Abstract
Background
The risk of severe acute respiratory syndrome‐related coronavirus 2 (SARS‐CoV‐2) infection and clinical outcomes of coronavirus disease (COVID‐19) in inflammatory bowel disease are unclear.
Methods
We searched PubMed and Embase with the keywords: inflammatory bowel disease, Crohn's disease, ulcerative colitis and COVID‐19, novel coronavirus and SARS‐CoV‐2. We included studies reporting the frequency of COVID‐19 infection and outcomes (hospitalisation, need for intensive care unit care and mortality) in patients with inflammatory bowel disease. We estimated the pooled incidence of COVID‐19 in inflammatory bowel disease and comparative risk vis‐a‐vis the general population. We also estimated the pooled frequency of outcomes and compared them in patients who received and did not receive drugs for inflammatory bowel disease.
Results
Twenty‐four studies were included. The pooled incidence rate of COVID‐19 per 1000 patients of inflammatory bowel disease and the general population were 4.02 (95% confidence interval [CI, 1.44–11.17]) and 6.59 [3.25–13.35], respectively, with no increase in relative risk (0.47, 0.18–1.26) in inflammatory bowel disease. The relative risk of the acquisition of COVID‐19 was not different between ulcerative colitis and Crohn's disease (1.03, 0.62–1.71). The pooled proportion of COVID‐19‐positive inflammatory bowel disease patients requiring hospitalisation and intensive care unit care was 27.29% and 5.33% while pooled mortality was 4.27%. The risk of adverse outcomes was higher in ulcerative colitis compared to Crohn's disease. The relative risks of hospitalisation, intensive care unit admission and mortality were lower for patients on biological agents (0.34, 0.19–0.61; 0.49, 0.33–0.72 and 0.22, 0.13–0.38, respectively) but higher with steroids (1.99, 1.64–2.40; 3.41, 2.28–5.11 and 2.70, 1.61–4.55) or 5‐aminosalicylate (1.59, 1.39–1.82; 2.38, 1.26–4.48 and 2.62, 1.67–4.11) use.
Conclusion
SARS‐CoV‐2 infection risk in patients with inflammatory bowel disease is comparable to the general population. Outcomes of COVID‐19‐positive inflammatory bowel disease patients are worse in ulcerative colitis, those on steroids or 5‐aminosalicylates but outcomes are better with biological agents.
Keywords: coronavirus, Crohn's disease, SARS‐CoV‐2, ulcerative colitis
Key Summary
Current knowledge
Inflammatory bowel disease may be associated with an increased risk of various infections.
Certain inflammatory bowel disease therapies may predispose to an increased risk of infections (e.g., anti tumour necrosis factor and tuberculosis, tofacitinib and herpes zoster).
The effect of inflammatory bowel disease and inflammatory bowel disease therapies on coronavirus disease infection and outcomes is unclear.
What are the new findings?
In this meta‐analysis, we found that the risk of coronavirus disease in patients with inflammatory bowel disease is similar to the general population.
The risk of coronavirus disease does not seem to be affected by the underlying type of inflammatory bowel disease, that is, Crohn's disease or ulcerative colitis.
The risk of adverse outcomes in inflammatory bowel disease is more in patients receiving steroids and 5‐aminosalicylates.
Biological agent use seems to be protective against adverse outcomes of coronavirus disease in inflammatory bowel disease patients.
1. INTRODUCTION
The coronavirus disease (COVID‐19) pandemic has brought forth a multitude of challenges for patients having inflammatory bowel disease (IBD) and healthcare practitioners involved in the care of patients with IBD. IBDs, both ulcerative colitis (UC) and Crohn's disease (CD), are associated with an increased risk of infections, especially in elderly patients, active IBD and those on immunosuppressive medications. 1 In many regions, national‐wide lockdowns have had effects on the availability of drugs and access to healthcare. To add to this, patients have faced psychological problems such as anxiety because of the concerns about their health in the presence of underlying IBD and ongoing treatment with immunosuppressive drugs. 2 While COVID‐19 has high infectivity and carries the risk of significant morbidity and mortality, information on the risk of infection by severe acute respiratory syndrome‐related coronavirus 2 (SARS‐CoV‐2) in IBD patients and the outcomes is limited.
Angiotensin‐converting enzyme 2 (ACE‐2) receptor, which has a role in viral entry into host cells, is expressed in the human intestine and the expression could be upregulated after infection with SARS‐ CoV‐2. There are conflicting data on changes in the expression of ACE‐2 in ileal and colonic mucosa of patients with active IBD. 3 , 4 In addition, the therapeutic agents used in IBD could affect ACE‐2 expression. 4 Therefore, a complex interplay of receptors, physiological processes and therapies may define the risk of acquisition and clinical outcomes in these patients.
Furthermore, published evidence regarding the risk of the acquisition of coronaviruses and clinical outcomes in infected patients with IBD was unavailable prior to this pandemic. Various organisations, in this state of evidence vacuum, have provided expert consensus‐based guidance for clinicians and patients with IBD. 5 The published literature on IBD and COVID‐19 is limited by cohort studies and case series including a small number of patients. This makes it difficult to assess the actual risk of COVID‐19 infection in patients with IBD and the consequences of such an infection. Another important concern is the effect of various therapeutic agents used in IBD on the risk of acquisition and the clinical course of COVID‐19.
Therefore, we planned to do a systematic review of observational studies on the risk of the acquisition of SARS‐CoV‐2 in patients with IBD, and to estimate whether the drugs affect the risk of acquisition and outcomes of SARS‐CoV‐2 infection.
2. METHODS
This meta‐analysis was conducted in accordance with the Meta‐Analysis of Observational Studies in Epidemiology guidance and Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidance.
2.1. Database search
We searched electronic databases using PubMed and Embase from 1 December 2019 to 29 July 2020. The keywords used for the search were inflammatory bowel disease, ulcerative colitis, Crohn disease, Crohn's disease combined using the operator “AND” Coronavirus, COVID‐19, SARS‐COV‐2, nCOV, coronaviridae infection or coronavirus disease 2019 (detailed strategy as described in Table S1). The bibliographies of included studies and reviews were searched for additional eligible studies. The authors of unpublished data of which we were aware were contacted for data. The eligible titles were combined and the duplicates were removed. The titles and abstracts were then reviewed by two reviewers (Anupam Kumar Singh and Anuraag Jena). After screening of the titles and abstracts, papers were selected for full text screening. Differences, if any, were resolved after discussion with a third reviewer (Vishal Sharma).
2.2. Inclusion and exclusion criteria
We included all relevant articles which fulfilled the inclusion criteria irrespective of the type (original paper, abstract, letter, correspondence), format or the language of publication. We included studies which reported at least one of the two key outcomes: (a) risk or frequency of acquisition of SARS‐CoV‐2 infection in IBD patients with or without comparison to the general population; or (b) outcomes (hospitalisation, need for intensive care unit (ICU) care or mortality) in IBD patients infected with SARS‐COV‐2. We excluded studies which did not have relevant outcome data or the data were incomplete. We also excluded single patient case reports, reviews, editorial and commentaries.
2.3. Data collection
Data were extracted from the included studies by the two reviewers (Anupam Kumar Singh and Anuraag Jena) and any discrepancy was resolved by discussion with the third reviewer (Vishal Sharma). Extracted data included publication details (author and year), place of study, overall population of IBD patients and COVID‐19‐positive IBD patients, age, gender, disease type (CD or UC), presence of comorbidities, current treatment including 5‐aminosalicylic acid (5‐ASA), immunomodulators (thiopurines, calcineurin inhibitors and methotrexate), steroids, biological agents (antitumour necrosis factor [TNF], vedolizumab and ustekinumab) and small molecule inhibitors and details of their outcomes (hospitalisation, need for ICU and mortality). The definitions of COVID‐19 infection used in various studies were also recorded.
2.4. Data analysis
The analysis was conducted using R statistical software version 4.0.1 and meta package was used additionally. The inverse variance method with a random‐effect approach was used for computing the pooled summary of incidence. The incidence was logit transformed for computing summary and the Clopper–Pearson confidence interval was used for individual studies. Similarly, the overall risk ratio was computed by the inverse variance method with a random effect approach. Continuity correction of 0.5 was applied for cells with 0 value. The heterogeneity was determined by I 2 and p value of heterogeneity. The DerSimonian–Laird estimator was used for computing τ. 2 We used a random‐effect approach irrespective of I 2 considering that heterogeneity was present among the studies at the level of study design and approach towards the research question.
We calculated the pooled incidence of COVID‐19 infection in patients with IBD and in the general population. We calculated the pooled risk ratio of acquisition of COVID‐19 in IBD as compared to the general population. Among the IBD population, the risk of acquiring the COVID‐19 infection was assessed for the subtype of IBD; that is, UC and CD. We also did subgroup analysis for assessing the impact of the age of patients on the incidence of COVID‐19 in IBD patients based on a cut‐off of 45 years. We also calculated the risk of infection in IBD patients based on the medication used for the treatment of IBD (5‐ASA, immunomodulators, steroids and biological agents (anti‐TNF, vedolizumab and ustekinumab)) at the time of acquiring the COVID‐19 infection. For the purpose of analysis, the thiopurines, calcineurin inhibitors and antimetabolites (methotrexate) were grouped together as immunomodulators.
We estimated the pooled frequency of various outcomes of interest: hospitalisation, need for ICU care and mortality in the patients with IBD infected with COVID‐19 and similarly for the subtypes of IBD (UC and CD) and the pooled risk of each outcome in patients receiving or not receiving various medications used for the treatment of IBD (5‐ASA, immunomodulators, steroids and biological agents). In the outcome analysis, the biological agents (anti‐TNF, vedolizumab and ustekinumab) were clubbed together as “biologics”. The risk ratio was calculated for hospitalisation, need for ICU and mortality in patients with UC and CD infected with COVID‐19. For the outcomes which were found to be heterogeneous (I 2 > 50%), assessment of heterogeneity by the Baujat plot and leave‐one‐out analysis was done.
2.5. Methodological quality and risk of bias assessment
Two of the investigators (Anupam Kumar Singh and Anuraag Jena) independently assessed the methodological quality and risk of bias for each study. We used the Joanna Briggs Institute critical appraisal checklist for studies reporting the incidence and outcome data. 6 , 7 The Joanna Briggs appraisal for incidence data includes questions about the appropriateness of study sample and selection, description of setting and subjects, completeness of provided data and analysis and the appropriateness of measuring the condition. For outcome data, the Joanna Briggs appraisal for case series includes questions about inclusion, standard and similar methods of diagnosing the condition and consecutiveness and completeness of participant data and outcomes. Any discordance in quality assessment was resolved by mutual agreement of both the investigators (Anupam Kumar Singh and Anuraag Jena) in discussion with a third reviewer (Vishal Sharma).
3. RESULTS
After the database search a total of 390 titles were identified and two additional papers were identified from other sources. In all there were 157 duplicates. Therefore, a total of 235 articles were screened for title and abstract and 35 papers underwent full text screening (Figure 1). Eventually, data from 24 studies were used for analysis. This also includes data extracted from the SECURE‐IBD registry (on 29 July 2020) and one study identified by manual search. Table 1 provides the details of the included studies including location, number of subjects, age, basis of diagnosis, comorbidities and the eventual inclusion in one of the two analyses. 2 , 8 , 29 The definitions used for COVID diagnosis were based on real time polymerase chain reaction (RT‐PCR) testing or clinical symptoms consistent with COVID‐19 with radiological evidence of pneumonia in most studies (Table 1). Table S2 lists the reasons for the exclusion of studies.
FIGURE 1.
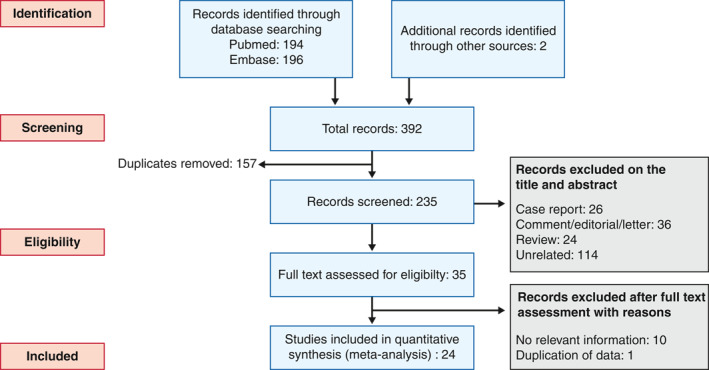
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow chart showing selection process of the studies
TABLE 1.
Summary of various included studies, patient characteristics and the data provided for analysis
| Study | Location | No. of total IBD | No. of COVID IBD and basis of diagnosis | Male: female | Age (years) | Comorbid conditions | UC and CD | Drugs | Outcome | Data used for analysis | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Risk of COVID‐19 | Outcome of COVID‐19 | ||||||||||
| Taxonera C | Madrid, Spain | 1918 | 12 RT PCR | 1:3 | 52 ±16 | Comorbidities 5 (41.6%)
|
UC 5 | ASA 4 (33.3%) | Hosp 8 (66.7%) | Yes | Yes |
| CD 7 | Steroid 0 IMM 6 (50%) | ICU 1 (8.3%) | |||||||||
| Biologicals 5 (41.6%) | Death 2 (16.7%) | ||||||||||
| Anti‐TNF 3 (25%) | |||||||||||
| Hormati A | Qom, Iran | 150 | 8 RT PCR | NA | NA | NA | NA | NA | NA | Yes | No |
| Lukin DJ | New York, USA | 119 as a cohort; additional 80 cases as part of case–control design | 29 (9 RT PCR positive and 20 highly suspected) | 1:1.42 | NA | NA | UC 14 | ASA 11 (37.9%) | NA | Yes | No |
| CD 15 | Steroid 19 (65.5%) | ||||||||||
| IMM 2 (6.9%) | |||||||||||
| Biologicals 21 (72.4%) | |||||||||||
| Rodriguez‐Lago 1 | Basque country, Spain | NA | 40 RT PCR | 3:2 | 59 (48–68) | Comorbidities 25 (62.5%) | UC 27 | ASA 26 (65%) | Hosp 21 (52.5%) | No | Yes |
| CD 13 | Steroid 4 (10%) | ICU 0 Death 2 (5%) | |||||||||
| IMM 13 (32.5%) | |||||||||||
| Biologicals 9 (22.5%) | |||||||||||
| Anti‐TNF 4 (10%) | |||||||||||
| Haberman R | New York, USA | NA | 37 | NA | NA | NA | UC 17 | ASA 7 (18.9%) | Hosp 4 (10.8%) | No | Yes |
| CD 20 | Steroid 1 (2.7%) | ICU 0 | |||||||||
| IMM 1 (2.7%) | |||||||||||
| Biologicals 24 (64.8%) | |||||||||||
| Anti‐TNF 19 (51.3%) | |||||||||||
| Turner D | China (paediatric) | 1431 | 0 (suspected or confirmed) | NA | NA | NA | NA | NA | NA | No | @ |
| Turner D | South Korea (paediatric) | 272 | 0 | NA | NA | NA | NA | NA | NA | No | @ |
| Axelrad JE | New York, USA | NA | 83 (confirmed or highly suspected) | 1.13:1 | 35 (27–45) | Asthma/COPD‐lO (12%) | UC 27 | ASA 13 (15.6%) | Hosp 5 (6.0%) | No | Yes |
| Hypertension 3 (3.6%) | CD 56 | Steroid 10 (12%) | Death 1 (1.2%) | ||||||||
| DM 1 (1.2%) | IMM 6 (7.2%) | ||||||||||
| CKD 1 (1.2%) | Biologicals 58 (69.8%) | ||||||||||
| Malignancy 1 (1.2%) | Anti‐TNF 44 (53%) | ||||||||||
| Post‐transplant 2 (2.4%) | |||||||||||
| Khan N | Nation‐wide Veteran Cohort, USA | 37857 | 36 ICD code based | NA | 60.9 ± 17.1 | NA | NA | IMM 2 (5.5%) | NA | Yes | No |
| Anti‐TNF 3 (8.3%) | |||||||||||
| Mosli M | Saudi Arabia | 1156 | 6 testing | 1:1 | NA | Comorbidities 0 | UC 1 | ASA 3 (50%) | NA | Yes | No |
| CD 5 | Steroid 1 (16.7%) | ||||||||||
| IMM 1 (16.7%) | |||||||||||
| Biologicals 2 (33.3%) | |||||||||||
| Anti‐TNF 2 (33.3%) | |||||||||||
| Scaldaferri F | Italy | 1451 | 5 | NA | NA | NA | NA | NA | Hosp 0 | Yes | Yes |
| ? Basis | ICU 0 | ||||||||||
| Death 0 | |||||||||||
| Allocca M | Italy and France | 6000 | 15 | 1:2.75 | 38.4 ± 10.54 | Comorbidities 9 (60%)
|
UC 6 | ASA 1 (6.6%) | Hosp 5 (33.3%) | Yes | Yes |
| CD 9 | Steroid 2 (13.3%) | ICU 0 Death 0 | |||||||||
| IMM 3 (20%) | |||||||||||
| Biologicals 12 (80%) | |||||||||||
| Anti‐TNF 8 (53.3%) | |||||||||||
| Grunert PC | Germany | 415 | 0 | NA | NA | NA | NA | NA | NA | Yes | No |
| Marafini 1 | Italy | 672 | 3 (RT PCR) | NA | NA | NA | NA | NA | Hosp 2 (66.6%) | Yes | Yes |
| Death 1 (33.3%) | |||||||||||
| Bezzio C | Italy | NA | 79 (RT PCR in 49 OR clinical plus radiology in 30) | 1.26:1 | 45(18–80) | Comorbidities 30 (37.9%)
|
UC 47 | ASA 24 (30%) | Hosp 22 (27.9%) | No | Yes |
| CD 32 | Steroid 9 (11.3%) | Death 6 (7.6%) | |||||||||
| IMM 7 (8.8%) | |||||||||||
| Biologicals 47 (59.5%) | |||||||||||
| Anti‐TNF 29 (36.7%) | |||||||||||
| Yu M | China, Wuhan | 102 | 0 | NA | NA | NA | NA | NA | NA | Yes | No |
| An P | Wuhan, China | 318 | 0 | NA | NA | NA | NA | NA | NA | Yes | No |
| Singh S | Multicentre, USA | 196,403 | 232 (RT PCR or ICD code based) | 1:1.25 | 51.2 ± 18.1 | Hypertension 121 (52.1%) | UC 131 | NA | Hosp 56 (24.1%) | Yes | Yes |
| DM 62 (26.7%) | CD 101 | ||||||||||
| CKD 38 (16.3%) | |||||||||||
| CV disease 86 (37%) | |||||||||||
| CVA 30 (12.9%) | |||||||||||
| COPD/asthma 91 (39.2%) | |||||||||||
| Gubatan J | North California, USA | 168 | 5 (RT PCR) | 2:3 | 70.6 (±4.2) | Hypertension 4 (80%) | UC 3 | ASA 4 (80%) | ICU 1 (20%) | Yes | Yes |
| DM 2 (40%) | CD 2 | Steroid 1 (20%) | Death 1 (20%) | ||||||||
| IMM 1 (20%) | |||||||||||
| Biologicals 1 (20%) | |||||||||||
| Anti‐TNF 1 (20%) | |||||||||||
| Norsa L | Northern Italy | 522 | 0 | NA | NA | NA | NA | NA | NA | Yes | No |
| Mak JWY | Hong Kong cohort | 2954 | 0 | NA | NA | NA | NA | NA | NA | Yes | No |
| Mak JWY | Taiwan cohort | 3091 | 0 | NA | NA | NA | NA | NA | NA | Yes | No |
| Foteinogiannopoulou | Greece | 78 | 0 | NA | NA | NA | NA | NA | NA | Yes | No |
| Gonzalez HA* | Multicentre (Italy, Spain, UK) | NA | 147 (RT PCR in 61, rest with consistent symptoms) | NA | NA | Comorbidities 45(30.6%)
|
UC 65 | ASA 41 (27.8%) | Hosp 44 (29.9%) | No | Yes |
| Steroid 12 (8.1%) | ICU 10 (6.8%) | ||||||||||
| CD 82 | IMM 71 (48.2%) | Death 2 (1.4%) | |||||||||
| Biologicals 96 (65.3%) | |||||||||||
| Anti‐TNF 39 (26.5%) | |||||||||||
| Grassia | Italy | 251 | 1 (not given) | NA | NA | NA | NA | NA | NA | Yes | No |
| SECURE IBD | Multicentre, multi‐ country registry | NA | 1830 | NA | NA | Comorbidities 669 (36.5%) | UC 812 | ASA/sulfasalazine572 (31.2%) | Hosp 512 (27.9%) | No | Yes |
| CD 1010 | Steroids 197 (10.7%) | ICU 99 (5.4%) | |||||||||
| Unknown 8 | IMM 360 (19.6%) | Death 63 (3.4%) | |||||||||
| Biologicals 1053 (57.5%) | |||||||||||
| Anti‐TNF 536 (29.2%) | |||||||||||
Note. @provides a case series of seven paediatric IBD‐COVID positive cases used in outcomes analysis.
Abbreviations: anti‐TNF, anti‐tumour necrosis factor agents; ASA, aminosalicylates; CD, Crohn's disease; COVID, coronavirus disease; Hosp, hospitalisation; IBD, IMM, immunomodulators; inflammatory bowel disease; ICU, intensive care unit; NA, not applicable; RT PCR, real‐time polymerase chain reaction; UC, ulcerative colitis.
*Unpublished.
3.1. Risk of COVID‐19 in IBD patients
Seventeen studies provided the information on the incidence rate of COVID‐19 in IBD. The pooled incidence rate of COVID‐19 in patients with IBD was 4.02 (95% confidence interval (CI) 1.44–11.17; 72 = 98%) per 1000 population (Figure 2). The corresponding rate of infection as reported in the general population was 6.59 (3.25–13.35; I 2 = 100%) in the six participating studies (Figure 2). The pooled relative risk (RR) of the acquisition of COVID‐19 in patients with IBD was not different from the general population (0.47, 0.18–1.26; I 2 = 89%, Figure 2). For studies in which the mean/median age of IBD patients was provided, we calculated the pooled incidence of COVID separately for studies with a mean/median age of 45 years or less and over 45 years. While the pooled incidence in studies with a mean/median age of 45 years or less was 2.06% (0.77–5.54; I 2 = 18), it was 4.44% (0.99–19.61; I 2 = 96%) for the studies with an age over 45 years (Figure S1). In addition, the studies which provided information on the impact of age on COVID‐19 infection in IBD are shown in Table S3.
FIGURE 2.
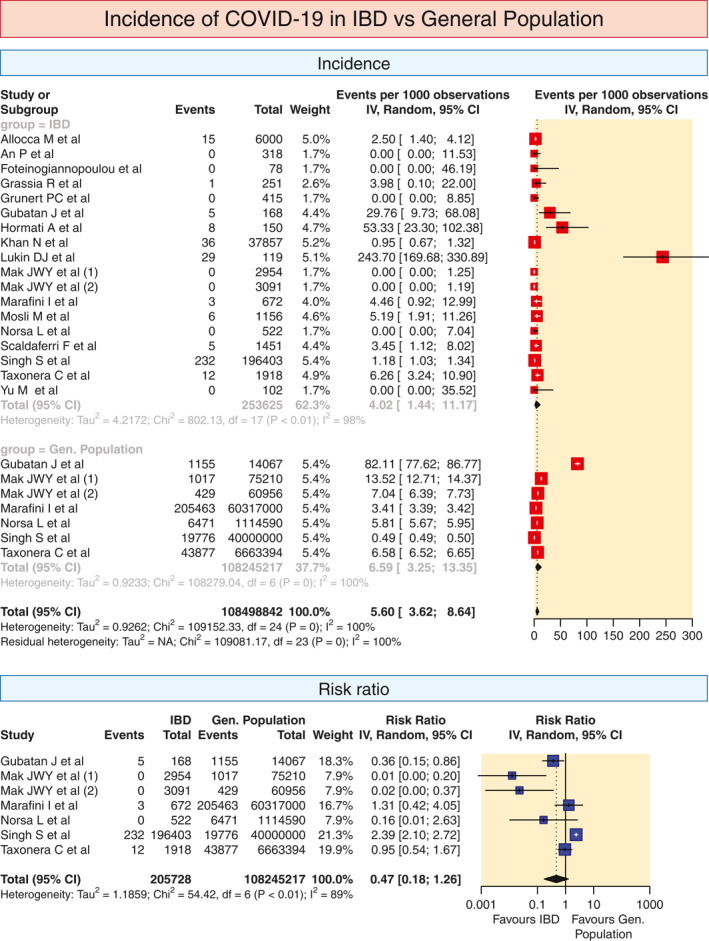
Pooled incidence of COVID in IBD and the general population and relative risk of COVID infection in IBD patients as compared to the general population. The pooled summary was computed by a random effect approach. CI, confidence interval; COVID, coronavirus disease; IBD, inflammatory bowel disease
The overall incidence of COVID‐19 in patients with UC was 4.55 (0.76–26.80; I 2 = 93%) per 1000 cases (Figure S2). The overall incidence rate of COVID‐19 in patients with CD was 6.66 (1.4929.35; I 2 = 92%) per 1000 cases (Figure S2). When the nine studies reporting the risk in UC and CD were compared for the overall RR, the risk was not different between the two (RR 1.03, 0.62–1.71; I 2 = 0; Figure S2).
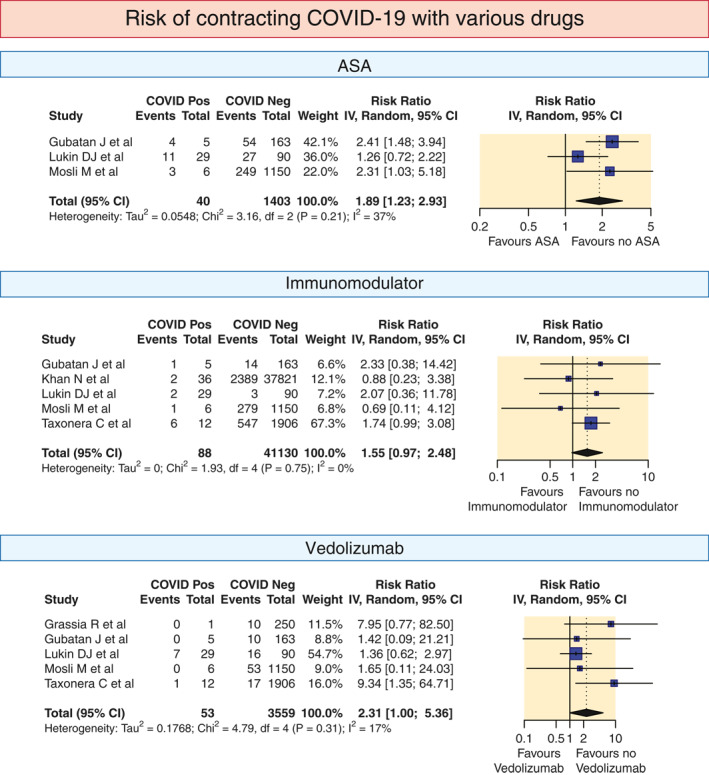
Some studies provided data regarding the use of various drugs in patients acquiring COVID‐19 and those who did not acquire COVID‐19. On pooled analysis of this data, the risk ratios of contracting COVID‐19 with various drugs were as follows: for 5‐ASA 1.89 (1.232.93; I 2 = 37%); steroids 1.64 (1–2.7; I 2 = 0%); immunomodulator 1.55 (0.97–2.48; I 2 = 0%); anti‐TNF 1.08 (0.68–1.71; I 2 = 0%); vedolizumab 2.31 (1–5.36; I 2 = 17%) and ustekinumab 3.16 (0.55–18.07; I 2 = 72%; Figure 3).
FIGURE 3.
Pooled risk ratio of COVID infection in IBD patients depending on use of various drugs (5‐ASA, steroids, immunomodulators, biological agents, anti‐TNF, vedolizumab and ustekinumab). The pooled summary was computed by a random effect approach. 5‐ASA, aminosalicylic acid; CI, confidence interval; COVID, coronavirus disease; IBD, inflammatory bowel disease; TNF, tumour necrosis factor

3.2. Outcomes for IBD patients with COVID‐19
The hospitalisation rates in the 11 included studies varied from 0% to 66.67%. The pooled hospitalisation rate was 27.99% (21.92–34.99; I 2 = 76%; Figure 4).
FIGURE 4.
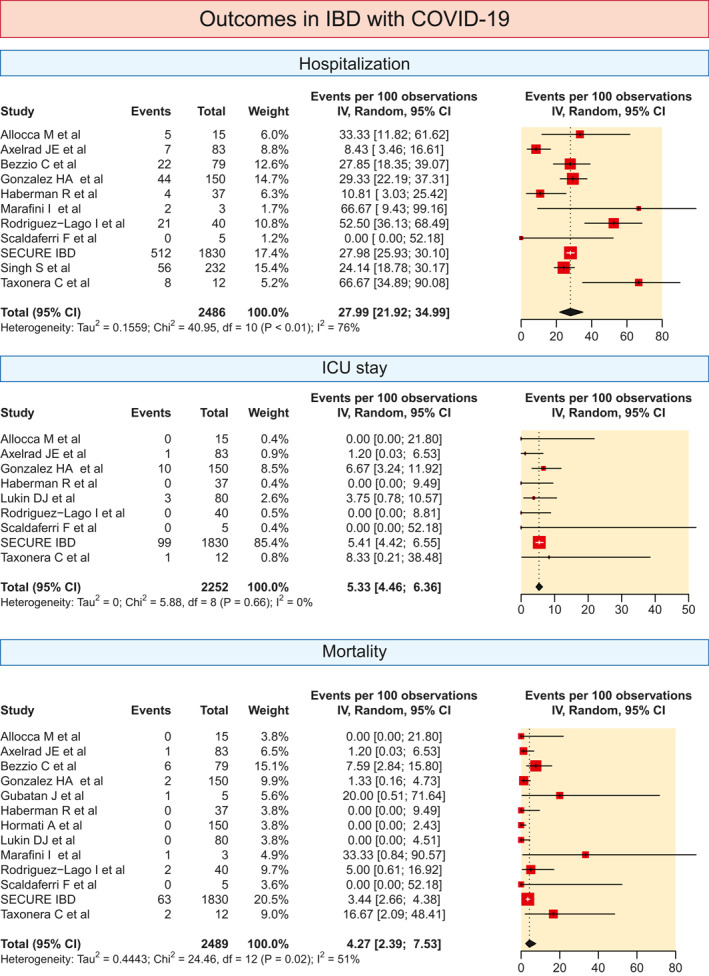
The pooled prevalence of various outcomes (hospitalisation, need for ICU and mortality) in IBD patients with COVID. The pooled summary was computed by a random effect approach. CI, confidence interval; COVID, coronavirus disease; IBD, inflammatory bowel disease; ICU, intensive care unit
The pooled proportion of patients needing ICU care was 5.33% (4.46–6.36; I 2 = 0) based on data from nine studies (Figure 4). The pooled mortality rate in patients with IBD with COVID‐19 was 4.27% (2.39–7.53; I 2 = 51%) and mortality rates varied from 0% to 33.3% in the 13 studies which were included (Figure 4).
The overall risk of hospitalisation was higher in patients with UC (RR 1.55, 1.22–1.97; I 2 = 15%) (Figure 5).
FIGURE 5.

The pooled relative risk of various outcomes (hospitalisation, need for ICU and mortality) in UC versus CD. The pooled summary was computed by a random effect approach. CD, Crohn's disease; CI, confidence interval; ICU, intensive care unit; UC, ulcerative colitis
The risk of need for ICU care was statistically similar between the two groups (RR 1.42, 0.972.07; I 2 = 0%; Figure 5). However, the risk of mortality was higher in patients with UC infected with COVID‐ 19 as compared to CD (RR 1.94, 1.22–3.10; I 2 = 0%; Figure 5).
3.3. Impact of IBD drugs on COVID‐19 outcomes
The relative risk of hospitalisation (1.59, 1.39–1.82; I 2 = 0), need for ICU care (2.38, 1.26–4.48; I 2 = 18) and mortality (2.62, 1.67–4.11; I 2 = 0) were higher with the use of 5‐ASA (Figure 6). The RR of hospitalisation (1.99, 1.64–2.40; I 2 = 3%), need for ICU (3.41, 2.285.11; I 2 = 0) and mortality (2.70, 1.61–4.55; I 2 = 0) were higher with the use of steroids (Figure 6). The RR of hospitalisation (0.89, 0.37–2.10; I 2 = 83%), need for ICU (0.71, 0.17–3.02; I 2 = 45%) and mortality (1.18, 0.23–6.01; I 2 = 55%) were similar irrespective of the use of immunomodulators (Figure 6). The RR of hospitalisation (0.34, 0.19–0.61; I 2 = 67%), need for ICU (0.49, 0.33–0.72; I 2 = 0) and mortality (0.22, 0.13‐0.38; I 2 = 0) were lower with the use of biological agents (Figure 6).
FIGURE 6.
The pooled relative risk of various outcomes (hospitalisation, need for ICU and mortality) in IBD COVID patients with respect to the use of various drugs (5‐ASA, steroids, immunomodulators, biological agents, anti‐TNF, vedolizumab and ustekinumab). The pooled summary was computed by a random effect approach. 5‐ASA, aminosalicylic acid; CI, confidence interval; IBD, inflammatory bowel disease; ICU, intensive care unit; TNF, tumour necrosis factor



3.4. Assessment of heterogeneity
The heterogeneity assessment was conducted for the incidence of COVID‐19 among the IBD and general population and outcome assessment (mortality and hospitalisation) among the patients with IBD with COVID‐19. The leave‐one‐out analysis for the incidence of COVID‐19 among IBD and the general population did not lead to a significant change in heterogeneity. The detailed information is provided in Figures S3 and S4.
3.5. Risk of bias
The risk of bias of included studies for the incidence of COVID‐19 infection and the outcomes of COVID‐19 infection in IBD patients is summarised in Tables S4 and S5. As the Joanna Briggs guidance suggests against using a score cut‐off for quality assessment we also did not score the studies. 30
4. DISCUSSION
IBD is associated with an increased risk of infection and this risk is related to many factors such as disease activity, malnutrition and the use of immunosuppressive drugs. It is important for clinicians and patients to be aware if there is a heightened risk of COVID‐19 infection in IBD and if it affects the outcomes. An increased expression of ACE‐2 in the intestinal tract (even higher than the lung alveoli) has been demonstrated and proposed as an alternative pathway of acquiring coronavirus infection. 31 The studies reporting about changes in ACE‐2 expression in the intestine in patients with IBD have conflicting results. 3 , 4 It is pertinent to note that although an increased expression of ACE‐2 in colonic mucosa was shown in IBD patients on immunohistochemistry, the functional activity was significantly lower in the inflamed areas. 32 The ACE‐2 which acts as a receptor for SARS‐CoV‐2 virus is distinct from the soluble form of ACE‐2, and the soluble form could prevent binding of the viral particles to the surface ACE‐2. 33
The findings of the present meta‐analysis suggest that the risk of COVID‐19 in IBD is not different from the general population. The risk of acquisition also does not seem to be affected by the type of IBD; that is, UC or CD. Furthermore, the risk of acquisition of COVID‐19 in IBD is not affected by the drugs used for treatment of IBD except for 5‐ASA. In COVID‐19‐ positive patients with IBD, hospitalisation was needed in 27% of patients while the mortality rate was under 5%. The risk of adverse outcomes (hospitalisation and mortality) were higher in patients with UC. The use of 5‐ASA or steroids was also associated with adverse outcomes (hospitalisation, ICU admission and mortality) while biological agents were protective.
The increased risk of adverse outcomes in UC as compared to CD could be due to the fact that patients with UC are more likely to be of older age. The usage of various drugs is also likely to be different in UC and CD, with patients having UC being more likely to receive 5‐ASA whereas those with CD are more likely to receive biological agents. 34 It is also unclear if biological differences in the two conditions, including the differences in expression of ACE‐2 and transmembrane protease serine‐2, could be responsible for differences in the outcomes. 3 , 4 The reasons for the increased risk of COVID‐19 infection with 5‐ASA are unclear but this may be related to the fact that 5‐ASA use may be a proxy for underlying UC. It has been shown that the expression of ACE‐2 receptor is increased to a larger degree in patients with UC. 3 In addition, a higher proportion of older IBD patients have UC and hence are more likely to be tested due to a higher likelihood of symptomatic disease.
Another notable finding is the association of drugs used in IBD with clinical outcomes following COVID‐ 19. While the use of 5‐ASA and steroids was associated with an increased risk of hospitalisation, ICU admission and mortality, the use of biological agents was associated with a reduction in these outcomes. These findings support the recommendations of various expert groups to limit the use of steroids and lower the dosages in the setting of the pandemic. Conversely, dexamethasone has been shown in a well‐powered randomised study (RECOVERY trial) to improve the outcomes in patients with severe COVID disease. Therefore, the findings of our meta‐analysis could represent the fact that steroid use is a proxy for the subset of patients with active IBD who are predisposed to adverse outcomes. Another finding is that 5‐ASA use is associated with adverse outcomes, which is difficult to explain by the biological action of 5‐ASA. 5‐ASA acts through peroxisome proliferator‐activated receptor‐γ which should attenuate the inflammatory response. However, 5‐ASA use could be an indicator of underlying UC and active disease and thereby associated with adverse outcomes. Finally, the use of biological agents was associated with a reduction in adverse outcomes. Because of the limited number of studies, we did not stratify this comparison for various groups of biological agents. However, it has been suggested that anti‐TNF could be beneficial in COVID‐19 disease by attenuating the hyperinflammatory response known as cytokine storm. 35 The drug, anecdotally, has been shown to be efficacious in improving COVID‐19 disease and is subject to a controlled trial.
This systematic review provides some guidance for the care of these patients and suggests that steroid use may be avoided in the setting of the pandemic while the use of biological agents can be continued. Furthermore, with the potential of new surges in various locations, the results of our meta‐analysis could guide clinicians and patients regarding the continuation of IBD medication in such scenarios. However, the results of this study should be looked at taking into consideration the limitations. Incidence in the included studies is reported from different geographical locations with different genetic composition of the population, medication used for IBD, comorbidities and hygiene practices, which may affect the underlying risk of acquisition of SARS‐CoV‐2 infection. Also, some studies evaluated only the symptomatic individuals for COVID‐19. Because of the limited availability of data, the confounding effect of older age, comorbidities, active disease and the combination of IBD medications on the risk of COVID‐19 acquisition and outcome could not be evaluated. In particular, one study which evaluated the age standardised incidence of COVID in IBD patients suggested that the risk in the IBD population may be overestimated. 8 Unfortunately, as similar data were not available from other studies, an analysis to account for differences of risk with age could not be performed. However, analysis from studies with a mean age of less than 45 years or over 45 years suggests that the incidence of COVID was higher in studies with a greater mean age. Furthermore, as SECURE‐IBD are registry‐based data, there may be a risk of the duplication of data. Also, the number of studies was limited especially for some analyses like the effect of various drug treatments on the outcomes.
A number of unanswered questions remain and require further research. Prospective studies should evaluate the risk of COVID‐19 and its outcome based on the underlying disease activity of IBD stratified by treatments. To know the true risk of asymptomatic infection, further research should use serological testing to identify the actual infection rate in IBD as well as the general population. Basic research should also focus on differences in intestinal mucosal ACE‐2 expression in relation to various drugs and their impact on clinical outcomes.
To conclude, the present meta‐analysis suggests that the risk of COVID in IBD patients is not higher than the general population. Also, the outcomes of COVID in IBD may be adversely affected by the type of disease (UC) and the use of 5‐ASA or steroids. The use of biological agents, in contrast, seems to be associated with better outcomes.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ETHICS APPROVAL
Not applicable as the paper is a systematic review and did not involve any primary research.
AUTHOR CONTRIBUTIONS
Conception: Vishal Sharma and Shaji Sebastian. Literature search: Anupam Kumar Singh and Vishal Sharma. Screening: Anupam Kumar Singh, Anuraag Jena and Vishal Sharma. Data extraction and RoB: Anupam Kumar Singh and Anuraag Jena. Data analysis: Praveen Kumar‐M. Initial draft: Anupam Kumar Singh, Praveen Kumar‐M, Anuraag Jena and Vishal Sharma. Manuscript revision for important intellectual content: Vishal Sharma and Shaji Sebastian. Final approval: all authors.
INFORMED CONSENT
Not applicable as the paper does not involve primary research on human subjects.
Supporting information
Supplementary material
Vishal Sharma and Shaji Sebastian are co‐senior authors.
DATA AVAILABILITY STATEMENT
The data are available upon reasonable request to the corresponding author.
REFERENCES
- 1. Kirchgesner J, Lemaitre M, Carrat F, et al. Risk of serious and opportunistic infections associated with treatment of inflammatory bowel diseases. Gastroenterology. 2018;155:337–46. [DOI] [PubMed] [Google Scholar]
- 2. Mosli M, Alourfi M, Alamoudi A, et al. A cross‐sectional survey on the psychological impact of the COVID‐19 pandemic on inflammatory bowel disease patients in Saudi Arabia. Saudi J Gastroenterol. 2020;26:263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Krzysztof NJ, Christoffer LJ, Rahul K, et al. Age, inflammation and disease location are critical determinants of intestinal expression of SARS‐CoV‐2 receptor ACE2 and TMPRSS2 in inflammatory bowel disease. Gastroenterology. 2020;159:1151–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burgueño JF, Reich A, Hazime H, et al. Expression of SARS‐CoV‐2 entry molecules ACE2 and TMPRSS2 in the gut of patients with IBD. Inflamm Bowel Dis. 2020;26:797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rubin DT, Abreu MT, Rai V, et al. International Organization for the Study of Inflammatory Bowel Disease. Management of patients with Crohn's disease and ulcerative colitis during the coronavirus disease‐ 2019 pandemic: results of an international meeting. Gastroenterology. 2020;159:6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Munn Z, Moola S, Lisy K, et al. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and incidence data. Int J Evid Base Healthc. 2015;13:147–53. [DOI] [PubMed] [Google Scholar]
- 7. Moola S, Munn Z, Tufanaru C, et al. Chapter 7: Systematic reviews of etiology and risk. In: Aromataris E, Munn Z, editors. Joanna Briggs Institute Reviewer's Manual. The Joanna Briggs Institute; 2017. https://wiki.jbi.global/display/MANUAL/Chapter+73A+Systematic+reviews+of+etiology+and+risk. Accessed 4 August 2020. [Google Scholar]
- 8. Taxonera C, Sagastagoitia I, Alba C, et al. Novel coronavirus disease (COVID‐19) in patients with inflammatory bowel diseases. Aliment Pharmacol Ther. 2019;52:276–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hormati A, Ghadir MR, Zamani F, et al. Are there any association between COVID‐19 severity and immunosuppressive therapy? Immunol Lett. 2020;224:12–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lukin DJ, Kumar A, Hajifathalian K, et al. Baseline disease activity and steroid therapy stratify risk of COVID‐ 19 in patients with inflammatory bowel disease. Gastroenterology. 2020;159:1541–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rodríguez‐Lago I, Ramírez de la Piscina P, Elorza A, et al. Characteristics and prognosis of patients with inflammatory bowel disease during the SARS‐CoV‐2 pandemic in the Basque country (Spain). Gastroenterology. 2020;159:781–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Haberman R, Axelrad J, Chen A, et al. Covid‐19 in immune‐mediated inflammatory diseases––case series from New York. N Engl J Med. 2020;383:85–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Turner D, Huang Y, Martín‐de‐Carpi J, et al. Corona virus disease 2019 and paediatric inflammatory bowel diseases: global experience and provisional guidance (March 2020) from the Paediatric IBD Porto Group of European Society of Paediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2020;70:727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Axelrad JE , Malter L , Hong S , et al. From the American Epicenter: coronavirus disease 2019 in patients with inflammatory bowel disease in the New York City metropolitan area. Inflamm Bowel Dis. 10.1093/ibd/izaa162. [DOI] [PMC free article] [PubMed]
- 15. Khan N, Patel D, Xie D, et al. Impact of anti‐TNF and thiopurines medications on the development of COVID‐19 in patients with inflammatory bowel disease: a nationwide VA cohort. study. 2020;159:1545–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scaldaferri F, Pugliese D, Privitera G, et al. Impact of COVID‐19 pandemic on the daily management of bio‐ technological therapy in inflammatory bowel disease patients: reorganisational response in a high‐volume Italian inflammatory bowel disease centre. United Eur Gastroenterol J. 2020;8:775–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Allocca M, Fiorino G, Zallot C, et al. Incidence and patterns of COVID‐19 among inflammatory bowel disease patients from the Nancy and Milan cohorts. Clin Gastroenterol Hepatol. 2020;18:2134–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grunert PC, Reuken PA, Stallhofer J, et al. Inflammatory bowel disease in the COVID‐19 pandemic ‐ the patients' perspective. J Crohns Colitis. 2020. 10.1093/ecco-jcc/jjaa126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marafini I, Salvatori S, Sena G, et al. Low frequency of COVID‐19 in inflammatory bowel diseases. Dig Liver Dis. 2020;52:1234–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bezzio C, Saibeni S, Variola A, et al. Outcomes of COVID‐19 in 79 patients with IBD in Italy: an IG‐IBD study. Gut. 2020;69:1213–7. [DOI] [PubMed] [Google Scholar]
- 21. Yu M, Ye Z, Chen Y, et al. Questionnaire assessment helps the self‐management of patients with inflammatory bowel disease during the outbreak of coronavirus disease 2019. Aging. 2020;12:12468–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. An P, Ji M, Ren H, et al. Prevention of COVID‐19 in patients with inflammatory bowel disease in Wuhan, China. Lancet Gastroenterol Hepatol. 2020;5:525–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Singh S, Khan A, Chowdhry M, et al. Risk of severe COVID‐19 in patients with inflammatory bowel disease in United States. A multicenter research network study. Gastroenterology. 2020;159:1575–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gubatan J, Levitte S, Balabanis T, et al. SARS‐CoV‐2 testing, prevalence, and predictors of COVID‐19 in patients with inflammatory bowel disease in Northern California. Gastroenterology. 2020;159:1141–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Norsa L, Indriolo A, Sansotta N, et al. Uneventful course in patients with inflammatory bowel disease during the severe acute respiratory syndrome coronavirus 2 outbreak in Northern Italy. Gastroenterology. 2020;159:371–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mak JWY, Weng MT, Wei SC, et al. Zero COVID‐19 infection in inflammatory bowel disease patients: findings from population‐based inflammatory bowel disease registries in Hong Kong and Taiwan. J Gastroenterol Hepatol. 2020. 10.1111/jgh.15164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Foteinogiannopoulou K, Orfanoudaki E, Koutroubakis IE. Keeping on the high quality of healthcare in Greek inflammatory bowel disease patients in the SARS‐CoV‐2 era. Clin Gastroenterol Hepatol. 2020. 10.1016/j.cgh.2020.04.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Grassia R, Soro S, Conti CB. Inflammatory bowel diseases and biological treatment in SARS‐CoV‐2 era. Why not? Inflamm Bowel Dis. 2020;26:e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brenner EJ, Ungaro RC, Colombel JF, et al. SECURE‐IBDDatabase Public Data Update; 2020. https://covidibd.org/current-data/. Accessed 29 July. [Google Scholar]
- 30. Munn Z, Barker TH, Moola S, et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Database System Rev Implement Rep. 2019. 10.11124/JBISRIR. [DOI] [PubMed] [Google Scholar]
- 31. Hamming I, Timens W, Bulthuis ML, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Garg M, Royce SG, Tikellis C, et al. Imbalance of the renin‐angiotensin system may contribute to inflammation and fibrosis in IBD: a novel therapeutic target? Gut. 2020;69:841–51. [DOI] [PubMed] [Google Scholar]
- 33. Li W, Moore MJ, Vasilieva N, et al. Angiotensin‐converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Alulis S, Vadstrup K, Borsi A, et al. Treatment patterns for biologics in ulcerative colitis and Crohn's disease: a Danish Nationwide Register Study from 2003 to 2015. J Crohn's Colitis. 2020;14:S628. 10.1093/ecco-jcc/jjz203.928. [DOI] [PubMed] [Google Scholar]
- 35. Feldmann M, Maini RN, Woody JN, et al. Trials of anti‐tumour necrosis factor therapy for COVID‐19 are urgently needed. Lancet. 2020;395:1407–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
The data are available upon reasonable request to the corresponding author.


