Abstract
The relation between severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection and demyelinating Guillain‐Barre syndrome (GBS) has been defined. We aim to report the clinical features of a child with axonal GBS associated with SARS‐CoV‐2. A 6‐year‐old male presented with symmetric ascending paralysis progressed over a 4‐day course and 2 days of fever. He had bilateral lower and upper limb flaccid weakness of 1/5 with absent deep tendon reflexes. He had severe respiratory muscle weakness requiring invasive mechanical ventilation. On admission, SARS‐CoV‐2 returned as positive by real‐time polymerase chain reaction on a nasopharyngeal swab. Cerebrospinal fluid analysis showed elevated protein without pleocytosis. He was diagnosed with GBS associated with SARS‐CoV‐2 infection. The nerve conduction study was suggestive of acute motor axonal neuropathy. Ten consecutive therapeutic plasma exchange sessions with 5% albumin replacement followed by four sessions on alternate days were performed. On Day 12, methylprednisolone (30 mg/kg/day for 5 days) was given. On Day 18, intravenous immunoglobulin (2 g/kg/day) was given and repeated 14 days after due to severe motor weakness. On Day 60, he was discharged from the hospital with weakness of neck flexor and extensor muscles of 3/5 and the upper limbs and the lower limbs of 2/5 on home‐ventilation. Our patient is considered to be the youngest patient presenting with a possible para‐infectious association between axonal GBS and SARS‐CoV‐2 infection. The disease course was severe with a rapid progression, an earlier peak, and prolonged duration in weakness as expected in axonal GBS.
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) has become a global epidemic in our health system with more than 102 million confirmed cases worldwide. As of February 2021, approximately 2 500 000 COVID‐19 cases have been confirmed in Turkey. 1
The most common clinical presentations of COVID‐19 are fever, malaise, and respiratory symptoms, ranging from a mild cough to severe pneumonia. 2 However, there is increasing evidence that severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) may also affect the nervous system. Meningitis, encephalitis, acute disseminated encephalomyelitis, postinfectious brainstem encephalitis, myositis, acute necrotizing hemorrhagic encephalopathy, and anosmia have been defined as the neurological manifestations of SARS‐CoV‐2. 3
Guillain‐Barre syndrome (GBS) is an immune‐mediated polyneuropathy, mostly triggered by a viral or bacterial infection. 4 The most common microorganisms related to GBS include Epstein–Barr virus, Campylobacter jejuni, Influenza A virus, cytomegalovirus, Haemophilus influenza, and Mycoplasma pneumonia. Recently, Zika virus has been associated with GBS. 5 The main subtypes of GBS were defined as acute inflammatory demyelinating polyneuropathy (AIDP) and acute motor axonal neuropathy (AMAN). These two subtypes present differences in immunopathogenesis, clinical course, and treatment response. 6 The relation between SARS‐CoV‐2 infection and AIDP has been defined in a case series. 7 Herein, we aim to report a child with axonal GBS associated with SARS‐CoV‐2.
1.1. Case report
A 6‐year‐old male presented with symmetric ascending paralysis progressed over a 4‐day course and 2 days of fever. His immunization status was appropriate to his age, and his previous medical history was unremarkable. He had contact with a relative diagnosed with COVID‐19 1‐week before.
He was admitted to the pediatric intensive care unit (PICU). On physical examination, he was conscious and oriented. The cranial nerves were intact bilaterally. There was no weakness of bulbar muscles. He had bilateral lower and upper limb flaccid weakness of 1/5 affecting proximal and distal muscles equally with absent deep tendon reflexes and weakness of neck flexor and extensor muscles. He had severe respiratory muscle weakness requiring invasive mechanical ventilation. On admission, SARS‐CoV‐2 returned as positive by real‐time polymerase chain reaction (PCR) on a nasopharyngeal swab. On Days 7 and 11, SARS‐CoV‐2 remained positive by real‐time PCR. On Day 14, the nasopharyngeal swab test for SARS‐CoV‐2 was negative. The laboratory analysis revealed lymphopenia. Cerebrospinal fluid (CSF) analysis showed elevated protein (51 mg/dl) without pleocytosis. The anti‐ganglioside antibodies were negative. He was diagnosed with COVID‐19 and GBS, based on the clinical features, CSF findings, and molecular tests. The chest X‐ray was normal (Figure 1). On Day 1, spinal magnetic resonance imaging revealed contrast enhancement of cauda equina and nerve roots (Figure 2). On Day 14, the nerve conduction study was suggestive of AMAN (Tables 1 and 2).
Figure 1.
On admission, the chest x‐ray of the patient shows normal findings
Figure 2.
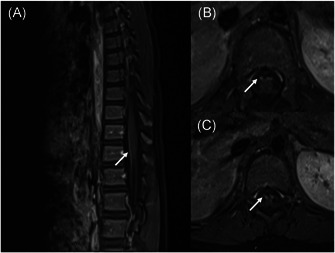
Contrast‐enhanced (A) sagittal and (B, C) axial T1‐weighted magnetic resonance imaging of the lumbar spine demonstrates marked enhancement of the cauda equina and anterior nerve roots
Table 1.
Motor nerve conduction studies
| Segment | Distal latency (ms) | Amplitude (mV) | NCV (m/s) | F latency (ms) | |
|---|---|---|---|---|---|
| Right median nerve | Wrist | NR (normal ≤ 3.8) | NR (normal ≥ 4) | ‐ | Absent |
| Elbow | NR | NR | ‐ | (normal ≤ 30) | |
| Right ulnar nerve | Wrist | NR (normal ≤ 3.8) | NR (normal ≥ 4) | ‐ | Absent |
| Elbow | NR | NR | ‐ | (normal ≤ 31) | |
| Right peroneal nerve | Ankle | 5.3 (normal ≤ 5.6) | 0.1(normal ≥ 2.8) | ‐ | Absent |
| Head of fibula | 9.7 | 0.2 | 47.1 (normal ≥ 40) | (normal ≤ 56) | |
| Left peroneal nerve | Ankle | 4.4 (normal ≤ 5.6) | 0.0 (normal ≥ 2.8) | ‐ | Absent |
| Head of fibula | 8.7 | 0.1 | 50.0 (normal ≥ 40) | (normal ≤ 56) | |
| Right tibial nerve | Ankle | 3.9 (normal ≤ 5.6) | 4.4 (normal ≥ 3.6) | ‐ | Absent |
| Knee | 8.4 | 4.4 | 53.3 (normal ≥ 40) | (normal ≤ 56) | |
| Left tibial nerve | Ankle | 3.8 (normal ≤ 5.6) | 7.4 (normal ≥ 3.6) | ‐ | Absent |
| Knee | 8.3 | 5.3 | 55.6 (normal ≥ 40) | (normal ≤ 56) |
Abbreviations: NCV, nerve conduction velocity; NR, no response.
Table 2.
Sensory nerve conduction studies
| Segment | Latency (ms) | Amplitude (mV) | NCV (m/s) | |
|---|---|---|---|---|
| Right median nerve | Wrist | 1.6 (normal ≤ 2.5) | 23.8 (normal ≥ 20) | 65.8 (normal ≥ 50) |
| Elbow | 2.5 (normal ≤ 2.5) | 42.7 (normal ≥ 20) | 74.7 (normal ≥ 50) | |
| Right ulnar nerve | Wrist | 1.4 (normal ≤ 2.5) | 12.8 (normal ≥ 20) | 62.9 (normal ≥ 50) |
| Elbow | 2.4 (normal ≤ 2.5) | 66.7 (normal ≥ 20) | 72.9 (normal ≥ 50) | |
| Right sural nerve | Lat mall | 1.6 (normal ≤ 2.6) | 19.8 (normal ≥ 6) | 64.5 (normal ≥ 40) |
| Left sural nerve | Lat mall | 1.9 (normal ≤ 2.6) | 23.9 (normal ≥ 6) | 57.9 (normal ≥ 40) |
Abbreviation: NCV, nerve conduction velocity.
Ten consecutive therapeutic plasma exchange sessions with 5% albumin replacement followed by four sessions on alternate days were performed using Prismaflex TPE 2000 filter set (Gambro Lundia AB). On Day 12, methylprednisolone (30 mg/kg/day for 5 days) was given. On Day 18, intravenous immunoglobulin (IVIG) (2 g/kg/day) was given and repeated 14 days after due to severe motor weakness. On Day 30, a tracheostomy was performed while in the PICU. On Day 50, the nerve conduction study demonstrated an increase in the amplitude of the tibial nerve compared to previous findings, compatible with recovery from motor axonal neuropathy. On Day 60, he was discharged from the hospital with weakness of neck flexor and extensor muscles of 3/5 and the upper limbs and the lower limbs of 2/5 on home‐ventilation. His reflexes remained absent.
Written informed consent to publication has been obtained from the parents on behalf of the patient.
2. DISCUSSION
We, herein, describe a 6‐year‐old boy presented with axonal GBS associated with SARS‐CoV‐2 infection. In our case, there was a temporal relation between fever, lymphopenia, a positive test for SARS‐CoV‐2, and muscle weakness, indicating the possible association of GBS and SARS‐CoV‐2 infection in a para‐infectious profile. As of Jan 2021, more than 60 patients with GBS associated with SARS‐CoV‐2 were reported. In the majority of these patients, the neurological symptoms emerge in 3–24 days after SARS‐CoV‐2 infection.8, 9 A few adult patients with COVID‐19 presented with GBS, resembling a course of para‐infectious profile.10, 11, 12 The disease course was severe in two of them who required mechanical ventilation lasting 1 month.11, 12 The nerve conduction study was available in one, indicating inflammatory demyelinating polyneuropathy. 11 Compared to these patients presenting with a para‐infectious profile, our patient had the most severe disease course with a rapid progression, an earlier peak, and prolonged duration in weakness.10, 11, 12 The clinical features of our patient were consistent with axonal GBS, which explains why the disease course is more severe than other patients with a para‐infectious profile. Axonal GBS is characterized by rapid progress and an earlier peak in weakness compared to demyelinating GBS. 6 The disease severity seems to correlate with the underlying immunopathogenesis, irrespective of post‐infectious or para‐infectious profile. The possible explanation for a severe disease course of GBS associated with SARS‐CoV‐2 infection could be an exacerbated immune response against nervous system antigens triggered by SARS‐CoV‐2 infection. 13
The common clinical and electrophysiological features of GBS associated with SARS‐CoV‐2 infection were defined as the presence of albuminocytological dissociation, demyelinating GBS, and favorable outcomes on discharge. 8 Our patient did not present the typical features of GBS associated with SARS‐CoV‐2 infection other than the presence of albuminocytological dissociation. Axonal GBS presented in a minority of patients with SARS‐CoV‐2 infection, and most of them were adults.8, 14 Until now, three children with GBS associated with SARS‐CoV‐2 infection have been reported, 2 of them with demyelinating GBS and one with axonal GBS.15, 16, 17 To the best of our knowledge, our patient was the youngest patient with axonal GBS associated with SARS‐CoV‐2 infection.
On the other hand, one can suggest that this patient suffered from critical illness polyneuropathy, manifesting with axonal polyneuropathy. However, the patient did not have multiorgan dysfunction or respiratory symptoms, and the chest x‐ray was normal. These findings make unlikely the possibility of the critical illness polyneuropathy. 18
In conclusion, our patient is considered to be the youngest patient presenting with a possible para‐infectious association between axonal GBS and SARS‐CoV‐2 infection. The disease course was severe with a rapid progression, an earlier peak, and prolonged duration in weakness as expected in axonal GBS.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/jmv.27018
Akçay N, Menentoğlu ME, Bektaş G, Şevketoğlu E. Axonal Guillain‐Barre syndrome associated with SARS‐CoV‐2 infection in a child. J Med Virol. 2021;93:5599–5602. 10.1002/jmv.27018
DATA AVAILABILITY STATEMENT
The gathered data supporting the findings of this study are available from the authors upon request.
REFERENCES
- 1. World Health Organization (WHO) Coronavirus disease (COVID‐19) dashboard. February 1, 2021. https://covid19.who.int/
- 2. Hoang A, Chorath K, Moreira A, et al. COVID‐19 in 7780 pediatric patients: a systematic review. EClinicalMedicine. 2020;24:100433. 10.1016/j.eclinm.2020.100433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rimensberger PC, Kneyber MCJ, Deep A, et al. Caring for critically Ill children with suspected or proven coronavirus disease 2019 infection: recommendations by the Scientific Sections' Collaborative of the European Society of Pediatric and Neonatal Intensive Care. Pediatr Crit Care Med. 2021;22:56‐67. 10.1097/PCC.0000000000002599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Levison LS, Thomsen RW, Markvardsen LK, Christensen DH, Sindrup SH, Andersen H. Pediatric Guillain‐Barre syndrome in a 30‐year nationwide cohort. Pediatr Neurol. 2020;107:57‐63. 10.1016/j.pediatrneurol.2020.01.017 [DOI] [PubMed] [Google Scholar]
- 5. Willison HJ, Jacobs BC, van Doorn PA. Guillain‐Barré syndrome. Lancet. 2016;388:717‐727. 10.1016/S0140-6736(16)00339-1 [DOI] [PubMed] [Google Scholar]
- 6. Kuwabara S, Yuki N. Axonal Guillain‐Barré syndrome: concepts and controversies. Lancet Neurol. 2013;12:1180‐1188. 10.1016/S1474-4422(13)70215-1 [DOI] [PubMed] [Google Scholar]
- 7. Manganotti P, Bellavita G, D'Acunto L, et al. Clinical neurophysiology and cerebrospinal liquor analysis to detect Guillain‐Barré syndrome and polyneuritis cranialis in COVID‐19 patients: a case series. J Med Virol. 2021;93:766‐774. 10.1002/jmv.26289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hasan I, Saif‐Ur‐Rahman K, Hayat S, et al. Guillain‐Barré syndrome associated with SARS‐CoV‐2 infection: a systematic review and individual participant data meta‐analysis. J Peripher Nerv Syst. 2020;25:335‐343. 10.1111/jns.12419 [DOI] [PubMed] [Google Scholar]
- 9. Finsterer J, Scorza FA, Fiorini AC. SARS‐CoV‐2‐associated Guillain‐Barre syndrome in 62 patients. Eur J Neurol. 2021;28:e10‐e12. 10.1111/ene.14544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhao H, Shen D, Zhou H, Liu J, Chen S. Guillain‐Barré syndrome associated with SARS‐CoV‐2 infection: causality or coincidence? Lancet Neurol. 2020;19:383‐384. 10.1016/S1474-4422(20)30109-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gale A, Sabaretnam S, Lewinsohn A. Guillain‐Barré syndrome and COVID‐19: association or coincidence. BMJ Case Rep. 2020;13:e239241. 10.1136/bcr-2020-239241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abrams RMC, Kim BD, Markantone DM, et al. Severe rapidly progressive Guillain‐Barré syndrome in the setting of acute COVID‐19 disease. J Neurovirol. 2020;26:797‐799. 10.1007/s13365-020-00884-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. García LF. Immune response, inflammation, and the clinical spectrum of COVID‐19. Front Immunol. 2020;11:1441. 10.3389/fimmu.2020.01441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rahimi K. Guillain‐Barre syndrome during COVID‐19 pandemic: an overview of the reports. Neurol Sci. 2020;41:3149‐3156. 10.1007/s10072-020-04693-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Curtis M, Bhumbra S, Felker MV, et al. Guillain‐Barre syndrome in a child with COVID‐19 infection. Pediatrics. 2020:e2020015115. 10.1542/peds.2020-015115 [DOI] [PubMed] [Google Scholar]
- 16. Khalifa M, Zakaria F, Ragab Y, et al. Guillain‐Barré syndrome associated with severe acute respiratory syndrome coronavirus 2 detection and coronavirus disease 2019 in a child. J Pediatric Infect Dis Soc. 2020;9:510‐513. 10.1093/jpids/piaa086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Frank CHM, Almeida TVR, Marques EA, et al. Guillain‐Barré syndrome associated with SARS‐CoV‐2 infection in a pediatric patient. J Trop Pediatr. 2020: 1‐6. 10.1093/tropej/fmaa044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Latronico N, Bolton CF. Critical illness polyneuropathy and myopathy: a major cause of muscle weakness and paralysis. Lancet Neurol. 2011;10:931‐941. 10.1016/S1474-4422(11)70178-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The gathered data supporting the findings of this study are available from the authors upon request.


