CONFLICT OF INTERESTS
None to declare.
AUTHOR CONTRIBUTIONS
Lorenzo Azzi: Conceptualization; Investigation; Writing‐original draft. Marco Toia: Data curation; Writing‐review & editing. Nicole Stevanello: Investigation. Fabrizio Maggi: Supervision. Greta Forlani: Conceptualization; Supervision; Writing‐review & editing.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/odi.13874.
To the Editor:
The case here reported presents some clinical signs and symptoms which could be associated with the first dose administration of the ChAdOx1 COVID‐19 vaccine, thus suggesting the onset of side effects not described so far.
A 31‐year‐old woman was referred to our clinic complaining of moderate, burning pain in her mouth. Three days prior to her visit, she had received the first dose of the ChAdOx1 COVID‐19 vaccine, batch ABV2856, and twenty‐four hours after vaccination, she had reported symptoms such as fever, headache, myalgia, arthralgia and fatigue, commonly associated with the vaccine administration.
The clinical examination of her mouth showed the presence of diffuse, erythematous and swollen red lesions on her buccal mucosa, tongue, gums and palate (Figure 1).
FIGURE 1.
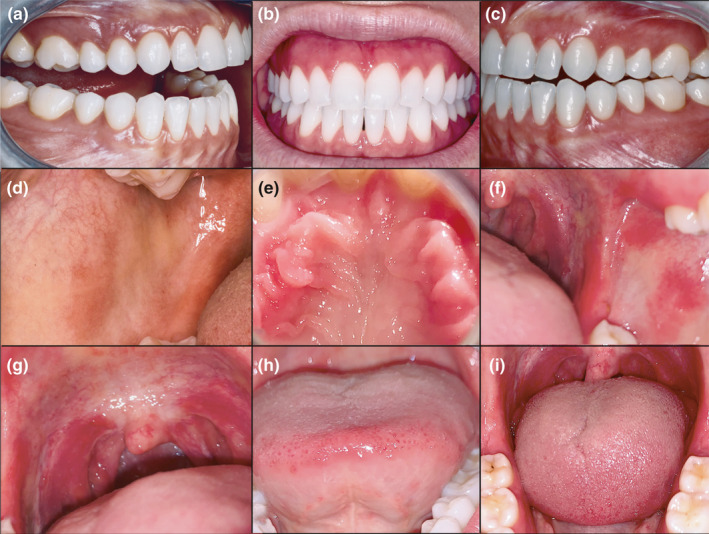
Diffuse oral mucositis which flared up the day after the injection of the first dose of the ChAdOx1 COVID‐19 vaccine. Multiple erythematous, swollen and painful lesions were observed on the gums (a‐c), buccal mucosa (d,f), palate (g), tongue (h) and retromolar trigone (i)
The patient's medical history revealed a single episode of deep vein thrombosis in her right arm in June 2020, and the presence of heterozygous Factor V Leiden mutation. More importantly, she had never been diagnosed with SARS‐CoV‐2 infection, either by NAAT or by serological testing. She only took an oral contraceptive.
The lesions were successfully treated with topical corticosteroids (i.e. Betamethasone effervescent tablets 1 mg three times per day, with progressive dose reduction) and topical miconazole oral gel 2%. They healed after three days.
ChAdOx1 is one of the approved COVID‐19 vaccines and is currently being distributed in the general population in many countries (Voysey et al., 2021).
The most common side effects reported by clinical trials are fever, headache, fatigue and myalgia. These symptoms are reported especially in young subjects after getting the first dose (Ramasamy et al., 2021).
However, in the last weeks, several countries have temporarily banned the administration of ChAdOx1 after the report of severe cases of thromboembolism in people who had been vaccinated (Oldenburg et al., 2021).
The European Medicines Agency has claimed that at present there is no indication that these episodes could be directly caused by the administration of the ChAdOx1 vaccine, while investigations into this issue still continue, at least for the ABV2856 batch (European Medicines Agency 2021, https://www.ema.europa.eu/en/news/astrazenecas‐covid‐19‐vaccine‐ema‐finds‐possible‐link‐very‐rare‐cases‐unusual‐blood‐clots‐low‐blood).
Since the onset of the current pandemic, several authors have reported cases of oral lesions developing in patients affected by COVID‐19. These lesions often vary in clinical manifestation depending on the site of the tissue damage; therefore, the correlation with SARS‐CoV‐2 infection is still controversial. However, some studies conducted in a large cohort of COVID‐19 patients have highlighted common histopathological features of the oral lesions, such as the presence of thrombotic vascular occlusion of small‐ and medium‐sized vascular structures (Favia et al., 2020). Moreover, diffuse thrombotic disease in the lungs of patients with COVID‐19 has been commonly reported (Lipcsey et al., 2021). Of note, Favia et al. showed that in COVID‐19 patients affected by both the moderate and more severe form of the disease, the vascular occlusion often causes mucosal ulceration.
In addition, Tomo et al Tomo et al. (2020). recently described the case of a young woman with a mild form of COVID‐19 disease who developed a diffuse erythema involving the lateral borders of the tongue and accompanied by a burning sensation, suggesting that these clinical manifestations could be associated with an oral mucositis triggered by a mucosal hypersensitivity to the viral infection in the epithelium. The clinical features of those lesions were very similar to those described in our report.
The COVID‐19 vaccination triggers a specific adaptive immune response against the virus, producing neutralizing antibodies directed against SARS‐CoV‐2 Spike protein. Thus, we may suggest that, similarly to the COVID‐19 patient described by Tomo et al., the induction of the specific immune response followed to the SARS‐CoV‐2 vaccination could be associated with a mucosal hypersensitivity characterized by underlying vascular events without epithelial damage.
Consistently, our patient was diagnosed with a heterozygous Factor V Leiden mutation, a condition that is associated with a 5‐fold to 10‐fold increase in thromboembolic events when compared with the non‐affected population (Zermatten et al., 2020). This condition may have favoured the onset of oral mucositis. However, oral mucositis can be regarded as a minor side effect, and the benefit of the immune protection acquired after vaccination outweighs the risks linked to COVID‐19 thromboembolism, which is more common in this group of patients.
Further reports are needed to assess whether oral mucositis may be considered a minor side effect of ChAdOx1 vaccine, while treatment with topical corticosteroids has proved to be effective in reducing the patient's discomfort and speeding up the healing process.
ACKNOWLEDGEMENTS
Prof. Marina Tettamanti supervised the English language in this paper.
REFERENCES
- European Medicines Agency . (2021). AstraZeneca’s COVID‐19 vaccine: EMA finds possible link to very rare cases of unusual blood clots with low blood platelets. https://www.ema.europa.eu/en/news/astrazenecas‐covid‐19‐vaccine‐ema‐finds‐possible‐link‐very‐rare‐cases‐unusual‐blood‐clots‐low‐blood. Accessed on April 7, 2021 [Google Scholar]
- Favia, G. , Tempesta, A. , Barile, G. , Brienza, N. , Capodiferro, S. , Vestito, M. C. , Crudele, L. , Procacci, V. , Ingravallo, G. , Maiorano, E. , & Limongelli, L. (2020). Covid‐19 symptomatic patients with oral lesions: Clinical and histopathological study on 123 cases of the University Hospital Policlinic of Bari with a purpose of a new classification. Journal of Clinical Medicine, 10, 757. 10.3390/jcm10040757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipcsey, M. , Persson, B. , Eriksson, O. , Blom, A. M. , Fromell, K. , Hultström, M. , Huber‐Lang, M. , Ekdahl, K. N. , Frithiof, R. , & Nilsson, B. O. (2021). The outcome of critically ill COVID‐19 patients is linked to thromboinflammation dominated by the kallikrein/kinin system. Frontiers in Immunology, 12, 627579. 10.3389/fimmu.2021.627579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenburg, J. , Klamroth, R. , Langer, F. , Albisetti, M. , von Auer, C. , Ay, C. , Korte, W. , Scharf, R. E. , Pötzsch, B. , & Greinacher, A. (2021). Diagnosis and management of vaccine‐related thrombosis following AstraZeneca COVID‐19 vaccination: Guidance statement from the GTH. Hämostaseologie. 10.1055/a-1469-7481. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- Ramasamy, M. N. , Minassian, A. M. , Ewer, K. , Flaxman, A. L. , Folegatti, P. M. , Owens, D. R. , Voysey, M. , Aley, P. K. , Angus, B. , Babbage, G. , Belij‐Rammerstorfer, S. , Berry, L. , Bibi, S. , Bittaye, M. , Cathie, K. , Chappell, H. , Charlton, S. , Cicconi, P. , & Clutterbuck, E. A. … Oxford COVID Vaccine Trial Group (2021). Safety and immunogenicity of ChAdOx1 nCoV‐19 vaccine administered in a prime‐boost regimen in young and old adults (COV002): A single‐blind, randomized, controlled, phase 2/3 trial. Lancet, 396, 1979–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomo, S. , Miyahara, G. I. , & Simonato, L. E. (2020). Oral mucositis in a SARS‐CoV‐2‐infected patient: Secondary or truly associated condition?. Oral Diseases. 10.1111/odi.13570 [DOI] [PubMed] [Google Scholar]
- Voysey, M. , Costa Clemens, S. A. , Madhi, S. A. , Weckx, L. Y. , Folegatti, P. M. , Aley, P. K. , Angus, B. , Baillie, V. L. , Barnabas, S. L. , Bhorat, Q. E. , Bibi, S. , Briner, C. , Cicconi, P. , Clutterbuck, E. A. , Collins, A. M. , Cutland, C. L. , Darton, T. C. , Dheda, K. , & Dold, C. … Oxford COVID Vaccine Trial Group (2021). Single‐dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV‐19 (AZD1222) vaccine: A pooled analysis of four randomized trials. Lancet, 397, 881–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zermatten, M. G. , Bertaggia Calderara, D. , Aliotta, A. , & Alberio, L. (2020). Thrombin generation in a woman with heterozygous factor V Leiden and combined oral contraceptoves: A case report. Res Pract Thromb Haemost, 4, 429–432. [DOI] [PMC free article] [PubMed] [Google Scholar]


