Abstract
Background
In Sweden, home care services is a major external contact for older persons.
Methods
Five home care service companies in Stockholm, Sweden, enrolled 405 employees to a study including serum IgG to SARS‐CoV‐2 and SARS‐CoV‐2 virus in throat swabs.
Results
20.1% (81/403) of employees were seropositive, about twice as many as in a simultaneously enrolled reference population (healthcare workers entirely without patient contact, n = 3671; 9.7% seropositivity). 13/379 employees (3.4%) had a current infection (PCR positivity). Amongst these, 5 were also seropositive and 3 were positive with low amounts of virus. High amounts of virus and no antibodies (a characteristic for presymptomatic COVID‐19) were present in 5 employees (1.3%).
Conclusions
Personnel providing home services for older persons appear to be a risk group for SARS‐CoV‐2. Likely presymptomatic employees can be readily identified by screening. Increased protection of employees and of the older persons they serve is warranted.
Keywords: COVID‐19, home care services for older persons, SARS‐CoV‐2
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has since its first report in Wuhan, China, in December 2019 escalated to a pandemic, affecting all countries around the world [1, 2]. As of 23 November 2020, over 61.8 million confirmed coronavirus disease 2019 (COVID‐19) cases caused by SARS‐CoV‐2, and over 1.4 million deaths have been reported worldwide [3]. The older segment of the population (>70 years of age) is a particularly vulnerable group. They have been shown to be more susceptible to severe infections and thus are at higher risk of dying due to SARS‐CoV‐2 infection [4, 5].
Several public health measures have been introduced to prevent and control the spread of the virus in different countries [6]. Countries such as France, Spain and Italy declared total lock downs in March 2020, whilst other countries such as South Korea and Sweden had different approaches to control the spread without opting for lock downs [7]. However, all countries without exception have implemented strategies to protect older persons from SARS‐CoV‐2 infections.
The Public Health Agency of Sweden has at the time of writing reported more than 547 000 confirmed SARS‐CoV‐2 cases and more than 11 000 deaths due to COVID‐19 in Sweden. People aged over 70 years comprise about 12% of total confirmed cases and almost 90% of total deaths due to COVID‐19 in Sweden (Folkhälsomyndigheten.se data, accessed on 23 November 2020). The Swedish government introduced a protection order for residential care centres and the Public Health agency of Sweden advised against visits to older persons, neither at home nor at residential care centres [8].
Many reports have indicated a higher prevalence of SARS‐CoV‐2 virus amongst healthcare personnel compared with the general public [9]. A study from Stockholm, Sweden, screened care personnel for older persons with rapid detection of IgG against SARS‐CoV‐2 and showed that 20.3% of employees were tested positive [10]. In Sweden, whilst some persons over age 70 live in residential care centres, most of them receive home care services instead. About 8% of all subjects 65 years or older and 23% of all subjects 80 years or older receive care at home [11]. In January 2020, almost 80 000 persons were living at residential care centres, whilst about 190 000 people over age 70 had received home care services in Sweden [12]. Hence, it is of great value to gain knowledge on the spread of SARS‐CoV‐2 amongst home care services personnel in order to design strategies to control the infection amongst this group and the personnel working with them.
The aim of the study was to investigate past (serum antibody positivity) or current (presence of viral RNA) SARS‐CoV‐2 infection in personnel working for home care services for older persons during the COVID‐19 outbreak.
Materials and methods
Subjects and samples
Employees who were on duty from five different home care service companies for older persons (n = 438) in the region of Stockholm were invited to participate in the study during the period 11 May–17 June 2020. The number of employees at the companies ranged from 27 to 291 (Table 1). The study did not specifically inquire about symptoms, but the rules were clear that employees were not allowed to work if they had symptoms. Home help services were given in the homes of the old subjects. Services included help with personal hygiene, nutrition, household chores such as cleaning and errands, for example food stores, pharmacies or post office. The actual precautions taken to prevent spread may have differed between companies and may never be fully known. A resume by one of us (Å.S.) may serve as an illustrative example: the situation was characterized by shortage of personal protective equipment (PPE) and widespread notions that face masks did not work and that only symptomatic subjects were infectious. An initial guideline stipulating the use of face masks, visors and aprons was after a few days replaced by a guideline stipulating to only use face masks, and these were made available only if the old person had symptoms. A local dentist volunteered face masks, but we had to reuse them for multiple customers. We made visors from overhead sheets attached to elastic bands. Volunteers provided aprons. When travelling, scarfs covered our faces. There were rules restricting visits to old persons at institutions, but they did not apply to old persons at home. As far as we could see, neither relatives nor the ambulatory nurses administrating daily drugs (e.g. insulin) used PPE.
Table 1.
Characteristics of healthcare workers at home care services for older persons and samples collected
| Home care service company | Number of total employees | Number of employees participating in the study | Number of swabs collected | Number of blood samples | Number of men | Number of women | Median age total (men/women) |
|---|---|---|---|---|---|---|---|
| Hemtjänst 24 Omsorg | 28 | 28 | 28 | 28 | 14 | 14 | 32 (32.5/32) |
| Hägersten‐Liljeholmen‐Älvsjö Hemtjänst | 291 | 271 | 261 | 270 | 126 | 145 | 43 (44/43) |
| Roo Hemtjänst AB | 72 | 67 | 65 | 67 | 35 | 32 | 43 (42/44) |
| Saras Omsorg | 20 | 18 | 16 | 18 | 10 | 8 | 37 (42.5/35) |
| Vardaga Hemtjänst | 27 | 21 | 17 | 20 | 7 | 14 | 44 (44/40) |
| All companies | 438 | 405 | 387 | 403 | 192 | 213 | 43 (42.5/40) |
The participants were asked to provide a throat swab sample for SARS‐CoV‐2 RNA detection and a blood sample to perform serological analysis of antibodies for SARS‐CoV‐2.
Written informed consent was obtained from all participants in the study. The study was approved by the National Ethical Review Agency of Sweden (decision numbers 2020‐01620 and 2020‐01881). Trial registration number is as follows: ClinicalTrials.gov NCT04411576. All methods were carried out in compliance with the Helsinki declaration.
As described in a parallel paper [13], we assembled a reference group of 3671 hospital employees who had no patient contact whatsoever. They were simultaneously enrolled at the Karolinska University Hospital, also in Stockholm, Sweden. The hospital employees without patient contact were, for example, working with administration, economy, information technology, engineering, research and innovation, or at the hospital laboratories. As hospital employees are very motivated to participate (92% participated in the parallel study) [13], we considered that the use of hospital employees without patient contact would be a rapid strategy to obtain a large reference group with minimum selection bias because of nonparticipation that could be considered as approximately representative of the underlying degree of SARS‐CoV‐2 exposure in the region.
SARS‐CoV‐2 nucleic acid detection
The participants were requested to give a throat swab sample as described in the user manual (Beaver Biomedical Engineering: stratech.co.uk/wp‐content/uploads/2020/04/BEAVER‐IFU‐43903‐Sample‐Collection‐Kit19324.pdf). The samples were inactivated by incubation at 75 degrees for 50 minutes. The viral RNA was extracted according to the manufacturer’s protocol by performing MGISP‐960 automated extraction standard workflow with MGIEasy Magnetic Beads Virus DNA/RNA Extraction Kit (Wuhan MGI Tech Co, Ltd). Real‐time polymerase chain reaction was performed on QuantStudio 5 instruments and software (Design and Analysis Software v1.5.1; Thermo Scientific), using the BGI 2019‐nCoV Detection Kit (BGI Real‐Time RT‐PCR for detecting 2019 nCoV) according to the manufacturer’s protocol along with internal parameters for testing process and sampling quality. Every step of the laboratory work followed validated standard operating protocols for reproducibility, sensitivity, specificity and lack of cross‐reactivity with other strains of coronavirus. For the sample preparations, the safety routines according to BSL2 requirements were followed. Results of the PCR tests were classified as positive, negative or inconclusive/censored.
Serological analysis of antibodies for SARS‐CoV‐2
Blood was collected in tubes containing serum separating gel and centrifuged at 2000 g for 10 minutes at room temperature to collect the serum. To inactivate the samples, they were incubated at 56 degrees for 30 minutes before being stored at minus 20 degrees until further analysis. Sera were analysed against three different variants of viral proteins, which included spike trimers that contained prefusion‐stabilized spike glycoprotein ectodomain [14], spike S1 domain, and nucleocapsid protein.
The serum samples were analysed with FLEXMAP 3D instruments (Luminex Corp) using a multiplex antigen bead array in a high‐throughput 384‐plate format [15]. Samples were assigned IgG‐positive if the sera were reactive against at least two of the three viral antigens. For each antigen, the cut‐off for seropositivity was defined as mean + 6SD of 12 negative control samples that were included in each analysis run.
The serology assay was evaluated based on the analyses of 243 samples from COVID‐19 convalescents (defined as PCR‐positive individuals sampled more than 16 days after positive PCR test) and 442 negative samples (defined as samples collected 2019 or earlier in the same region, including 26 individuals with confirmed infections of other coronaviruses than SARS‐CoV‐2). The assay had a 99.2% sensitivity and 99.8% specificity based on these samples.
Results are presented as descriptive statistics examining the prevalence of antibodies and viral infection by home care service company.
Results
A total of 438 employees in five home care service companies for older persons were invited, and 405 employees (92.5%) were enrolled in the study. The participants provided 387 swab throat samples and 403 serum samples between 11 May and 17 June 2020. In total, 375 participants provided both swab and blood samples with valid results (Fig. 1). The mean age of the participants was 43 years. The median age ranged between 32 and 44 at the different home care companies (Table 1).
Fig. 1.
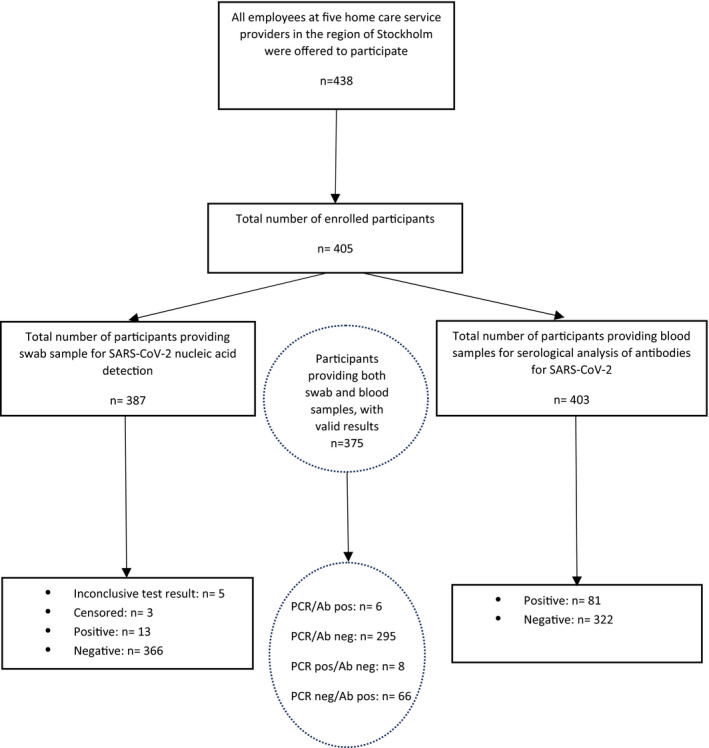
Flow chart summarizing the screening of care personnel for older persons for nucleic acid detection and serological analysis of antibodies for SARS‐CoV‐2. Results of the PCR test were classified as positive, negative or inconclusive/censored. Results were censored if a repeat sample had been taken and the results differed. Inconclusive means that the test result was ambiguous. PCR = polymerase chain reaction, Ab = antibodies.
SARS‐CoV‐2 nucleic acid detection in care personnel for older persons
The overall prevalence of confirmed SARS‐CoV‐2 by real‐time PCR was 3.4% (13/379 participants), with 2 out of 5 companies having no PCR confirmed case of SARS‐CoV‐2 at all (Table 2). The prevalence of confirmed PCR positivity was higher amongst men (6.1%, n = 11) compared with women (n = 2) (Table 2).
Table 2.
Results of SARS‐CoV‐2 PCR and serology testing from employees at home care service companies for older persons
| Home care service company | SARS‐CoV‐2 PCR test results | SARS‐CoV‐2 serology test results | ||||
|---|---|---|---|---|---|---|
| Positive | Prevalence (%) | Total | Positive | Prevalence (%) | Total | |
| Hemtjänst 24 Omsorg | 3 | 11.5 | 26 | 14 | 50 | 28 |
| Hägersten‐Liljeholmen‐Älvsjö Hemtjänst | 9 | 3.5 | 257 | 50 | 18.5 | 270 |
| Roo Hemtjänst AB | 1 | 1.6 | 63 | 12 | 17.9 | 67 |
| Saras Omsorg | 0 | 0 | 16 | 0 | 0 | 18 |
| Vardaga Hemtjänst | 0 | 0 | 17 | 5 | 25 | 20 |
| Total women | 2 | 1 | 199 | 37 | 17.5 | 212 |
| Total men | 11 | 6.1 | 180 | 44 | 23 | 191 |
| Total | 13 | 3.4 | 379 | 81 | 20.1 | 403 |
Serological analysis of antibodies for SARS‐CoV‐2
The total seroprevalence amongst the care personnel for older persons was 20.1% (81/403 employees were seropositive). One home care service company for older persons had 14/28 employees showing antibody positivity to SARS‐CoV‐2, but another home service company did not have any seropositive employees at all (Table 2).
By comparison, the simultaneously enrolled reference group of employees without patient contact (n = 3671) had a 9.7% seropositivity rate when tested by exactly the same serum test (OR for seropositivity when employed in home care services compared with the reference group: 2.3 (95% CI: 1.8–3.1), P < 0.0001 [13]).
Amongst the 13 SARS‐CoV‐2 PCR‐positive home services employees, eight were negative for SARS‐CoV‐2 antibodies. Five of these participants had cycle threshold (Ct) values lower than 27, indicating a strong positivity for SARS‐CoV‐2 (data not shown).
Discussion
We find that personnel working in companies providing home services for older persons are a risk group for SARS‐CoV‐2 infection, with an about twice as high risk of seropositivity as the reference group. The comparatively high seroprevalence in combination with a rather low proportion of PCR‐positive subjects in our study suggests that most of the first wave of SARS‐CoV‐2 infections had already started to subside when the study was performed. This is well in line with the statistics showing a sharp peak of COVID‐19‐related death in the region in early April [16]. Indeed, a substantial proportion of PCR‐positive subjects either were seropositive or had very low amounts of virus (signs of a previous infection or a lingering infection after resolution of symptoms). The large variation in seroprevalences between different home service companies is in line with the known propensity for COVID‐19 to primarily spread in clusters.
A total of five individuals (5/405) had the typical presymptomatic pattern of being PCR‐positive with high amounts of virus in combination with lack of antibodies. Presymptomatic subjects are potential ‘super spreaders’ [17]. We thus find that in a rather large cohort of home service employees there existed 1.2% of potentially superspreading individuals (five individuals, a low number). The point estimate of the proportion potentially contagious (1.3%) was actually higher than at the Karolinska University Hospital, where 57/9449 (0.6%) employees had a similar profile [18]. These were easily identified by the screening, and the fact that they were identified amongst subjects who serve multiple older clients suggests that such identification of potential superspreaders could be considered.
A strength of our study is that it was performed early on, at a time when large‐scale testing was not available in Sweden and that we exploited high performance assays in experienced academic laboratories. Although we find that the first wave of the epidemic was already declining in the region, there was still evidence of actively ongoing transmission at that time. Furthermore, a large‐scale reference group without any contact with patients could be assembled at the same time and used for comparison.
The Swedish Public Health Agency performed a weekly serosurvey in primary care and amongst blood donors in the Stockholm region. During the time of this study, the seroprevalences in blood donors varied between 8 and 11.4%, but with wide confidence intervals (from 2% to 21%) [19], whereas the seroprevalences amongst adults in primary care varied between 8.7% and 11.6% (95% confidence limits ranging from 5.7% to 15.9%). These data are therefore in good agreement with the seroprevalences in our reference cohort (9.87%). There is also an analysis of determinants of seropositivity, which found that professions involving meeting many individuals were risk groups [20].
Weaknesses of our study include the fact that only 5 companies providing home care services participated in the study. There are 12 times as many companies providing home care services in the region, and the companies that participated were not selected at random, but participation was decided on a first‐come, first‐serve basis (only a minority of the companies had decided to participate when the start date of the study was imminent). Also, the older persons who received the home care services were not enrolled, limiting our ability to document whether services provided by infectious home services personnel may have transmitted infections to the users. We did not enrol the older persons because of issues regarding complexity in obtaining a fully informed and coercion‐free consent and issues regarding requirements for documentation of testing results of the users and issues that were not readily resolved.
Our findings on the seropositivity amongst home care service employees are similar to another study [10], implying that there are now independent studies pointing to that this is a risk group for SARS‐CoV‐2 infection.
Conclusion
In summary, we find that employees in home care services were a risk group for contracting SARS‐CoV‐2 infection, as evidenced by a higher seroprevalence compared with the reference group. We also find that there were only a few employees with the typical testing result of presymptomatic subjects, who may be potential ‘super spreaders’.
Increased attention for protection and screening of personnel as well as of the older persons they serve is warranted.
Conflict of interest
All authors declare no conflict of interest.
Acknowledgements
The authors would like to thank Camilla Lagheden, Carina Eklund, Suyesh Amatya, Eni Andersson, Helena Andersson, Sofia Bergström, Emine Eken, Pedram Farsi, Cecilia Hellström, Yasmin Hussein, Roxana Merino Martinez, Jennie Olofsson, Björn Pfeifer, Ulla Rudsander, Balazs Szakos, Hanna Tegel and Emel Yilmaz for excellent technical assistance. This research was supported by the County Council of Stockholm. The paper was made available at the preprint server medRXiv.org concomitantly with submission to Journal of Internal Medicine.
Hassan SS, Seigerud Å, Abdirahman R, Arroyo Mühr LS, Nordqvist Kleppe S, Pin E, Månberg A, Hober S, Nilsson P, Engstrand L, Miriam Elfström K, Blomqvist J, Conneryd Lundgren K, Dillner J (Karolinska Institutet, Stockholm; Roo Home Services, Stockholm; KTH Royal Institute of Technology, Stockholm; Science for Life Laboratory, Stockholm; and Karolinska University Hospital, Stockholm, Sweden). SARS‐CoV‐2 infections amongst personnel providing home care services for older persons in Stockholm, Sweden (Brief Report). J Intern Med 2021; 290: 430–436. 10.1111/joim.13274
References
- 1. Lipsitch M, Swerdlow DL, Finelli L. Defining the epidemiology of covid‐19 ‐ studies needed. N Engl J Med. 2020;382(13):1194–6. [DOI] [PubMed] [Google Scholar]
- 2. Guarner J. Three emerging coronaviruses in two decades. Am J Clin Pathol. 2020;153(4):420–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. World Health Organization . COVID‐19 Weekly Epidemiological Update. [cited 2020 Nov 23]. Available from: https://www.who.int/publications/m/item/weekly‐epidemiological‐update‐‐‐1‐december‐2020.
- 4. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Verity R, Okell LC, Dorigatti I, Winskill P, Whittaker C, Imai N, et al. Estimates of the severity of coronavirus disease 2019: a model‐based analysis. Lancet Infect Dis. 2020;20(6):669–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: Summary of a report of 72314 cases from the chinese center for disease control and prevention. JAMA. 2020;323(13):1239–42. [DOI] [PubMed] [Google Scholar]
- 7. Ghosal S, Bhattacharyya R, Majumder M. Impact of complete lockdown on total infection and death rates: A hierarchical cluster analysis. Diabetes Metab Syndr. 2020;14(4):707–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. The Publich Health Agency of Sweden . Decisions and guidelines in the Ministry of Health and Social Affairs’ policy areas to limit the spread of the COVID‐19 virus. 2020. [cited 2020 Nov 23]. Available from: https://www.government.se/articles/2020/04/s‐decisions‐and‐guidelines‐in‐the‐ministry‐of‐health‐and‐social‐affairs‐policy‐areas‐to‐limit‐the‐spread‐of‐the‐covid‐19‐virusny‐sida/.
- 9. Ladhani SN, Jeffery‐Smith A, Patel M, Janarthanan R, Fok J, Crawley‐Boevey E, et al. High prevalence of SARS‐CoV‐2 antibodies in care homes affected by COVID‐19: Prospective cohort study, England. EClinicalMedicine. 2020;28:100597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lindahl JF, Hoffman T, Esmaeilzadeh M, Olsen B, Winter R, Amer S, et al. High seroprevalence of SARS‐CoV‐2 in elderly care employees in Sweden. Infect Ecol Epidemiol. 2020;10(1):1789036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. The Swedish National Board of Health and Welfare . 2019. [cited 2020 Nov 23]. Available from: https://wwwsocialstyrelsense/globalassets/sharepoint‐dokument/artikelkatalog/statistik/2019‐5‐7pdf#:~:text=De%20vanligaste%20insatserna%20enligt%20socialtj%C3%A4nstlagen%20till%20personer%2065,%C3%A5r%20Drygt%20321%20000%20%C3%A4ldre%20personer%20har%20socialtj%C3%A4nstinsatser.
- 12. The Swedish National Board of Health and Welfare . 2019. [cited 2020 Nov 23]. Available from: https://www.socialstyrelsen.se/globalassets/1‐globalt/covid‐19‐statistik/engelska‐sidan/faktablad‐statistik‐covid‐19‐bland‐aldre‐eng.pdf.
- 13. Dillner J, Elfström MK, Blomqvist J, Eklund C, Lagheden C, Nordqvist‐Kleppe S, et al. Antibodies to SARS‐CoV‐2 and risk of future sickness. medRxiv. 2020. 10.1101/2020.09.14.20194308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, et al. Cryo‐EM structure of the 2019‐nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rudberg AS, Havervall S, Manberg A, Jernbom Falk A, Aguilera K, Ng H, et al. SARS‐CoV‐2 exposure, symptoms and seroprevalence in healthcare workers in Sweden. Nat Commun. 2020;11(1):5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pimenoff VN, Elfstrom M, Baussano I, Bjornstedt M, Dillner J. Estimating total excess mortality during a COVID‐19 outbreak in Stockholm, Sweden. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arons MM, Hatfield KM, Reddy SC, Kimball A, James A, Jacobs JR, et al. Presymptomatic SARS‐CoV‐2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382(22):2081–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dillner J, Elfström KM, Blomqvist J, Engstrand L, Uhlén M, Eklund C, et al. Screening for high amounts of SARS‐CoV‐2 identifies pre‐symptomatic subjects among healthy healthcare workers. medRxiv. 2020. 10.1101/2020.12.13.20248122. [DOI] [Google Scholar]
- 19. The Publich Health Agency of Sweden . 2019. [cited 2021 Jan 25]. Available from: https://wwwfolkhalsomyndighetense/publicerat‐material/publikationsarkiv/p/pavisning‐av‐antikroppar‐efter‐genomgangen‐covid‐19‐hos‐blodgivare‐delrapport‐2/.
- 20. The Publich Health Agency of Sweden . 2019. [cited 2021 Jan 25]. Available from: https://wwwfolkhalsomyndighetense/publicerat‐material/publikationsarkiv/f/forekomsten‐av‐antikroppar‐mot‐sars‐cov‐2‐i‐stadsdelen‐rinkeby‐kista‐stockholm‐2224‐juni‐2020/.


