Abstract
Aim
On December 31, 2019, an unknown outbreak of pulmonary disease was reported in China. The novel coronavirus SARS‐CoV‐2 was the etiologic agent of this disease, and responsible of the current pandemic of COVID‐19. Accumulated evidence on placental features is based most on case‐reports and small case‐series, with differing results.
Methods
We gathered a cohort of 29 infected pregnant mothers who delivered 32 newborns, and had placentas available for pathologic examination. Placentas were compared with a control group.
Results
Of the 29 mothers, clinical and radiological features were similar to what was already described in COVID‐19. Pregnancy modified some analytical parameters. One of the mothers succumbed to the disease. Of the 32 newborns, 1 developed an early infection, with positive reverse‐transcriptase polymerase chain reaction (RT‐PCR) at 48 h of life, with an initial RT‐PCR negative. SARS‐CoV‐2 presence was assessed on placental tissue with immunohistochemistry and RT‐PCR, both were negative. All newborns had good clinical outcomes. No differences in morphological placental findings were found among both groups.
Conclusion
Lack of statistically significant differences among case and control groups suggest that placentas from SARS‐CoV‐2 infected mothers represent a cohort of normal placentas only submitted because of maternal SARS‐CoV‐2 status. To the best of our knowledge, no irrefutable cases of vertical transmission have been yet described. Other authors have failed to demonstrate presence of viral RNA in placental tissue. Accumulated knowledge suggests that if vertical transmission is possible, it is a rare event.
Keywords: coronavirus, COVID‐19, placental pathology, SARS‐CoV‐2, vertical transmission
Introduction
On December 31, 2019, an unknown outbreak of pulmonary disease was reported in Wuhan, Hubei region of China. 1 A new Coronavirus, named SARS‐CoV‐2, phylogenetically related to SARS, 2 , 3 was identified as the etiologic agent of this disease, 2 termed Coronavirus Disease 2019, (COVID‐19). On 11th of March, the World Health Organization (WHO) declared the COVID‐19 a pandemic. 4 One year after initial reports, there are over 79,200,000 confirmed COVID‐19 cases with over 1,700,000 deaths. 5
Although knowledge of this disease is rapidly increasing, not much is known of the evolution of this disease in pregnant women and its risk of vertical transmission to the fetus. As the placenta is a vital organ in fetal development, and the gate of entry of both nutrients and pathogens to the fetus, further pathological examination could provide paramount information in understanding SARS‐CoV‐2 materno‐fetal infection dynamics.
Sharps et al. 6 conducted a systematic review on this topic, covering all published studies that assessed placental morphological features in COVID‐19 infected women. Their group found that most evidence was based on single case reports and case series with a few larger series that generally lacked a control group. A wide variety of histopathological abnormalities were described, with varying frequencies. As evidence tended to be weak, they pointed out the need for an improved evidence base from controlled studies.
In this study, we gathered a series of 29 SARS‐CoV‐2 positive pregnant women, infected in the third trimester of gestation, who gave birth 32 children in our center and had placenta available for pathological examination. Morphological findings were compared with a reference population. The objectives of this review were to study outcomes of third trimester SARS‐CoV‐2 infected pregnant women, assess potential for vertical transmission and evaluate pathologic features of infected women placentas versus control group placentas.
Material and Methods
All selected patients were pregnant women in the third trimester of gestation with COVID‐19 diagnosis made by positive reverse‐transcriptase polymerase chain reaction (RT‐PCR) for SARS‐CoV‐2 5 who gave birth in our center and had placenta available for pathological examination. Cases were collected from March 13 to May 11, 2020, with a total of 29 pregnant women and 32 newborns. Cases were numbered according to submission order of placenta to our Department. Clinical records were reviewed for data collection once SARS‐CoV‐2 infection was confirmed and placenta was submitted.
Maternal data collected were age, time of gestation in weeks and days, parity, previous history of abortions, previous history of in vitro fertilization (IVF), way of delivery, day of illness to delivery, days from diagnosis to delivery, symptoms, comorbidities, gestational diabetes, intensive care unit (ICU) admission, evolution after delivery, treatment received for COVID‐19, radiological examinations, analytical values at SARS‐CoV‐2 diagnosis, and time until discharge or death.
All RT‐PCR conducted on mothers were performed on nasopharyngeal swabs. At the beginning of the study RT‐PCR were conducted only on symptomatic women. As evidence progressively supported the existence of asymptomatic pregnant patients, 6 , 7 universal RT‐PCR screening was implemented.
Newborn data collected were sex, weight, cranial perimeter and height, Apgar score in minute 1 and 5, SARS‐CoV‐2 RT‐PCR results at birth and 48 h later, and clinical evolution.
Preventively, at birth, all newborns from infected mothers were isolated, kept in specifically designated incubators and attended by health professionals with personal protective equipment (PPE), who provided care focused on minimizing nosocomial transmission of the virus.
All newborns underwent SARS‐CoV‐2 RT‐PCR conducted on nasopharyngeal swabs at birth and at 48 h of life. As SARS‐CoV‐2 transmission on breast milk has not currently been reported, 8 , 9 , 10 , 11 maternal breastfeeding was encouraged. To prevent potential horizontal transmission, mothers self‐extracted milk and babies were fed by a healthy familiar. Donated bank milk was offered as an option until lactation started.
Placentas were submitted to Pathology Department according to the guidelines of the American College of Pathologists, published in 1997, 12 to which SARS‐CoV‐2 maternal infection was added. Placental data including trimmed weight and pathologic diagnoses were collected. Feto‐placental weight ratios were calculated. All placentas were fixed in 10% formalin solution for at least 48 h. Due to the critical context of this pandemic, no fresh tissue was submitted to the Microbiology Department. All placentas were measured, weighted and processed according to the Amsterdam consensus on placental pathology. 23 One cassette of rolled fetal membranes, one cassette with three sections of umbilical cord representing the placental end, fetal end and middle section, four cassettes of placental parenchyma, one specifically sampling the cord insertion area, and one cassette of three sections of basal plate were included in all cases. Cases of twin placentas included an additional cassette of a roll of the dividing membranes. Macroscopic lesions of interest were described, photographed, and included in an additional block. All cases were included in paraffin, stained with hematoxilin and eosin, and diagnosed by a team of one surgical pathologist (RMRZ, experienced in placenta) and two training pathologists (LBS and EMW), following diagnostic criteria presented in Supporting Information, Data S1.
A control group of 58 term placentas from controlled, uneventful pregnancies was collected. Controls were part of a study conducted in 2017. 14 Cases and controls were unmatched. Although both cases and controls were sampled adjusting to Amsterdam consensus recommendations, 13 slight differences in sampling impeded masking and randomization. All controls were reevaluated by the same team of pathologists, following the same criteria presented in Data S1.
Data are expressed in its corresponding units. Quantities are provided as mean and ranges/SD. If mean is noninformative, median is provided instead. As gestational age has an effect on fetal 15 and placental 16 , 17 , 18 development, to ensure data are comparable, percentiles corrected for gestational age were assigned. Categorical variables are compared using Fisher's exact test. Quantitative variables are compared with Student's t test or Mann–Whitney U test. A two‐tailed p‐value <0.05 was considered statistically significant.
This study was performed following the Declaration of Helsinki principles, and authorization of the local Institutional Review Board was obtained.
Results
Pregnant women
Data collected from mothers are presented in Data S2. Twenty‐nine pregnant patients’ data were collected. Mean age was 31.9 years (range: 18–43). Mean gestational age at delivery was 37 + 2 weeks (range: 33 + 1 to 41 + 3 weeks). Fourteen (48%) pregnancies had caesarean delivery. Three cases (10%) were dichorionic‐diamniotic twin gestations. Thirteen (45%) patients had relevant comorbidities, presented in Data S2. Regarding gestation‐related comorbidities, cases 11, 16, 23, and 27 had gestational diabetes. Cases 6, 13, 16, and 23 had a history of prolonged rupture of membranes (rupture of membranes >12 h prior to delivery).
Most common symptoms were persistent cough (18 patients, 62%) or fever (8 patients, 26%). There were eight (26%) asymptomatic patients. Of the 22 (76%) patients who had a chest radiographic study, 8 (36%) had changes compatible with COVID‐19 pneumonia.
In analysis conducted at diagnosis, 14/29 (48.2%) patients had neutrophilia (>7.700 neutrophils/mL), 4/29 (13.8%) had absolute lymphopenia (<1.000 lymphocytes/mL), 28/28 (100%) had hyperfibrinogenemia, 4/22 (18.2%) had hypertransaminasemia, 18/22 (81.8%) had elevation of lactate dehydrogenase (LDH), 18/18 (100%) had elevation of D‐Dimer, 22/23 (95.7%) had elevation of C‐reactive protein (CRP), and 0/7 (0%) had elevation of procalcitonin (PCT). In additional analysis, two more patients had lymphopenia and two more patients had elevation of PCT.
All patients, except cases 7 and 23, had an oxygen saturation by pulse oximetry ranging from 96% to 100% on the day of delivery or 1 day later. Case 7 had saturations of 91%–92% with 100% fraction of inspired of oxygen. Case 23 maintained saturations of 95%–98% with low flows of supplementary oxygen.
Clinical evolution was good in 28 (97%) patients. One of the patients (case 7), a 26‐year‐old, obese woman, with an uneventful pregnancy controlled in other hospital, was admitted in our center with severe COVID‐19 pneumonia. Her clinical course complicated with bacterial superinfection, acute respiratory distress syndrome (ARDS), and macrophage activation syndrome‐like, treated with ceftriaxone, hydroxychloroquine, dexamethasone, and tocilizumab (a humanized anti‐IL‐6R antibody).
In this context, when the patient was in day +7 of infection and in 33 + 0 weeks of pregnancy, caesarean delivery was proposed as the patient had hypotension, anemia, and high oxygen demands. Lung maturation therapy with betamethasone was initiated and caesarean delivery was performed on week 33 + 2 without incidences.
However, after some apparent initial improvement, the patient worsened with progressively higher oxygen demands, finally needing ICU admission and intubation due to severe ARDS. Although optimal ICU care was provided, a septic shock and multiorganic failure with renal insufficiency and disseminated intravascular coagulation (DIC) supervened. Even under heparin prophylaxis, a massive pulmonary embolism took place and the patient died 15 days after the onset of symptoms and 12 after admission.
Newborns
Data collected from newborns are presented in Data S3. Thirty‐two newborns were delivered. Twenty‐six (81%) newborns were singletons. Thirteen newborns (41%) were female. Mean weight at birth of singleton babies was 3145 g (range: 1775–4030 g). Four babies (13%) were small for gestational age (weight at birth below p10), one of them corresponding to a twin pregnancy. Four babies (13%) were born preterm (born before 37 + 0 weeks of gestation).
Twenty‐nine newborns (91%) had Apgar score in minute 1 was ≥8 and ≥9 in minute 5. Cases 7, 18, and 26, had lower Apgar scores, with immediate recovery. General clinical evolution of all newborns was good. All of the four preterm babies had immediate respiratory distress, and three of them (75%) needed temporary ventilator support with continuous positive airways pressure (CPAP).
All cases except case 10 (97%) had both negative RT‐PCR. Case 10 was delivered by caesarean procedure, which was uneventful, and had an inconclusive RT‐PCR at birth, another test was conducted at 24 h of life, which was negative. However, at 48 h of life, routine RT‐PCR yielded a positive result. The patient was asymptomatic and radiological examinations showed no alterations. Analytical alterations found in seriated controls were decreasing ultrasensitive Troponine I levels and elevated D‐Dimer levels with normal coagulation analysis, rest of analyses were normal. A follow‐up RT‐PCR conducted at 21 days of life was negative. Clinical evolution was good.
Placentas
Data collected from SARS‐CoV‐2 infected women and controls are presented in Table 1. Cases and controls were comparable, although control gestations tended to be ended later than COVID‐19 gestations (p = 0.005).
Table 1.
Clinical features of pregnancies in SARS‐CoV‐2 and control pregnancies
| Cases (n = 29) | Controls (n = 58) | p‐Value | |
|---|---|---|---|
| Maternal age, years (mean ± SD) | 31.9 ± 5.7 | 33.6 ± 5.3 | 0.157 |
| Fetal weight, grams (median; range) | 2940 (1775–4030) | 3370 (2460–4230) | 0.503 |
| Gestational age, days (mean ± SD) | 261 ± 15.2 | 279 ± 8.0 | 0.005 |
| Placental weight (median; range) | 518.5 (289–942) | 486 (283–755) | 0.646 |
| FP ratio (median; range) | 6.3 (5.0–8.6) | 6.7 (5.0–13.3) | 0.230 |
| Twin pregnancy | 3 | 0 | |
| Placenta increta | 1 | 0 |
Note: Bold value indicates that reached statistical significance (p < 0.05).
Abbreviation: FP, Feto‐placental.
Placental weight ranged from 351 g (p3‐5) to 777 g (>97). Three cases were dichorionic‐diamniotic twin pregnancies. There was one case of placenta increta. There was one case of placentomegalia (weight > p97). One case had chronic infarctions macroscopically visible. Rest of the cases showed no macroscopical abnormalities. Complete pathologic diagnoses are presented in Table 2. Most cases showed focal features of villous chorangiosis (VC, 19 cases, 59%) or focal features of delayed villous maturation (DVM, 17 cases, 53%), usually in association (16 cases, 50%). Besides, there were five (16%) cases of acute chorioamnionitis (AC), six (19%) cases of chronic villitis (CV), and five (16%) cases without relevant lesions. Rest of diagnoses made corresponded to one or two cases each. In all cases at least focally dystrophic calcifications, patches of fibrin among villi and syncytial knots were found. No chronic intervillositis or viral inclusions were found. Microscopic features are presented in Figure 1.
Table 2.
Diagnosis made in SARS‐CoV‐2 infected women and controls
| Diagnosis | Cases (n = 32 a ) | Controls (n = 58) | Fisher's exact test |
|---|---|---|---|
| No lessions | 5 (15.6) | 8 (13.8) | 1.000 |
| Acute chorioamnitis | 5 (15.6) | 23 (39.7) | 0.020 |
| Stage 1 | 4 (12.5) | 18 (31.0) | 0.072 |
| Stage 2 | 1 (3.1) | 5 (8.6) | 0.416 |
| Stage 3 | 0 (0) | 0 (0) | N/A |
| Acute funisitis | 1 (3.1) | 13 (22.4) | 0.016 |
| Stage 1 | 1 (3.1) | 6 (10.3) | 0.414 |
| Stage 2 | 0 (0) | 7 (12.1) | 0.048 |
| Stage 3 | 0 (0) | 0 (0) | N/A |
| Villitis of unknown etiology | 6 (18.8) | 9 (15.6) | 0.771 |
| Low grade | 4 (12.5) | 3 (5.1) | 0.241 |
| High grade | 2 (6.3) | 6 (10.3) | 0.707 |
| Plasma cell deciduitis | 2 (6.3) | 0 (0) | 0.124 |
| Presence of DVM | 17 (53.1) | 23 (39.7) | 0.270 |
| Presence of villous chorangiosis | 19 (59.4) | 22 (37.9) | 0.076 |
| Villous edema | 1 (3.1) | 7 (12.1) | 0.251 |
| MVM | 1 (3.1) | 7 (12.1) | 0.251 |
| FVM | 1 (3.1) | 1 (1.7) | 1.000 |
| Meconium in chorial plate | 2 (6.3) | 0 (0) | 0.707 |
| Placentomegaly | 1 (3.1) | 0 (0) | 0.356 |
Note: Bold values indicate that reached statistical significance (p < 0.05)
Abbreviations: DVM, delayed villous maturation; FVM, fetal vascular malperfusion; MVM, maternal vascular malperfusion.
Although there are 29 pregnancies in the SARS‐CoV‐2 group, 32 children were delivered.
Figure 1.
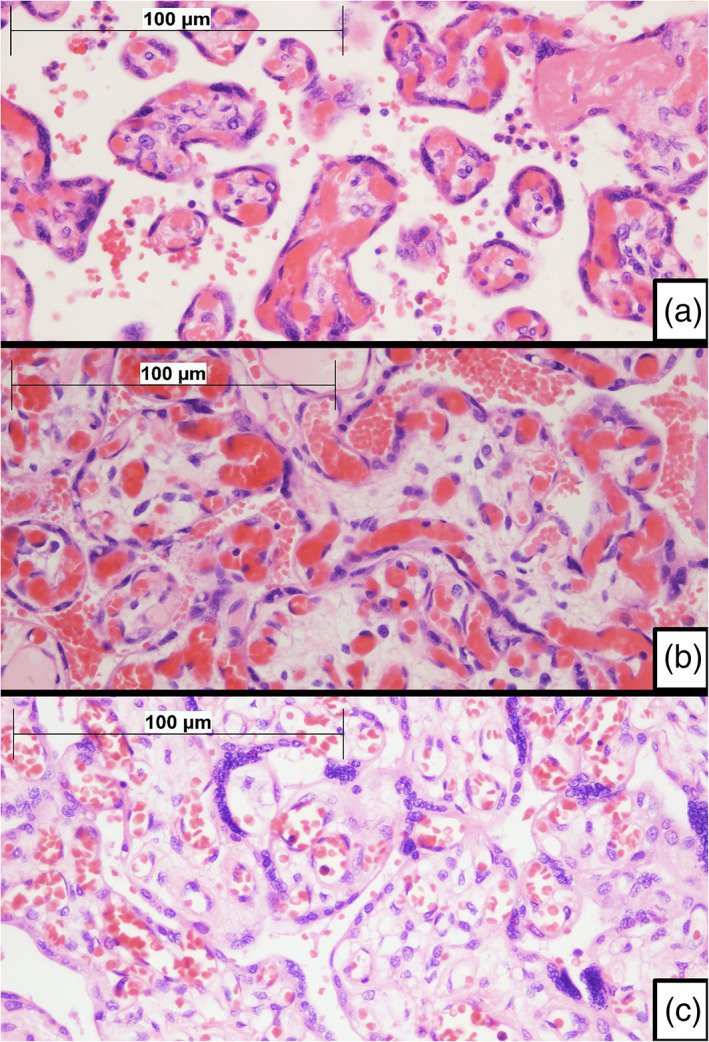
Microscopic features of placentas. (a) Normal term villi. Figure shows slender villi with a high proportion of capillary vessels forming vasculosyncytial membranes, with little intervening stroma. A thin layer of intervening syncytiotrophoblast can be seen. Although congestive, villi do not fulfill criteria for diagnosis of villous chorangiosis (H&E ×400). (b) Villous chorangiosis. Villi show an increased number of capillary cross‐sectional profiles per villus (per definition, at least 10 capillaries per villus are required to fulfill minimal criteria for villous chorangiosis) (H&E ×400). (c) Delayed villous maturation. Terminal villi show features that resemble immature intermediate villi. Villi tend to be larger, with an increased amount of stroma, which may be edematous. There is usually a thick layer of syncytiotrophoblast with scattered persistent cytotrophoblast cells. Villous capillaries are nonperipherical and do not to merge with the overlying syncytiotrophoblast, failing to form vasculosyncytial membranes. Delayed villous maturation may be seen in association with villous chorangiosis. Compare villous features with A (H&E ×400)
As case 10 corresponded to a child who developed positive PCR 48 h after delivery, IHC stains for SARS‐CoV‐2 Spike and NP proteins were performed on placental tissue. These stains were negative in two separate occasions, both with a positive external control. Additionally, SARS‐CoV‐2 PCR was conducted on deparaffinized tissue, with negative results.
As neutrophilia is related to AC, 19 and hypoxia could play a role in the development of VC, 20 a statistical analysis was performed that showed no association of these entities in the COVID‐19 group (data not shown).
Regarding controls, mean maternal age was 33.6 years (range: 22–46). Mean gestational age was 39 + 6 weeks (range: 37 + 2 to 41 + 4). There was one case of gestational diabetes. All pregnancies had good maternal and fetal outcomes. Placental weight ranged from 283 (<p3) to 755 g (>p97). Placental weight percentiles and feto‐placental ratios were found in similar distributions in both groups (p = 0.646 and p = 0.230).
Complete diagnoses and statistical analysis are presented in Table 2. AC and acute funisitis (AF), were more common in the control group, reaching statistical significance (p = 0.020 and p = 0.016). Rest of diagnoses and morphological features (including presence of dystrophic calcifications, patches of fibrin among villi and syncytial knots) were similar among groups, lacking statistical significance.
Discussion
COVID‐19 is a global concern in which risk for pregnant women and the fetus is not well‐defined. In our series, symptoms and radiographic findings were similar to what is reported in pregnant patients 6 and in the general population. 21 , 22 , 23 , 24 Eight asymptomatic cases were diagnosed thanks to implementation of SARS‐CoV‐2 universal screening in pregnant patients. Most frequently altered analytical values found in our cohort, with the exception of absolute lymphopenia, which has been described in COVID‐19, 21 , 24 , 25 could be explained by most tests being conducted in the immediate postpartum, thus modifying neutrophil count, 26 fibrinogen levels, 26 , 27 D‐Dimer levels, 26 , 28 and CRP concentration. 27 , 29
Regarding maternal prognosis, one patient (3%) succumbed to the illness. Although the patient was young, presence of obesity is a risk factor for mortality. 30 Series of other authors have not reported maternal mortality. 6
Clinical outcome of all newborns of our series was good, similar to what is described in other series. 6 Considering potential risk of vertical transmission, in our series there are no proven cases of vertical transmission. Only one newborn (case 10) was a SARS‐CoV‐2 positive case, detected at 48 h of life. Newborn was delivered by an uneventful caesarean procedure, following all preventive measures stated before.
Vertical transmission is highly unlikely, as negativity of the two RT‐PCR conducted at birth and at 24 h of life suggest absence of virus at birth. Besides, viral presence was not demonstrated on placental tissue, as both IHC and PCR for SARS‐CoV‐2 were negative.
Although safety protocols aimed at reducing nosocomial transmission were strictly followed, infections can still happen. Even when following all protective measures, health professionals may become infected in the hospital setting, and the same could happen to patients. This was one of the arguments that called into question the two possible vertical transmission cases reported in Wuhan, where community or nosocomial acquired infection could not be completely ruled‐out. 31 , 32
In this case, all evidence gathered stablished that there was no vertical transmission. With current knowledge and our data we believe that SARS‐CoV‐2 is inefficient crossing the placental barrier, and occurrence of vertical transmission, if possible, is an extremely rare event. 31
Further evidence that supports lack of vertical transmission is that some case series assessed presence of viral RNA with RT‐PCR conducted on vaginal secretions, 9 , 11 amniotic fluid, 8 , 9 , 10 , 33 umbilical cord blood, 8 , 9 , 10 , 11 , 33 placenta, 9 , 10 , 11 , 33 , 34 and breast milk. 8 , 9 , 10 , 11 In none of those cases viral presence was demonstrated. However, although this evidence is suggestive of lack of vertical transmission, more studies systematically reviewing the presence of viral RNA shedding on different fluids and in the placenta should be conducted.
Nevertheless, COVID‐19 could still have indirect fetal effects, direct consequence of the severity of the disease on the mother. However, as COVID‐19 is a disease generally well tolerated by the mothers, 6 severity of the disease does not seem to play a relevant role in fetal prognosis.
Lesions typically associated with viral infection, 35 like viral inclusions or chronic histiocytic intervillositis, were not identified in the COVID‐19 group. Although CV may be associated with viral infection, 35 there were no statistically significant differences among groups. Incidence of CV was similar to other published series. 36 , 37 , 38 Most cases of CV are diagnosed in normal term pregnancies with normal newborns. 37 , 38
There was a higher proportion of AC and AF in the control group. As described by Roberts et al., 19 the vast majority of histologic AC in term pregnancies are noninfectious. Besides, AC was diagnosed in a similar proportion in our series, compared to her results (31% stage 1 and 8% stage 2 versus 27% stage 1 and 8% stage 2 of her population). We consider these findings not related to COVID‐19, and explainable by gestations in the control group being ended later.
There was a relatively high presence of features of DVM and VC in both case and control placentas, usually found in association. 39 There were no statistically significant differences among COVID‐19 and control groups. Both DVM and VC are associated to a variety of conditions, none of them related with viral infections. 20 , 39 Although COVID‐19 is a pathology associated with hypoxia, which is a cause for VC, 20 no association was found for hypoxia and VC in the COVID‐19 group, as the vast majority of patients had good oxygen saturation measurements.
The presence of patches of fibrin, 40 syncytial knots, 41 and dystrophic calcifications may be found in the mature placenta, and is not considered specific of any entity per se. Syncytial knots density increases with gestational age, and at term, 27%–29% are accepted as normal. 41
Our results show lack of specific placental findings in the pregnant patients infected with SARS‐CoV‐2. We believe that our cohort of placentas from infected SARS‐CoV‐2 mothers represent normal placentas that would not have been submitted if the patients were uninfected, adjusting to the submission criteria described before. 12
These results contrast with the results of the many groups that have studied placental pathology in COVID‐19. As Sharps et al. pointed, 6 most studies were small case series that lacked control groups. Besides, most studies, including those that had a control group, were aware of the COVID‐19 status of the patients. As a result, placental lesions in COVID‐19 patients may be overestimated. Furthermore, as described by Romero et al, 38 up to 78% of normal at term pregnancies may have low‐grade inflammatory or vascular malperfusion lesions (both maternal or fetal), with no repercussion on fetal outcome. An adequate control group is vital to avoid spurious associations of COVID‐19 and placental pathology.
An explanation for this lack of actual viral pathology in placentas could be that the placenta is not a target organ of SARS‐CoV‐2 infection. The Angiotensin‐Converting Enzyme 2 (ACE2), the receptor of the Spike protein of SARS‐CoV‐2, 3 is an enzyme expressed in the lungs, 42 but also expressed in the syncytiotrophoblast of the placenta. 43 , 44 Given the lack of vertical transmission cases among COVID‐19 pregnant women, there are probably more undescribed factors that are playing a role in placental dynamics during SARS‐CoV‐2 infection, opening promising lines of investigation.
A limitation of this study is its retrospective design. Minor disparities were found when collecting data from clinical records. Furthermore, protocol variations were to be expected as SARS‐CoV‐2 body of knowledge rapidly evolved since the start of this study. Yet, clinical data and results obtained are similar to what other authors have reported. 6
It has to be considered that absence of statistical significance does not mean that both groups are equal. Actual demonstration of equality would require larger cohorts of cases and controls.
Although we were unable to blindly assess cases and controls, diagnostic homogeneity is increased as all cases and controls were reviewed by the same group, with defined diagnostic criteria.
In conclusion, in the pregnant population COVID‐19 is a disease generally well‐tolerated, with clinical and radiological features similar to the general population. Analytical values may be altered by pregnancy and delivery, however, lymphopenia is still typical of COVID‐19. Death of one patient serves as a reminder that COVID‐19 may present as severe disease, even in young pregnant patients.
Fetal and neonatal prognoses were excellent. There was one asymptomatic newborn that was SARS‐CoV‐2 positive, but vertical transmission could not be demonstrated.
Placentas from patients infected with SARS‐CoV‐2 did not have any specific features. We believe that our cohort of placentas represent normal term placentas, that were submitted to our Department only due to maternal SARS‐CoV‐2 infection.
Conflict of interest
The authors declare no conflict of interests.
Supporting information
Data S1 Diagnostic criteria and definitions
Data S2 Data collected from pregnant patients
Data S3 Data collected from newborns
Acknowledgments
The authors thank Dr. Álvaro López‐Janeiro for his invaluable help and support during preparation of this manuscript.
References
- 1. Wuhan Municipal Health Commission . Wuhan Municipal Health Commission on the current situation of pneumonia in our city (31, December, 2019). [Internet] [In Chinese, translated and cited 14 April 2020]. http://wjw.wuhan.gov.cn/front/web/showDetail/2019123108989
- 2. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xu X, Chen P, Wang J, Feng J, Zhou H, Li X, et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci. 2020;63:457–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. WHO . WHO Director‐General's opening remarks at the media briefing on COVID‐19 (2020, March 11). [Internet] [Cited 16 April 2020]. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19-11-march-2020
- 5. WHO . Weekly Epidemiological Update on COVID‐19 – 29 December 2020 [Internet] [cited 30 December 2020]. https://www.who.int/publications/m/item/weekly-epidemiological-update-29-december-2020
- 6. Sharps MC, Hayes DJL, Lee S, Zou Z, Brady CA, Almoghrabi Y, et al. A structured review of placental morphology and histopathological lesions associated with SARS‐CoV‐2 infection. Placenta. 2020;101:13–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Breslin N, Baptiste C, Gyamfi‐Bannerman C, Miller R, Martinez R, Bernstein K, et al. COVID‐19 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of new York City hospitals. Am J Obstet Gynecol MFM. 2020;2:100118. 10.1016/j.ajogmf.2020.100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID‐19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fan C, Lei D, Fang C, et al. Perinatal transmission of COVID‐19 associated SARS‐CoV‐2: should we worry? Clin Infect Dis. 2021;72:862–4. 10.1093/cid/ciaa226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li Y, Zhao R, Zheng S, Chen X, Wang J, Sheng X, et al. Lack of vertical transmission of severe acute respiratory syndrome coronavirus 2, China. Emerg Infect Dis. 2020;26:1335–6. 10.3201/eid2606.200287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu W, Wang Q, Zhang Q, et al. Coronavirus disease 2019 (COVID‐19) during pregnancy: a case series. Preprints. 2020;2020020373. https://www.preprints.org/manuscript/202002.0373/v1. [Google Scholar]
- 12. Langston C, Kaplan C, Macpherson T, Manci E, Peevy K, Clark B, et al. Practice guideline for examination of the placenta: developed by the placental pathology practice guideline development task force of the College of American Pathologists. Arch Pathol Lab Med. 1997;121:449–76. [PubMed] [Google Scholar]
- 13. Khong TY, Mooney EE, Ariel I, Balmus NCM, Boyd TK, Brundler MA, et al. Sampling and definitions of placental lesions: Amsterdam placental workshop group consensus statement. Arch Pathol Lab Med. 2016;140:698–713. [DOI] [PubMed] [Google Scholar]
- 14. Herrero Ruiz, B. Crecimiento longitudinal fetal en el tercer trimestre como predictor de la aparición de eventos perinatales y hallazgos placentarios significativos. [Internet] [In Spanish]. http://hdl.handle.net/10486/681858
- 15. Carrascosa A, Fernández JM, Ferrández A, et al. Spanish Growth Studies 2010. [Internet] [In Spanish, cited 16 April 2020]. https://www.seep.es/images/site/publicaciones/oficialesSEEP/Estudios_Españoles_de_Crecimiento_2010.pdf
- 16. Kraus FT, Redline RW, Gersell DJ. Appendix 3: placental weights: means, standard deviations, and percentiles by gestational age. In: Redline RW, editor. Placental and gestational pathology. Volume 61. 1st ed. New York, NY: Cambridge University Press; 2018. p. 336. [Google Scholar]
- 17. Pinar H, Sung CJ, Oyer CE, Singer DB. Reference values for singleton and twin placental weights. Pediatr Pathol Lab Med. 1996;16:901–7. [DOI] [PubMed] [Google Scholar]
- 18. Kraus FT, Redline RW, Gersell DJ. Appendix 4: fetal‐placental weight ratios, means, Stardard deviations, and percentiles by gestational age. In: Redline RW, editor. Placental and gestational pathology. Volume 61. 1st ed. New York, NY: Cambridge University Press; 2018. p. 337. [Google Scholar]
- 19. Roberts DJ, Celi AC, Riley LE, Onderdonk AB, Boyd TK, Johnson LC, et al. Acute histologic Chorioamnionitis at term: nearly always noninfectious. PLoS One. 2012;7(3):e31819. 10.1371/journal.pone.0031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Redline RW. Fetal Stromal‐Vascular Developmental Abnormalities. In: Redline RW, editor. Placental and gestational pathology. Volume 61. 1st ed. New York, NY: Cambridge University Press; 2018. p. 79–92. [Google Scholar]
- 21. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wong HYF, Lam HYS, Fong AHT, Leung ST, Chin TWY, Lo CSY, et al. Frequency and distribution of chest radiographic findings in COVID‐19 positive patients. Radiology. 2019;296:E72–8. 10.1148/radiol.2020201160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. CDC . Fact Sheet for Healthcare Providers. Coronavirus Disease 2019 (2020, March 15). [Internet] [cited 13 April 2020]. https://www.fda.gov/media/134920/download
- 24. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kakodkar P, Kaka N, Baig M. A comprehensive literature review on the clinical presentation, and Management of the Pandemic Coronavirus Disease 2019 (COVID‐19). Cureus. 2020;12:e7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chandra S, Tripathi AK, Mishra S, Amzarul M, Vaish AK. Physiological changes in hematological parameters during pregnancy. Indian J Hematol Blood Transfus. 2012;28:144–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Adler G, Duchinski T, Jasinska A, Piotrowska U. Fibrinogen fractions in the third trimester of pregnancy and in puerperium. Thromb Res. 2000;97:405–10. [DOI] [PubMed] [Google Scholar]
- 28. Epiney M, Boehlen F, Boulvain M, et al. D‐dimer levels during delivery and the postpartum. J Thromb Haemost. 2005;3:268–71. [DOI] [PubMed] [Google Scholar]
- 29. Keski‐Nisula L, Kirkinen P, Ollikainen M, Saarikoski S. C‐reactive protein in uncomplicated parturients delivered by cesarean section. Acta Obstet Gynecol Scand. 1997;76:862–7. [DOI] [PubMed] [Google Scholar]
- 30. Zhang F, Xiong Y, Wei Y, et al. Obesity predisposes to the risk of higher mortality in young COVID‐19 patients. J Med Virol. 2020;92(11):2536–42. 10.1002/jmv.26039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schwartz DA, Dhaliwal A. Infections in pregnancy with COVID‐19 and other respiratory RNA virus disease are rarely, if ever, transmitted to the fetus: experiences with coronaviruses, HPIV, hMPV RSV, and influenza. Arch Pathol Lab Med. 2020;144:920–8. 10.5858/arpa.2020-0211-SA. [DOI] [PubMed] [Google Scholar]
- 32. Schwartz DA, Graham AL. Potential maternal and infant outcomes from coronavirus 2019‐nCoV (SARS‐CoV‐2) infecting pregnant women: lessons from SARS, MERS, and other human coronavirus infections. Viruses. 2020;12:E194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang X, Zhou Z, Zhang J, Zhu F, Tang Y, Shen X. A case of 2019 novel coronavirus in a pregnant woman with preterm delivery. Clin Infect Dis. 2020;71(15):844–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen S, Huang B, Luo DJ, et al. Pregnant women with new coronavirus infection: a clinical characteristics and placental pathological analysis of three cases. Zhonghua Bing Li Xue Za Zhi. 2020;49(5):418–23.[In Chinese]. 10.3760/cma.j.cn112151-20200225-00138. [DOI] [PubMed] [Google Scholar]
- 35. Roberts DJ. Placental infections. In: Redline RW, editor. Placental and gestational pathology. Volume 61. 1st ed. New York, NY: Cambridge University Press; 2018. p. 115–36. [Google Scholar]
- 36. Knox WF, Fox H. Villitis of unknown aetiology: its incidence and significance in placentae from a British population. Placenta. 1984;5:395–402. [DOI] [PubMed] [Google Scholar]
- 37. Parast M. Chronic villitis/villitis of unknown tiology (VUE). In: Redline RW, editor. Placental and gestational pathology. Volume 61. 1st ed. New York, NY: Cambridge University Press; 2018. p. 137–44. [Google Scholar]
- 38. Romero R, Kim YM, Pacora P, Kim CJ, Benshalom‐Tirosh N, Jaiman S, et al. The frequency and type of placental histologic findings in term pregnancies with normal outcome. J Perinat Med. 2018;46:613–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Redline RW. Distal villous immaturity. Diagn Histopathol. 2012;18:189–94. [Google Scholar]
- 40. Fox H. Perivillous fibrin deposition in the human placenta. Am J Obstet Gynecol. 1967;98:245–51. [DOI] [PubMed] [Google Scholar]
- 41. Loukeris K, Sela R, Baergen RN. Syncytial knots as a reflection of placental maturity: reference values for 20 to 40 weeks gestational age. Pediatr Dev Pathol. 2010;13:305–9. [DOI] [PubMed] [Google Scholar]
- 42. Hamming I, Timens W, Bulthuis M, Lely A, Navis G, Goor HV. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li M, Chen L, Zhang J, Xiong C, Li X. The SARS‐CoV‐2 receptor ACE2 expression of maternal‐fetal interface and fetal organ by single‐cell transcriptome study. PLoS One. 2020;15(4):e0230295. 10.1371/journal.pone.0230295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Valdés G, Neves L, Anton L, et al. Distribution of angiotensin‐(1‐7) and ACE2 in human placentas of normal and pathological pregnancies. Placenta. 2006;27:200–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 Diagnostic criteria and definitions
Data S2 Data collected from pregnant patients
Data S3 Data collected from newborns


