID‐19: an interim analysis of a randomized, double‐blinded, placebo controlled clinical trial. medRxiv. 2020. 10.1101/2020.08.06.20169573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pinzon MA, Arango DC, Betancur JP, et al. Clinical outcome of patients with COVID‐19 pneumonia treated with corticosteroids and colchicine in Colombia. Research Square. 2020. 10.21203/rs.3.rs-94922/v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rodriguez‐Nava G, Trelles‐Garcia DP, Yanez‐Bello MA, Chung CW, Trelles‐Garcia VP, Friedman HJ. Atorvastatin associated with decreased hazard for death in COVID‐19 patients admitted to an ICU: a retrospective cohort study. Crit Care. 2020;24(1):429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sandhu T, Tieng A, Chilimuri S, Franchin G. A case control study to evaluate the impact of colchicine on patients admitted to the hospital with moderate to severe COVID‐19 infection. Can J Infect Dis Med Microbiol. 2020;2020:8865954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scarsi M, Piantoni S, Colombo E, et al. Association between treatment with colchicine and improved survival in a single‐centre cohort of adult hospitalised patients with COVID‐19 pneumonia and acute respiratory distress syndrome. Ann Rheum Dis. 2020;79(10):1286‐1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tardif JC, Bouabdallaoui N, L'Allier PL, et al. Efficacy of colchicine in non‐hospitalized patients with COVID‐19. medRxiv. 2020. 10.1101/2021.01.26.21250494 [DOI] [Google Scholar]
- 22. Della‐Torre E, Della‐Torre F, Kusanovic M, et al. Treating COVID‐19 with colchicine in community healthcare setting. Clin Immunol. 2020;217:108490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Della‐Torre E, Ramirez GA, Dagna L, Tresoldi M. Colchicine treatment in community healthcare setting to prevent severe COVID‐19. Ann Rheum Dis. 2020. 10.1136/annrheumdis-2020-218759 [DOI] [PubMed] [Google Scholar]
- 24. Schlesinger N, Firestein BL, Brunetti L. Colchicine in COVID‐19: an old drug, new use. Curr Pharmacol Rep. 2020;6:137‐145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li X, Liu C, Mao Z, et al. Predictive values of neutrophil‐to‐lymphocyte ratio on disease severity and mortality in COVID‐19 patients: a systematic review and meta‐analysis. Crit Care. 2020;24(1):647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Piantoni S, Colombo E, Airò P, et al. The rationale for the use of colchicine in COVID‐19: comments on the letter by Cumhur Cure M et al. Clin Rheumatol. 2020;39(8):2489‐2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reyes AZ, Hu KA, Teperman J, et al. Anti‐inflammatory therapy for COVID‐19 infection: the case for colchicine. Ann Rheum Dis. 2020. 10.1136/annrheumdis-2020-219174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Della‐Torre E, Campochiaro C, Cavalli G, et al. Interleukin‐6 blockade with sarilumab in severe COVID‐19 pneumonia with systemic hyperinflammation: an open‐label cohort study. Ann Rheum Dis. 2020;79(10):1277‐1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available in PubMed at https://pubmed.ncbi.nlm.nih.gov/, reference number 14–21.


