Abstract
Background
Although there are many asymptomatic patients, one of the problems of COVID‐19 is early recognition of the disease. COVID‐19 symptoms are polymorphic and may include upper respiratory symptoms. However, COVID‐19 symptoms may be mistaken with the common cold or allergic rhinitis. An ARIA‐EAACI study group attempted to differentiate upper respiratory symptoms between the three diseases.
Methods
A modified Delphi process was used. The ARIA members who were seeing COVID‐19 patients were asked to fill in a questionnaire on the upper airway symptoms of COVID‐19, common cold and allergic rhinitis.
Results
Among the 192 ARIA members who were invited to respond to the questionnaire, 89 responded and 87 questionnaires were analysed. The consensus was then reported. A two‐way ANOVA revealed significant differences in the symptom intensity between the three diseases (p < .001).
Conclusions
This modified Delphi approach enabled the differentiation of upper respiratory symptoms between COVID‐19, the common cold and allergic rhinitis. An electronic algorithm will be devised using the questionnaire.
Keywords: allergic rhinitis, common cold, cough, COVID‐19, smell
1. INTRODUCTION
Although there are many asymptomatic patients, one of the problems of COVID‐19 is early recognition of the disease. Pre‐medical visit screening and symptom evaluation have to be implemented quickly to minimise the risk of seeing COVID‐19 patients unprepared. Furthermore, testing for coronavirus is still widely restricted due to the shortage of available PCR tests in many countries. 1 Testing capacities have improved dramatically since the beginning of the pandemic, with the recent addition of antigen‐based testing. Some of these tests are home‐based and have only just obtained FDA approval. However, they still represent a bottleneck, 2 with the subsequent waiting periods leading to large groups of people at risk of infection requiring quarantine. To prevent unnecessary closure of critical facilities, for example schools and public services, triage requires further improvement in terms of speed and accuracy.
COVID‐19 symptoms are polymorphic. Typically, COVID‐19 induces shortness of breath, cough, fever, nasal congestion and general malaise. 3 However, SARS‐coronavirus‐2 (SARS‐CoV‐2) infection has been linked to a number of other symptoms afflicting several organ systems, including muscle and joint pain, sore throat, headache, nausea, vomiting and diarrhoea, as well as coagulopathy. 4 Impaired sense of smell and taste has emerged as an alarming symptom of SARS‐CoV‐2 infection in the West, but not so much in Asia. 5 , 6 , 7 , 8 , 9 Presentation in the upper respiratory tract has also been described as extremely variable across age groups, 10 making it difficult to distinguish COVID‐19 from common upper respiratory infections (e.g. croup in children 10 ).
Therefore, besides the management of severe COVID‐19, one of the major problems of the infection is how to screen citizens with possible COVID‐19 and distinguish them from patients with similar symptoms caused by allergic rhinitis 11 , 12 or other common viral infections of the respiratory tract. A digital tool enabling a rapid distinction is needed for this approach and may be of great importance during the winter with the co‐existence of COVID‐19, flu, common cold or other respiratory viral infections and house dust mite‐induced rhinitis.
Systematic reviews and meta‐analyses have been produced for many COVID‐19 symptoms including differentiation between flu and COVID‐19. 13 However, there is insufficient knowledge on consensus across the international medical community regarding nasal symptoms that may enable differentiation between COVID‐19, common cold and allergic rhinitis. An ARIA (Allergic Rhinitis and its Impact on Asthma)‐EAACI (European Academy of Allergy and Clinical Immunology)‐GA2LEN (Global Allergy and Asthma European Network) initiative was carried out to establish consensus on a set of questions aimed at distinguishing these diseases. From this consensus, an algorithm will be proposed and digitalised using a method already validated in MASK. 14 , 15 , 16 The current paper presents the results of the consensus.
This is a new paper of the series of ARIA‐EAACI papers on COVID‐19. 17 , 18 , 19 , 20 , 21
2. METHODS
A modified Delphi was carried out. 22 A questionnaire developed by JB, WC, LK and JM was sent to all ARIA members by GLO. Those seeing COVID‐19 patients were requested to answer within a week.
The questionnaire included items related to upper and lower airway symptoms for COVID‐19, common cold and allergic rhinitis (Table 1). In the questionnaire, the respondents were asked to assess five nasal symptoms, three ocular symptoms, taste, smell, cough, wheezing and sore throat. For each question, there was a statement on frequency and severity. For this, participants were asked to grade the severity from 0 to 10. Then, they gave a global assessment from 0 to 10 according to whether they agreed on the suggested severity grading for the three diseases. A level of 6 or higher was considered as agreement. Suggestions for questions/statements were able to be added to the questionnaire.
TABLE 1.
The original survey with 15 items
| Question | COVID−19 | Common cold | Allergic rhinitis | Level Agreement MEAN | SD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Occurrence | Characteristics | Max VAS (mean) | SD | Occurrence | Characteristics | Max VAS (mean) | SD | Occurrence | Characteristics | Max VAS (mean) | SD | ||||
| 1 | Runny nose (anterior rhinorrhea) | Very rare | If present, mild symptoms (VAS<5/10) | 3.98 | 0.15 | Always | Anterior and posterior rhinorrhea | 9.93 | 0.54 | Often | Profuse anterior rhinorrhea | 5.41 | 1.22 | 8.50 | 1.90 |
| 2 | Sneezing | Very rare | Not in bursts | 3.99 | 0.11 | Common | Not in burst | 5.02 | 0.21 | Very common | In burst | 9.99 | 0.11 | 9.37 | 1.09 |
| 3 | Stuffy nose | Not uncommon | If present, mild symptoms (VAS<5/10) | 4.10 | 0.68 | Always | Often severe | 10.00 | 0.00 | Very common | May be severe | 8.07 | 0.36 | 8.86 | 1.51 |
| 4 | Nasal pruritus | NO | 0.00 | 0.00 | NO | 0.08 | 0.53 | Very common | Variable in intensity | 8.02 | 0.21 | 9.22 | 1.38 | ||
| 5 | Nasal pain | Possible | 2.99 | 0.11 | Sometimes | 3.00 | 0.00 | NO | 0.00 | 0.00 | 8.21 | 2.22 | |||
| 6 | Ocular itch | NO | 2.94 | 0.38 | NO | 3.00 | 0.00 | Common | 10.00 | 0.00 | 9.31 | 1.41 | |||
| 7 | Ocular pain | Possible | 3.09 | 0.78 | 3.00 | 0.00 | NO | 0.06 | 0.53 | 8.14 | 2.43 | ||||
| 8 | Ocular redness | Possible | 3.07 | 0.54 | NO | 3.05 | 0.30 | Common | 9.98 | 0.21 | 8.36 | 2.29 | |||
| 9 | ≥3 nasal symptoms | NO | N/A | YES | N/A | YES | N/A | 8.92 | 1.82 | ||||||
| 10 | Smell dysfunction | Not uncommon | Usually anosmia whereas in other diseases it is hyposmia. Associated with other COVID−19 symptoms, it is likely to be a significant diagnostic criterion | 10.00 | 0.00 | Sometimes | 6.98 | 0.21 | Rare | Anosmia very seldom | 6.95 | 0.30 | 8.88 | 1.88 | |
| 11 | Taste dysfunction | Not uncommon | Dysgeusia rather than loss of taste. Associated with other COVID−19 symptoms, it is likely to be a significant diagnostic criterion | 10.00 | 0.00 | Rare | 3.00 | 0.00 | Very rare | 2.00 | 0.00 | 9.24 | 1.34 | ||
| 12 | Dyspnea | Relatively common | May start as an isolated mild symptom but may rapidly become severe with respiratory rate>24/min | 10.00 | 0.00 | Rare | 5.00 | 2.92 | Sometimes if asthma | 10.00 | 0.00 | 9.08 | 1.35 | ||
| 13 | Cough | Relatively common | May start as an isolated mild symptom (2–4 episodes of dry cough per hour) but rapidly becomes severe | 10.00 | 0.00 | Common | Follows the nasal symptoms | 7.60 | 2.06 | Sometimes if asthma | 10.00 | 0.00 | 9.22 | 1.22 | |
| 14 | Wheezing | Not uncommon | Rarely isolated, not severe in contradistinction to asthma | 4.99 | 0.11 | Rare | 3.50 | 1.12 | Sometimes if asthma | 10.00 | 0.00 | 8.77 | 1.73 | ||
| 15 | Sore throat | Not uncommon | 5.09 | 0.62 | Common | 8.25 | 1.09 | Rare | 4.33 | 2.87 | 8.84 | 1.66 | |||
A total of 87 answer sheets were included in this analysis. Any written comments were transformed into numeric changes where possible. To determine whether the participants agreed that the symptom/item was to be included in the tool, we collected the total number of participants agreeing as well as the total percentages. The same procedure was used for disagreement and missing/invalid data, respectively.
3. RESULTS
Among the 192 questionnaires sent out, 89 (46.3%) were returned within 7 days. The average monthly number of COVID‐19 consultations among the participants was 16.8 ± 20. The participants were from 37 different countries (Figure 1).
FIGURE 1.
Countries involved in the questionnaire
There was a high proportion of agreeing participants, with an average of 76.3% (range 69–83). The overall data quality was acceptable, and missing values for some of the questions were below 20% (Table 2).
TABLE 2.
Participants' agreement to the questionnaire items
| No. | Symptom | Disagree (≤6) | Agree (>6) | Missing/invalid answer | |||
|---|---|---|---|---|---|---|---|
| n = 87 | |||||||
| n | % | n | % | n | % | ||
| 1 | Runny nose (anterior rhinorrhea) | 12 | 13.8 | 62 | 71.3 | 13 | 14.9 |
| 2 | Sneezing | 3 | 3.4 | 72 | 82.8 | 12 | 13.8 |
| 3 | Stuffy nose | 8 | 9.2 | 68 | 78.2 | 11 | 12.6 |
| 4 | Nasal pruritus | 7 | 8.0 | 69 | 79.3 | 11 | 12.6 |
| 5 | Nasal pain | 14 | 16.1 | 61 | 70.1 | 12 | 13.8 |
| 6 | Ocular itch | 5 | 5.7 | 70 | 80.5 | 12 | 13.8 |
| 7 | Ocular pain | 16 | 18.4 | 60 | 69.0 | 11 | 12.6 |
| 8 | Ocular redness | 13 | 14.9 | 62 | 71.3 | 12 | 13.8 |
| 9 | ≥3 Nasal symptoms | 7 | 8.0 | 65 | 74.7 | 15 | 17.2 |
| 10 | Smell dysfunction | 8 | 9.2 | 67 | 77.0 | 12 | 13.8 |
| 11 | Taste dysfunction | 2 | 2.3 | 73 | 83.9 | 12 | 13.8 |
| 12 | Dyspnea | 5 | 5.7 | 67 | 77.0 | 15 | 17.2 |
| 13 | Cough | 4 | 4.6 | 69 | 79.3 | 14 | 16.1 |
| 14 | Wheezing | 7 | 8.0 | 64 | 73.6 | 16 | 18.4 |
| 15 | Sore throat | 8 | 9.2 | 67 | 77.0 | 12 | 13.8 |
| Mean | 9.1 | 76.3 | 14.6 | ||||
Participants were able to grade the maximum expected severity for each disease, and the average final VAS severity data are shown in Figure 2. A two‐way ANOVA revealed significant differences in symptom intensity between the three diseases (p < .001).
FIGURE 2.
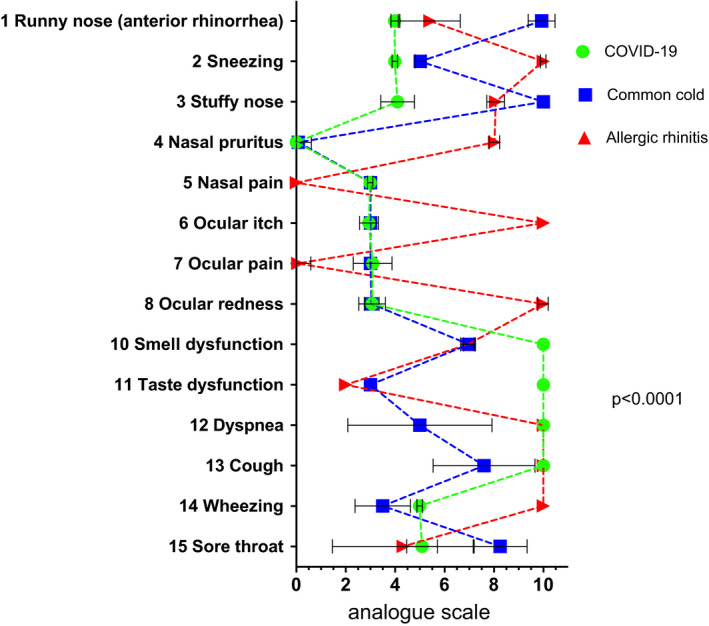
Maximum expected symptom severity. Analogue scale from 0 (not present) to 10 (maximum severity). Means ± SD are shown. A two‐way ANOVA revealed significant differences in VAS between diseases (p < .001)
Eye symptoms (7, 8) were among the most discussed statements, and the corresponding statements had relatively low levels of approval (Figure 1). Nasal pain (5) was regarded as impractical by six participants, which was also reflected by a relatively low level of agreement (8.21 ± 2.2; Figure 3). This was possibly caused by different interpretations of the item's description, and this issue needs to be addressed in further developments of the algorithm.
FIGURE 3.
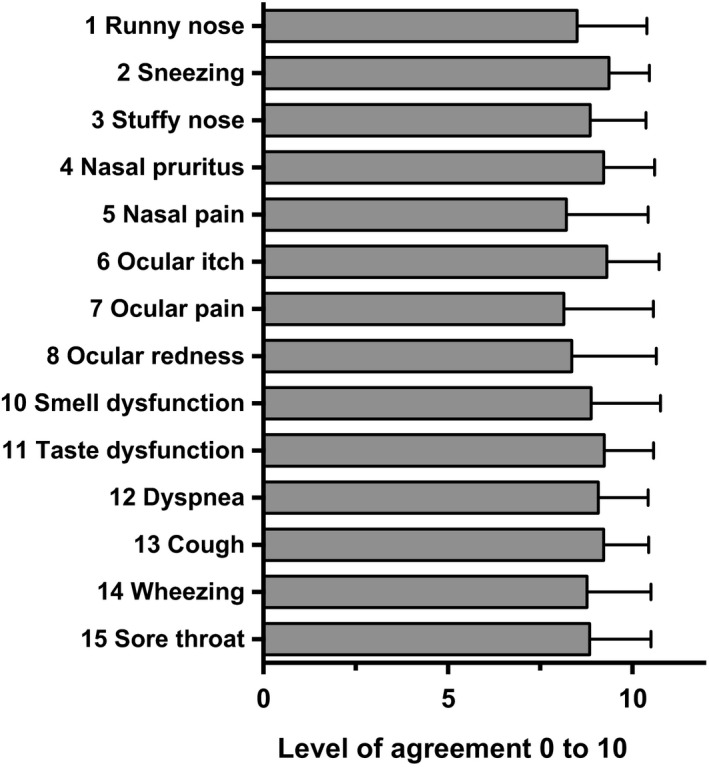
Mean level of agreement to suggested symptom severity. Analogue scale rating with range from 0 (disagreement) to 10 (complete agreement). A level of 6 or higher was considered as ‘agreement’. Means ± SD are shown
Additional common COVID‐19 symptoms will be considered for integration in the future algorithm development process (Table 3).
TABLE 3.
Additional items to be integrated in the algorithm
| Strenuous fatigue |
| Fever |
| COVID−19 comorbidities |
| Contact with COVID patient |
| Travel to ‘high‐risk’ region |
| Gastrointestinal symptoms |
| Muscle/body ache |
| Profound sweating |
4. DISCUSSION
This paper presents the results of a consensus initiative across the ARIA network of health professionals. The aim was to develop a set of questions on symptoms and their intensity in order to discriminate between classical rhinologic disorders and COVID‐19. The presentation of COVID‐19 is highly variable, ranging from a complete absence of symptoms to severe illness and critical organ dysfunction. The underlying mechanisms for this polymorphic behaviour are yet to be defined.
Within the ARIA network of specialists in upper and lower respiratory diseases, we asked 193 to respond to our consensus initiative, of whom 89 did. The response rate was under 50%, but many physicians were not seeing COVID‐19 patients. The strength of this paper is that the involved participants represented different medical specialties and many different countries, suggesting a generalisation of the study.
We found high levels of consensus among this community, with over 76% of participants agreeing to the symptoms presented in our questionnaire. VAS was found to be a useful and simple tool for discussing questions of symptom intensity in this large group of health professionals. Statistical analysis revealed a significantly different expected maximum VAS of the three diseases (two‐way ANOVA, p < .001). Hence, there are potential symptom constellations that allow discrimination between the three diseases.
The triage of patients with newly developed symptoms – any individual under suspicion of being at risk of SARS‐CoV‐2 infection – remains a challenge during this pandemic. Digital application‐based symptom reporting and triage have been evaluated in prospective trials in the UK, China and the US. 23 , 24 , 25 The improvement of triage will also (i) enhance pre‐test probability for SARS‐CoV‐2 PCR swabs or alternative test methods; (ii) increase the availability of tests in general to make current infection numbers more accurate; (iii) ease unnecessary quarantine; and (iv) reduce the closure of schools, child day care and public services.
ARIA‐MASK includes a decision‐making tool for allergic rhinitis. 14 With a broad user base of 39,670, there is an opportunity to provide newly developed tools for a large group of patients. The questionnaire, along with the participants’ comments, has to be transferred to a validation process. This process can be enhanced by already‐developed artificial intelligence (AI) in order to fine‐tune and improve symptom VAS thresholds. A final questionnaire and algorithm are open for use across the medical community, focussing on specialists treating upper and lower airway diseases and allergy, hence confronted with similar rhinologic, pneumologic and ophthalmologic symptoms. For allergy and respiratory tract specialists, undoubtedly at high risk of infection during examinations, recommendations for treatment and handling of the field of allergic diseases have been suggested by the European Academy of Allergy and Clinical Immunology (EAACI) in alliance with the global initiative ‘Allergic Rhinitis and its Impact on Asthma’ (ARIA). 17 , 19 , 20 , 21 , 26 It has been shown that digital decision‐making tools and app‐based algorithms can improve patient–doctor communication and therapy adherence for both patients and physicians. 27 , 28
In summary, our future COVID‐19 symptom tool may be a helpful device for improving active patient reporting and triage of patients when integrated in the ARIA MASK‐air App. We have asked the networks to circulate the tool to their members for testing, and we hope to be able to present the results and create more robust evidence in its practicality. This article presents a substantial consensus effort in COVID‐19‐treating physicians across the globe. Limitations arise from missing or inappropriate data in the returned questionnaires. However, the development process is followed by AI‐supported validation, and future studies have to show the power of such questionnaires.
CONFLICTS OF INTEREST
CA reports grants from Allergopharma, Idorsia, Swiss National Science Foundation, Christine Kühne‐Center for Allergy Research and Education, European Commission's Horison's 2020 Framework Programme, Cure, Novartis Research Institutes, Astra Zeneca, Scibase, advisory role in Sanofi/Regeneron, grants from Glakso Smith‐Kline, advisory role in Scibase. JB reports personal fees from Chiesi, Cipla, Hikma, Menarini, Mundipharma, Mylan, Novartis, Purina, Sanofi‐Aventis, Takeda, Teva, Uriach, other from KYomed‐Innov. VC reports personal fees from ALK, Allergy Therapeutics, LETI, Thermofisher, Merck, Astrazeneca, GSK. JCS reports Advisory Board from Boheringer Ingelheim, personal fees and Advisory Board from GSK, Bial, grants, personal fees and Advisory Board from AstraZeneca, non‐financial support from Mundipharma, personal fees from Sanofi, Advisory Board from Novartis. JCI reports personal fees from Faes Farma, Abbott Ecuador, Laboratorios Casasco, Laboratorios Bago Bolivia, Eurofarma Argentina, Sanofi. PK reports personal fees from Adamed, AstraZeneca, Berlin Chemie Menarini, Boehringer Ingelheim, Hal Allergy, Lekam, Mylan, Novartis, Polpharma, Sanofi, Teva, Chiesi, USP Pharmacia. VK reports personal fees from GSK, non‐financial support from AstraZeneca, DIMUNA, DLL reports personal fees from Allakos, Amstrong, Astrazeneca, Boehringer Ingelheim, Chiesi, DBV Technologies, Grunenthal, GSK, MEDA, Menarini, MSD, Novartis, Pfizer, Novartis, Sanofi, Siegfried, UCB, Alakos, Gossamer, grants from Sanofi, Astrazeneca, Novartis, UCB, GSK, TEVA, Boehringer Ingelheim, Chiesi, Purina institute. BL reports grants and personal fees from Mylan, Glenmark. JM reports personal fees and other from SANOFI‐GENZYME & REGENERON, NOVARTIS, ALLAKOS, grants and personal fees from MYLAN‐MEDA Pharma, URIACH Group, personal fees from MITSUBISHI‐TANABE, MENARINI, UCB, ASTRAZENECA, personal fees from GSK, MSD. NP reports personal fees from Novartis, Nutricia, HAL, MENARINI/FAES FARMA, SANOFI, MYLAN/MEDA, BIOMAY, AstraZeneca, GSK, MSD, ASIT BIOTECH, Boehringer Ingelheim, grants from Gerolymatos International SA, Capricare. OP reports grants and personal fees from ALK‐Abelló, Allergopharma, Stallergenes Greer, HAL Allergy Holding B.V./HAL Allergie GmbH, Bencard Allergie GmbH/Allergy Therapeutics, Lofarma, ASIT Biotech Tools S.A., Laboratorios LETI/LETI Pharma, Anergis S.A., Glaxo Smith Kline, personal fees Astellas Pharma Global, EUFOREA, ROXALL Medizin, Novartis, Sanofi‐Aventis and Sanofi‐Genzyme, Med Update Europe GmbH, streamedup! GmbH, grants from Pohl‐Boskamp, Inmunotek S.L.,personal fees from John Wiley and Sons, AS, personal fees from MEDA Pharma/MYLAN, Mobile Chamber Experts (a GA2LEN Partner), Indoor Biotechnologies. DP reports grants and personal fees from GlaxoSmithKline, personal fees from Menarini, Pliva, Belupo, AbbVie, Novartis, MSD, Chiesi, Revenio, AbbVie, Novartis, MSD, Chiesi, Revenio, non‐financial support from Philips, personal fees and non‐financial support from Boehringer Ingelheim, FP reports personal fees from SANOFI, ASTRAZENECA, NOVARTIS, GLAXO SMITHKLINE, STALLERGENES, ALLERGY THERAPEUTICS, HAL ALLERGY, MENARINI, MALESCI, GUIDOTTI, VALEAS, BOEHRINGER INGELHEIM, ALMIRALL, MUNDIPHARMA. NR reports and Advisory Board: Sanofi, Mylan, AstraZeneca, Speaker: Sanofi, Mylan, Chiesi. JS reports grants and personal fees from SANOFI, personal fees from GSK, NOVARTIS, ASTRA ZENECA, MUNDIPHARMA, FAES FARMA. Dr. Tsiligianni reports grants from GSK Hellas, ELPEN, Astra Zeneca Hellas, personal fees from GSK, Boehringer Ingelheim, Novartis, Astra Zeneca. DW reports personal fees from Optinose, ALK, Sanofi, and was co‐chair of Joint Task Force on Practice Parameters until July 2020. author of Rhinitis GRADE document (2017) and Rhinitis practice parameter update (2020). TZ reports personal fees from Bayer Health Care, FAES, Novartis, Henkel, AstraZeneca, AbbVie, ALK, Almirall, Astellas, Bayer Health Care, Bencard, Berlin Chemie, HAL, Leti, Meda, Menarini, Merck, MSD, Novartis, Pfizer, Sanofi, Stallergenes, Takeda, Teva, UCB, Kryolan L'Oréal.
Supporting information
Fig S1
Fig S2
APPENDIX 1.
ARIA MEMBER LIST
Amir Hamzah Abdul Latiff, Baharudin Abdullah, Werner Aberer, Nancy Abusada, Ian Adcock, Alejandro Afani, Ioana Agache, Xenofon Aggelidis, Jenifer Agustin, Cezmi A Akdis, Mübeccel Akdis, Mona Al‐Ahmad, Abou Al‐Zahab Bassam, Hussam Alburdan, Oscar Aldrey‐Palacios, Emilio Alvarez Cuesta, Hiba Alwan Salman, Ashraf Alzaabi, Salma Amade, Gene Ambrocio, Rosana Angles, Isabella Annesi‐Maesano, Ignacio J Ansotegui, Josep M. Anto, Paula Ara Bardajo, Stefania Arasi, Margarete Arrais, Hasan Arshad, Maria‐Cristina Artesani, Estrella Asayag, Francesca Avolio, Khuzama Azhari, Claus Bachert, Diego Bagnasco, Ilaria Baiardini, Nissera Bajrović, Petros Bakakos, Sergio Bakeyala Mongono, Christine Balotro‐Torres, Sergio Barba, Cristina Barbara, Elsa Barbosa, Bruno Barreto, Joan Bartra, Xavier Basagana, Eric D. Bateman, Lkhagvaa Battur, Anna Bedbrook, Martín Bedolla Barajas, Bianca Beghé, Antra Bekere, Elizabeth Bel, Ali Ben Kheder, Kazi S. Bennoor, Mikael Benson, Elena‐Camelia Berghea, Karl‐Christian Bergmann, Roberto Bernardini, David Bernstein, Mike Bewick, Slawomir Bialek, Artur Białoszewski, Thomas Bieber, Nils E. Billo, Maria‐Beatrice Bilo, Carsten Bindslev‐Jensen, Leif Bjermer, Hubert Blain, Irina Bobolea, Malgorzata Bochenska Marciniak, Christine Bond, Attilio Boner, Matteo Bonini, Sergio Bonini, Sinthia Bosnic‐Anticevich, Isabelle Bosse, Sofia Botskariova, Jacques Bouchard, Louis‐Philippe Boulet, Rodolphe Bourret, Philippe Bousquet, Fulvio Braido, Andrew Briggs, Christopher E Brightling, Jan Brozek, Luisa Brussino, Roland Buhl, Roxana Bumbacea, Rosalva Buquicchio, María‐Teresa Burguete Cabañas, Andrew Bush, William W Busse, Jeroen Buters, Fernan Caballero‐Fonseca, Moïses A Calderon, Mario Calvo, Paulo Camargos, Thierry Camuzat, FR Canevari, Antonio Cano, G Walter Canonica, Arnaldo Capriles‐Hulett, Luis Caraballo, Vicky Cardona, Kai‐Hakon Carlsen, Jonas Carmona Pirez, Jorge Caro, Warner Carr, Pedro Carreiro‐Martins, Fredelita Carreon‐Asuncion, Ana‐Maria Carriazo, Carme Carrion‐Ribas, Thomas Casale, Mary‐Ann Castor, Elizabeth Castro, A.G. Caviglia, Lorenzo Cecchi, Alfonso Cepeda Sarabia, Maciej Chalubinski, Ramanathan Chandrasekharan, Yoon‐Seok Chang, Victoria Chato‐Andeza, Lida Chatzi, Christina Chatzidaki, Niels H. Chavannes, Claudia Chaves Loureiro, Aurora‐Alejandra Chavez Garcia, Marta Chelninska, Yuzhi Chen, Lei Cheng, Sharon Chinthrajah, Tomas Chivato, Ekaterine Chkhartishvili, George Christoff, Henry Chrystyn, Derek K Chu, Antonio Chua, Alexander Chuchalin, Kian Fan Chung, Alberto Cicerán, Cemal Cingi, Giorgio Ciprandi, Ieva Cirule, Ana‐Carla Coelho, Enrico Compalati, Jannis Constantinidis, Jaime Correia de Sousa, Elisio Manuel Costa, David Costa, María del Carmen Costa Domínguez, André Coste, M. Cottini, Linda Cox, Carlos Crisci, Maria Angiola Crivellaro, Alvaro A Cruz, John Cullen, Adnan Custovic, Biljana Cvetkovski, Wienczyslawa Czarlewski, Gennaro D'Amato, Jane da Silva, Ronald Dahl, Sven‐Erik Dahlen, Vasilis Daniilidis, Louei Darjazini Nahhas, Ulf Darsow, Janet Davies, Frédéric de Blay, Giulia De Feo, Eloisa De Guia, José‐Ricardo de la Torre Navarrete, Chato de los Santos, Esteban De Manuel Keenoy, Govert De Vries, Diana Deleanu, Pascal Demoly, Judah Denburg, Philippe Devillier, Alain Didier, Sanja Dimic Janjic, Maria Dimou, Anh Tuan Dinh‐Xuan, Ratko Djukanovic, Maria Do Ceu Texeira, Dejan Dokic, Margarita Gabriela Domínguez Silva, Habib Douagui, Nikolaos Douladiris, Maria Doulaptsi, Gérard Dray, Ruta Dubakiene, Eve Mathieu‐Dupas, Stephen Durham, Marzia Duse, Mark Dykewicz, Didier Ebo, Natalija Edelbaher, Thomas Eiwegger, Patrik Eklund, Yehia El‐Gamal✝, Zeinab A. El‐Sayed, Shereen S. El‐Sayed, Magda El‐Seify, Regina Emuzyte, Lourdes Enecilla, Marina Erhola, Heidilita Espinoza, Jesús Guillermo Espinoza Contreras, John Farrell, Lenora Fernandez, Paola Fimbres Jimenez, Antje Fink Wagner, Alessandro Fiocchi, Wytske J Fokkens, Lenia Folletti, Joao A Fonseca, Jean‐François Fontaine, Francesco Forastiere, Jose Miguel Fuentes Pèrez, Emily Gaerlan‐Resureccion, Mina Gaga, José Luis Gálvez Romero, Amiran Gamkrelidze, Alexis Garcia, Cecilia Yvonne García Cobas, María de la Luz Hortensia García Cruz, Valeria Garcia Ortiz, Jacques Gayraud, Matteo Gelardi, Bilun Gemicioglu, Dimitra Gennimata, Sonya Genova, José Gereda, Roy Gerth van Wijk, Antonio Giuliano, René‐Maximiliano Gomez, Miguel‐Ange Gonzalez Ballester, Sandra González Diaz, Maia Gotua, Christos Grigoreas, Ineta Grisle, Marta Guidacci, Nick Guldemond, Zdenek Gutter, Antonieta Guzmán, Tari Haahtela, Ramsa Halloum, David Halpin, Eckard Hamelmann, Suleiman Hammadi, Richard Harvey, Enrico Heffler, Joachim Heinrich, Adnan Hejjaoui, Birthe Hellquist‐Dahl, Luiana Hernández Velázquez, Mark Hew, Elham Hossny, Peter Howarth, Martin Hrubiško, Yunuen Rocío Huerta Villalobos, Marc Humbert, Salina Husain, Michael Hyland, Guido Iaccarino, Moustafa Ibrahim, Natalia Ilina, Maddalena Illario, Cristoforo Incorvaia, Antonio Infantino, Carla Irani, Zhanat Ispayeva, Juan Carlos Ivancevich, Edgardo EJ Jares, Deborah Jarvis, Ewa Jassem, Klemen Jenko, Rubén Darío Jiméneracruz Uscanga, Sebastian L Johnston, Guy Joos, Maja Jošt, Kaja Julge, Ki‐Suck Jung, Jocelyne Just, Marek Jutel, Igor Kaidashev, Omer Kalayci, Fuat Kalyoncu, Jeni Kapsali, Przemyslaw Kardas, Jussi Karjalainen, Carmela A. Kasala, Michael Katotomichelakis, Loreta Kavaliukaite, Thomas Keil, Paul Keith, Musa Khaitov, Nikolai Khaltaev, You‐Young Kim, Bruce Kirenga, Jorg Kleine‐Tebbe, Ludger Klimek, Fanny Ko, Bernard Koffi N’Goran, Evangelia Kompoti, Peter Kopač, Gerard Koppelman, Anja Koren Jeverica, Seppo Koskinen, Mitja Košnik, Tomasz Kostka, Kosta V. Kostov, Marek L Kowalski, Tanya Kralimarkova, Karmen Kramer Vrščaj, Helga Kraxner, Samo Kreft, Vicky Kritikos, Dmitry Kudlay, Mikael Kuitunen, Inger Kull, Piotr Kuna, Maciej Kupczyk, Violeta Kvedariene, Marialena Kyriakakou, Nika Lalek, Massimo Landi, Stephen Lane, Désiree E. Larenas‐Linnemann, Susanne Lau, Daniel Laune, Jorge Lavrut, Lan TT Le, Martina Lenzenhuber, Gualtiero Leo, Marcus Lessa, Michael Levin, Jing Li, Philip Lieberman, Giuseppe Liotta, Brian Lipworth, Xuandao Liu, Rommel Lobo, Karin C Lodrup Carlsen, Carlo Lombardi, Renaud Louis, Stelios Loukidis, Olga Lourenço, Jorge A. Luna Pech, Bojan Madjar, Enrico Maggi, Antoine Magnan, Bassam Mahboub, Alpana Mair, Anke‐Hilse Maitland van der Zee, Mika Makela, Michael Makris, Hans‐Jorgen Malling, Mariana Mandajieva, Patrick Manning, Manolis Manousakis, Pavlos Maragoudakis, Gianluigi Marseglia, Gailen Marshall, Mohammad Reza Masjedi, Jorge F. Máspero, Juan José Matta Campos, Marcus Maurer, Sandra Mavale‐Manuel, Cem Meço, Erik Melén, Giovanni Melioli, Elisabete Melo‐Gomes, Eli O Meltzer, Enrica Menditto, Andrew Menzies‐Gow, Hans Merk, Jean‐Pierre Michel, Yann Micheli, Neven Miculinic, Luís Midão, Florin Mihaltan, Nikolaos Mikos, Manlio Milanese, Branislava Milenkovic, Dimitrios Mitsias, Bassem Moalla, Giuliana Moda, María Dolores Mogica Martínez, Yousser Mohammad, Frances‐Montserrat Moharra, Mostafa Moin, Mathieu Molimard, Isabelle Momas, Monique Mommers, Alessandro Monaco, Steve Montefort, Lucia‐Elvira Montenegro, Riccardo Monti, Dory Mora, Mario Morais‐Almeida, Ralph Mösges, Badr Eldin Mostafa, Joaquim Mullol, Lars Münter, Antonella Muraro, Ruth Murray, Antonio Musarra, Tihomir Mustakov, Robert Naclerio, Kari C. Nadeau, Rachel Nadif, Alla Nakonechna, Leyla Namazova‐Baranova, Gretchen Navarro‐Locsin, Hugo Neffen, Kristof Nekam, Angelos Neou, Eustachio Nettis, Daniel Neuberger, Laurent Nicod, Stefania Nicola, Verena Niederberger‐Leppin, Marek Niedoszytko, Antonio Nieto, Ettore Novellino, Elizabete Nunes, Dieudonné Nyembue, Robyn O’Hehir, Cvetanka Odjakova, Ken Ohta, Yoshitaka Okamoto, Kimi Okubo, Brian Oliver, Gabrielle L Onorato, Maria Pia Orru, Solange Ouédraogo, Kampadilemba Ouoba, Francisco‐Javier Padilla, Pier Luigi Paggiaro, Aris Pagkalos, Giovanni Pajno, Gianni Pala, SP Palaniappan, Isabella Pali‐Schöll, Susanna Palkonen, Stephen Palmer, Carmen Panaitescu Bunu, Petr Panzner, Nikolaos G Papadopoulos, Vasilis Papanikolaou, Alberto Papi, Bojidar Paralchev, Giannis Paraskevopoulos, Hae‐Sim Park, Giovanni Passalacqua, Vincenzo Patella, Ian Pavord, Ruby Pawankar, Soren Pedersen, Susete Peleve, Simona Pellegino, Ana Pereira, Mariana Pereira, Tamara Pérez, Andrea Perna, Diego Peroni, Oliver Pfaar, Nhân Pham‐Thi, Bernard Pigearias, Isabelle Pin, Konstantina Piskou, Constantinos Pitsios, Davor Plavec, Dagmar Poethig, Wolfgang Pohl, Antonija Poplas Susic, Todor A. Popov, Fabienne Portejoie, Paul Potter, Lars Poulsen, Alexandra Prados‐Torres, Fotis Prarros, David Price, Emmanuel Prokopakis, Francesca Puggioni, Elisa Puig‐Domenech, Robert Puy, Klaus Rabe, Silvia Rabotti, Filip Raciborski, Josephine Ramos, Cristina Recalcati, Marysia T. Recto, Shereen M. Reda, Frederico S Regateiro, Norbert Reider, Sietze Reitsma, Susana Repka‐Ramirez, Erminia Ridolo, Janet Rimmer, Daniela Rivero Yeverino, José Angelo Rizzo, Carlos Robalo‐Cordeiro, Graham Roberts, Karen Robles, Nicolas Roche, Mónica Rodríguez González, Eréndira Rodríguez Zagal, Giovanni Rolla, Christine Rolland, Regina Roller‐Wirnsberger, Miguel Roman Rodriguez, Antonino Romano, Jan Romantowski, Philippe Rombaux, Joel Romualdez, Jose Rosado‐Pinto, Nelson Rosario, Lanny Rosenwasser, Oliviero Rossi, Menachem Rottem, Philip Rouadi, Nikoleta Rovina, Irma Rozman Sinur, Mauricio Ruiz, Lucy Tania Ruiz Segura, Dermot Ryan, Hironori Sagara, Daiki Sakai, Daiju Sakurai, Wafaa Saleh, Johanna Salimaki, Konstantinos Samitas, Boleslaw Samolinski, María Guadalupe Sánchez Coronel, Mario Sanchez‐Borges✝, Jaime Sanchez‐Lopez, Melissa Sansonna, Codrut Sarafoleanu, Faradiba Sarquis Serpa, Joaquin Sastre‐Dominguez, Eleonora Savi, Agne Savonyte, Bisher Sawaf, Glenis K Scadding, Sophie Scheire, Peter Schmid‐Grendelmeier, Juan Francisco Schuhl, Holger Schünemann, Maria Schvalbová, Jorgen Schwarze, Nicola Scichilone, Gianenrico Senna, Cecilia Sepúlveda, Elie Serrano, Sara Shamai, Aziz Sheikh, Mike Shields, Vasil Shishkov, Nikos Siafakas, Alexander Simeonov, Estelle FER Simons, Juan Carlos Sisul, Brigita Sitkauskiene, Ingelbjorg Skrindo, Tanja Soklič Košak, Dirceu Solé, Martin Sondermann, Talant Sooronbaev, Manuel Soto‐Martinez, Manuel Soto‐Quiros, Bernardo Sousa Pinto, Milan Sova, Michael Soyka, Krzysztof Specjalski, Annette Sperl, Otto Spranger, Sofia Stamataki, Lina Stefanaki, Cristiana Stellato, Rafael Stelmach, Timo Strandberg, Petra Stute, Abirami Subramaniam, Charlotte Suppli Ulrik, Michael Sutherland, Silvia Sylvestre, Aikaterini Syrigou, Luis Taborda Barata, Nadejda Takovska, Rachel Tan, Frances Tan, Vincent Tan, Ing Ping Tang, Masami Taniguchi, Line Tannert, Pongsakorn Tantilipikorn, Jessica Tattersall, Filippo Tesi, Uta Thieme, Carel Thijs, Mike Thomas, Teresa To, Ana Maria Todo‐Bom, Alkis Togias, Peter‐Valentin Tomazic, Vesna Tomic‐Spiric, Sanna Toppila‐Salmi, Maria‐José Torres Jaen, Elina Toskala, Massimo Triggiani, Nadja Triller, Katja Triller, Ioanna Tsiligianni, M. Uberti, Ruxandra Ulmeanu, Jure Urbancic, Marilyn Urrutia Pereira, Martina Vachova, Felipe Valdés, Rudolf Valenta, Marylin Valentin Rostan, Antonio Valero, Arunas Valiulis, Mina Vallianatou, Erkka Valovirta, Michiel Van Eerd, Eric Van Ganse, Marianne van Hage, Olivier Vandenplas, Tuula Vasankari, Dafina Vassileva, Cesar Velasco Munoz, Maria Teresa Ventura, Cécilia Vera‐Munoz, Frédéric Viart, Dilyana Vicheva, Pakit Vichyanond, Petra Vidgren, Giovanni Viegi, Claus Vogelmeier, Leena Von Hertzen, Theodoros Vontetsianos, Dimitris Vourdas, Vu Tran Thien Quan, Martin Wagenmann, Samantha Walker, Dana Wallace, De Yun Wang, Susan Waserman, Katrin Wehner, Magnus Wickman, Sian Williams, Dennis Williams, Nicola Wilson, Gary Wong, Kent Woo, Lucyna Wozniak, John Wright, Piotr Wroczynski, Paraskevi Xepapadaki, Plamen Yakovliev, Masao Yamaguchi, Kwok Yan, Yoke Yeow Yap, Mais Yassin, Barbara Yawn, Panayiotis Yiallouros, Arzu Yorgancioglu, Shigemi Yoshihara, Ian Young, Osman M Yusuf, Asghar Zaidi, Fares Zaitoun, Petra Zalud, Heather Zar, M.T. Zedda, Mario E Zernotti, Luo Zhang, Nanshan Zhong, Mihaela Zidarn, Torsten Zuberbier, Celia Zubrinich.
REFERENCES
- 1. Han E, Tan MMJ, Turk E, et al. Lessons learnt from easing COVID‐19 restrictions: an analysis of countries and regions in Asia Pacific and Europe. Lancet. 2020;396(10261):1525‐1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gao J, Quan L. Current status of diagnostic testing for SARS‐CoV‐2 infection and future developments: a review. Med Sci Monit. 2020;26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mair M, Singhavi H, Pai A, et al. A meta‐analysis of 67 studies with presenting symptoms and laboratory tests of COVID‐19 patients. Laryngoscope. 2021;131(6):1254‐1265. [DOI] [PubMed] [Google Scholar]
- 4. Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of covid‐19 in New York City. N Engl J Med. 2020;382(24):2372‐2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rojas‐Lechuga MJ, Izquierdo‐Dominguez A, Chiesa‐Estomba C, et al. Chemosensory dysfunction in COVID‐19 out‐patients. Eur Arch Otorhinolaryngol. 2020;278(3):695‐702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pang KW, Chee J, Subramaniam S, Ng CL. Frequency and clinical utility of olfactory dysfunction in COVID‐19: a systematic review and meta‐analysis. Curr Allergy Asthma Rep. 2020;20(12):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hoang MP, Kanjanaumporn J, Aeumjaturapat S, Chusakul S, Seresirikachorn K, Snidvongs K. Olfactory and gustatory dysfunctions in COVID‐19 patients: a systematic review and meta‐analysis. Asian Pac J Allergy Immunol. 2020;38(3):162‐169. [DOI] [PubMed] [Google Scholar]
- 8. Liu X, Li X, Sun T, et al. East‐West differences in clinical manifestations of COVID‐19 patients: a systematic literature review and meta‐analysis. J Med Virol. 2021;93(5):2683‐2693. [DOI] [PubMed] [Google Scholar]
- 9. Mullol J, Alobid I, Marino‐Sanchez F, et al. The loss of smell and taste in the COVID‐19 outbreak: a tale of many countries. Curr Allergy Asthma Rep. 2020;20(10):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Venn AMR, Schmidt JM, Mullan PC. A case series of pediatric croup with COVID‐19. Am J Emerg Med. 2020. 10.1016/j.ajem.2020.09.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bousquet J, Anto JM, Bachert C, et al. Allergic rhinitis. Nat Rev Dis Primers. 2020;6(1):95. [DOI] [PubMed] [Google Scholar]
- 12. Bedard A, Basagana X, Anto JM, et al. Treatment of allergic rhinitis during and outside the pollen season using mobile technology. A MASK study. Clin Transl Allergy. 2020;10(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pormohammad A, Ghorbani S, Khatami A, et al. Comparison of influenza type A and B with COVID‐19: a global systematic review and meta‐analysis on clinical, laboratory and radiographic findings. Rev Med Virol. 2021;31(3):e2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Courbis AL, Murray RB, Arnavielhe S, et al. Electronic clinical decision support system for allergic rhinitis management: MASK e‐CDSS. Clin Exp Allergy. 2018;48(12):1640‐1653. [DOI] [PubMed] [Google Scholar]
- 15. Bousquet JJ, Schunemann HJ, Togias A, et al. Next‐generation ARIA care pathways for rhinitis and asthma: a model for multimorbid chronic diseases. Clin Transl Allergy. 2019;9:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bousquet J, Bedbrook A, Czarlewski W, et al. Guidance to 2018 good practice: ARIA digitally‐enabled, integrated, person‐centred care for rhinitis and asthma. Clin Transl Allergy. 2019;9:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bousquet J, Akdis C, Jutel M, et al. Intranasal corticosteroids in allergic rhinitis in COVID‐19 infected patients: an ARIA‐EAACI statement. Allergy. 2020;75(10):2440‐2444. [DOI] [PubMed] [Google Scholar]
- 18. Bousquet J, Jutel M, Akdis CA, et al. ARIA‐EAACI statement on asthma and COVID‐19 (June 2, 2020). Allergy. 2020:76(3):689‐697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Klimek L, Jutel M, Akdis C, et al. Handling of allergen immunotherapy in the COVID‐19 pandemic: an ARIA‐EAACI statement. Allergy. 2020;75(7):1546‐1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Klimek L, Pfaar O, Worm M, et al. Allergen immunotherapy in the current COVID‐19 pandemic: a position paper of AeDA, ARIA, EAACI, DGAKI and GPA: position paper of the German ARIA Group(A) in cooperation with the Austrian ARIA Group(B), the Swiss ARIA Group(C), German Society for Applied Allergology (AEDA)(D), German Society for Allergology and Clinical Immunology (DGAKI)(E), Society for Pediatric Allergology (GPA)(F) in cooperation with AG Clinical Immunology, Allergology and Environmental Medicine of the DGHNO‐KHC(G) and the European Academy of Allergy and Clinical Immunology (EAACI)(H). Allergol Select. 2020;4:44‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pfaar O, Klimek L, Jutel M, et al. COVID‐19 pandemic: practical considerations on the organization of an allergy clinic ‐ an EAACI/ARIA position paper. Allergy. 2020;76(3):648‐676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thompson M. Considering the implication of variations within Delphi research. Fam Pract. 2009;26(5):420‐424. [DOI] [PubMed] [Google Scholar]
- 23. Varsavsky T, Graham MS, Canas LS, et al. Detecting COVID‐19 infection hotspots in England using large‐scale self‐reported data from a mobile application: a prospective, observational study. Lancet Public Health. 2021;6(1):21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yu J, Zhang HW, Shao YK, et al. A smartphone‐based online tool for prehospital self‐triage of COVID‐19. Chin J Acad Radiol. 2020:16:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Antonelli M, Capdevila J, Chaudhari A, et al. Identification of optimal symptom combinations to trigger diagnostic work‐up of suspected COVID‐19 cases: analysis from a community‐based, prospective, observational cohort. medRxiv. 2020. 10.1101/2020.11.23.20237313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Klimek L, Hoffmann HJ, Kalpaklioglu AF, et al. In‐vivo diagnostic test allergens in Europe: a call to action and proposal for recovery plan ‐ An EAACI position paper. Allergy. 2020;75(9):2161‐2169. [DOI] [PubMed] [Google Scholar]
- 27. Tricco AC, Ashoor HM, Cardoso R, et al. Sustainability of knowledge translation interventions in healthcare decision‐making: a scoping review. Implement Sci. 2016;11:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stacey D, Hawker G, Dervin G, et al. Decision aid for patients considering total knee arthroplasty with preference report for surgeons: a pilot randomized controlled trial. BMC Musculoskelet Disord. 2014;15:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2


