Abstract
Background
ABO blood groups have been linked to susceptibility to infection with certain microorganisms, including coronaviruses. We examined the relationship between blood group and clinical outcomes in individuals infected with severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) and compared their blood group distribution with the general population.
Methods
At the inception of the pandemic, all individuals testing positive for SARS‐CoV‐2 in Kuwait were admitted to one designated coronavirus disease 2019 (COVID‐19) hospital and enrolled in a prospective registry. Patients admitted from February 24 to May 27, 2020, were stratified according to blood group. As a control, blood groups of 3,730,027 anonymized individuals representing almost Kuwait's entire population were obtained from a national database.
Results
Of 3305 SARS‐CoV‐2–positive patients, 37.1%, 25.5%, 28.9%, and 8.5% were groups O, A, B, and AB, respectively. Univariate analysis revealed no significant differences in severe clinical outcomes or death among the blood groups. However, multivariable analysis demonstrated that group A individuals had higher odds of developing pneumonia compared with non–group A (adjusted odds ratio 1.32, 95% confidence interval 1.02–1.72, p < .036). Compared with the general population, the COVID‐19 cohort had a lower frequency of group O, equivalent frequency of A, and higher frequency of B and AB. No significant difference in the RhD group was found.
Conclusion
This study supports potential involvement of the ABO blood group system in predisposing to infection with SARS‐CoV‐2 in an unselected population. Examination of the mechanistic link between blood group and COVID‐19 and its implications on controlling the current pandemic is warranted.
Keywords: ABO blood groups, coronavirus, COVID‐19, pneumonia, RhD antigen, SARS‐CoV‐2, severe COVID‐19 disease
1. INTRODUCTION
The factors determining inherent susceptibility to infection with the novel severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) and the risk of severe outcomes in infected individuals are poorly understood. Although certain clinical associations including advanced age, male sex, pulmonary disease, cardiovascular disease, obesity, and diabetes are now well established, 1 , 2 , 3 the mechanistic underpinnings of the varying effects of coronavirus disease 2019 (COVID‐19) on the global population remain intensely debated. 4 One hypothesis implicated ABO blood groups as a contributor to COVID‐19 susceptibility and severity. Differences in ABO blood groups can modify host susceptibility to infections through the interaction of the carbohydrate moieties defining ABO subgroups with microorganisms and the immune system. Numerous microorganisms have been implicated. However, the most robust evidence to support the ABO blood group's ability to modulate host infection risk or severity was found in infections with Plasmodium falciparum, Helicobacter pylori, and norovirus. 5 This relationship was extended to coronaviruses when a study of healthcare staff exposed to the index case of the 2003 SARS‐CoV‐1 outbreak demonstrated that group O participants were less likely to be infected than non–group O. 6
The potential link between ABO blood group and COVID‐19 received widespread media attention when a commercial genome‐profiling company issued a press release during the current pandemic reporting preliminary data from their genetic study of 750,000 participants associating blood group O with decreased susceptibility to COVID‐19. 7 Several studies 8 , 9 , 10 , 11 , 12 , 13 addressed this hypothesis using selected cohorts and controls subject to potential sampling bias. Nevertheless, they suggest a protective effect for blood group O, but data regarding a positive correlation between blood group A and increased risk of infection or severe clinical outcomes are conflicting. Further studies in large, unselected populations are promptly required, especially with the emergence of recent reports that contest the relationship between blood group and COVID‐19. 14 , 15 A better understanding of this relationship may further explain the mechanisms underlying vulnerability to severe infection.
Kuwait offered a unique population to study the association between blood group and COVID‐19. When neighboring Iran was declared as one of the early epicenters of COVID‐19 international dissemination at the beginnings of the COVID‐19 pandemic in February 2020, Kuwait adopted a broad testing strategy prior to diagnosing its index case on February 24, 2020. Mass mandatory testing of all returning travelers was implemented. With the subsequent rise of locally transmitted cases, inhabitants of high‐risk residential areas and all contacts of positive patients were also tested. 16 All SARS‐CoV‐2–positive individuals were admitted to the nationally designated COVID‐19 hospital, irrespective of symptoms or disease severity, as a method of quarantine at the beginning of the pandemic when the number of cases was low enough for them to be contained within one site. This provided an unselected cohort of individuals infected with SARS‐CoV‐2 in a single facility that can be studied. Clinical characteristics and outcomes were compiled in a prospective, national COVID‐19 registry. 16 Furthermore, blood group testing is mandated for all individuals on issuing a national identity card, which is required of all Kuwaiti citizens and resident expatriates. Thus, blood group data for 3.7 million people were obtainable from a national population database, serving as a general population control. As a result, Kuwait's population is ideally suited for investigating the relationship between blood group and COVID‐19. The aims of this study were to (1) examine the blood group distribution in our COVID‐19 cohort, (2) investigate the relationship between blood group and clinical outcomes, including severe COVID‐19 in infected individuals, and (3) compare the blood group frequencies in the COVID‐19 cohort to the general population.
2. METHODS
2.1. Study population and data source
Since the diagnosis of the first SARS‐CoV‐2–positive case in Kuwait on February 24, 2020, all patients testing positive for SARS‐CoV‐2 had been admitted to Jaber Al‐Ahmad Al‐Sabah Hospital (COVID‐19 hospital), Kuwait's nationally designated COVID‐19 medical center, as a method of quarantine. At the pandemic's onset, the Kuwait Ministry of Health rapidly repurposed this new, fully operational, 1114 bed hospital, which was at 20% occupancy due to its limited catchment area, into a medical facility that can hold large numbers of SARS‐CoV‐2–positive patients. Individuals were quarantined until they had two negative SARS‐CoV‐2 nasopharyngeal swabs over 48 h, which occurred within approximately 14 days in most cases. This practice was maintained until May 12, 2020, when the number of cases was low enough to be contained within one site, after which the hospital admission and SARS‐CoV‐2 testing criteria were revised to accommodate for the rising number of cases. Subsequently, patients requiring admission were accepted in other medical facilities or field hospitals.
All SARS‐CoV‐2–positive individuals admitted to the COVID‐19 hospital were enrolled in a prospective COVID‐19 registry. Demographic, clinical, and laboratory information on presentation and clinical outcomes during the patients' hospitalization were obtained by manual review of the medical records. Patients admitted between February 24 and May 27, 2020, were included in this study. The study's duration included 15 additional days after the admission policy had changed on May 12 because the majority of SARS‐CoV‐2–positive individuals were still captured within the COVID‐19 hospital population until May 27.
Ethical approval was obtained from the Kuwait Ministry of Health Ethical Review Board (approval number: 2020/1402), which waived the requirement for patient consent due to the retrospective, noninterventional nature of the study.
2.2. Blood group data
ABO and Rh antigen D (RhD) blood groups were obtained from the patient's national identity card, which were previously determined by serological testing in a Ministry of Health–certified laboratory, as a prerequisite before issuing the card. General population blood group data were available from the Kuwait Public Authority for Civil Information national database. An anonymized summary of the distribution of blood groups for residents living in Kuwait was obtained and used as a general population control. Kuwait's population consists of Kuwaiti citizens (Kuwaitis) and resident expatriates of other nationalities listed in Table S1 (non‐Kuwaitis).
2.3. SARS‐CoV‐2 test
SARS‐CoV‐2 testing was performed by reverse transcriptase‐polymerase chain reaction of a nasopharyngeal swab specimen using a commercially available testing kit (FLOQSwab sample collection kit by COPAN Diagnostics™ Murrieta, USA, ref: A305CS01). The protocol employed in our cohort was described recently. 17
2.4. Clinical outcomes and definitions
The frequency of selected clinical outcomes was compared among blood groups (complete list in Supplementary Methods S1). Severe COVID‐19 referred to patients requiring any method of respiratory support including supplemental oxygen, intubation with mechanical ventilation, or extracorporeal life support, as defined by Ellinghaus et al. 8 This accepted pragmatic definition was used to facilitate the comparison of disease severity among other study cohorts examining different populations. All patients requiring supplemental oxygen had a baseline oxygen saturation of less than or equal to 93% on room air at sea level. Pneumonia was defined per the American Thoracic Society and Infectious Diseases Society of America clinical practice guidelines. 18 Septic shock was defined using the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis‐3). 19 Acute respiratory distress syndrome was defined per the World Health Organization interim guidance: “clinical management of severe acute respiratory infection when COVID‐19 is suspected.” 20 Acute kidney injury was defined according to the Kidney Disease Improving Global Outcomes clinical practice guidelines. 21
2.5. Statistical analysis
A descriptive analysis was performed where parametric data were reported as mean and standard deviation, and nonparametric data were reported as median and range. Unless otherwise specified, comparisons across all blood groups were performed using the appropriate statistical test based on the aforementioned clinical outcomes. The following tests were used: Chi‐square for categorical variables, and one‐way analysis of variance or Kruskal–Wallis for continuous parametric or nonparametric variables, respectively. Normality was assessed using Kolmogorov–Smirnov and Shapiro–Wilk's tests. Multivariate logistic regression analysis was performed to determine relationships between the variables of interest and clinical outcomes. Selected variables included ones that had significant differences among the blood groups on univariate analysis of clinical or biologic relevance, further supplemented by specific variables without significant differences on univariate analysis but were identified as clinically relevant based on the literature. 2 A two‐tailed p‐value of <.05 was considered significant. The analysis was performed using SPSS IBM, version 26 (Armonk, New York, IBM Corp.).
3. RESULTS
3.1. ABO blood group distribution and baseline characteristics of the COVID‐19 cohort
A total of 3305 patients who were admitted to the COVID‐19 hospital during the study period were included. ABO and RhD blood group data were available for all patients; 1225 (37.1%), 843 (25.5%), 956 (28.9%), and 281 (8.5%) were groups O, A, B, and AB, respectively. Mean age was 42 years, 1017 (30.8%) were female, and 1590 (48.1%) were Kuwaiti, with blood group distribution according to nationality shown in Table S1. Of 1872 (56.7%) patients with available body mass index measurements, 573 (31%) were obese. The proportion of obese patients was comparable to that of Kuwait's general population. 22 The most common comorbidities were diabetes (n = 663, 20.1%) followed by hypertension (n = 709, 21.5%), similar across all the different blood groups. Univariate analysis demonstrated that of all the baseline characteristics, sex and nationality were the only variables that differed significantly across the blood groups (p < .009 and p < .001, respectively) (Table 1).
TABLE 1.
Baseline clinical characteristics of COVID‐19 patients on presentation stratified by ABO blood group
| Total | O | A | B | AB | p‐value a | |
|---|---|---|---|---|---|---|
| (n = 3305) | (n = 1225, 37.1%) | (n = 843, 25.5%) | (n = 956, 28.9%) | (n = 281, 8.5%) | ||
| Gender | <.009 | |||||
| Male, n (%) | 2288 (69.2) | 807 (65.9) | 589 (69.96) | 692 (72.4) | 200 (71.2) | |
| Female, n (%) | 1017 (30.8) | 418 (34.1) | 254 (30.1) | 264 (27.6) | 81 (28.8) | |
| Age, years, mean (±SD) | 42.4 (±17.2) | 42.1 (±17.9) | 42.8 (±17.2) | 42.2 (±16.7) | 42.3(±16.3) | .822 |
| Nationality, | <.001 | |||||
| Kuwaiti, n (%) | 1590 (48.1) | 673 (54.9) | 396 (47.0) | 423 (44.2) | 98 (34.9) | |
| Non‐Kuwaiti, n (%) | 1715 (51.9) | 552 (45.1) | 447 (53.0) | 533 (55.8) | 183 (65.1) | |
| Body mass index, kg/m2 | ||||||
| Evaluable, n (%) | 1872 (56.7) | 693 (56.4) | 490 (58.2) | 531 (55.6) | 158 (56.3) | .526 |
| <30 non‐obese, n (%) | 1299 (39.3) | 458 (37.3) | 339 (40.2) | 383 (40.1) | 119 (42.4) | |
| ≥30 obese, n (%) | 573 (17.4) | 235 (19.1) | 151 (18.0) | 148 (15.5) | 39 (13.9) | |
| Symptoms b , n (%) | ||||||
| None | 1130 (34.2) | 449 (36.7) | 256 (30.4) | 331 (34.6) | 94 (33.5) | <.030 |
| Chills | 1243 (37.6) | 445 (36.3) | 324 (38.4) | 372 (38.9) | 102 (36.3) | .572 |
| Cough | 1270 (38.4) | 447 (36.5) | 353 (41.9) | 359 (37.6) | 111 (39.5) | .085 |
| Sputum production | 63 (1.9) | 16 (1.3) | 29 (3.4) | 15 (1.6) | 3 (1.1) | <.002 |
| Shortness of breath | 318 (9.6) | 119 (9.7) | 78 (9.3) | 91 (9.5) | 30 (10.7) | .916 |
| Comorbidities n (%) | ||||||
| Diabetes | 663 (20.1) | 238 (19.4) | 180 (21.4) | 195 (20.4) | 50 (17.8) | .542 |
| Hypertension | 709 (21.5) | 270 (22.0) | 195 (23.1) | 191 (20.0) | 53 (18.9) | .26 |
| Coronary artery disease | 153 (4.6) | 66 (5.4) | 35 (4.2) | 41 (4.3) | 11 (3.9) | .456 |
| Chronic obstructive | 15 (0.5) | 9 (0.7) | 0 (0.0) | 4 (0.4) | 2 (0.7) | .093 |
| Pulmonary disease | ||||||
| Asthma | 213 (6.4) | 85 (6.9) | 49 (5.8) | 62 (6.5) | 17 (6.0) | .77 |
| Cancer | 54 (1.6) | 25 (2.0) | 16 (1.9) | 11 (1.2) | 2 (0.7) | .212 |
| Chronic kidney disease | 64 (1.9) | 31 (2.5) | 18 (2.1) | 11 (1.2) | 4 (1.4) | .114 |
| Vital Signs, mean (±SD) | ||||||
| Heart rate, beats per minute | 88.1 (±15) | 88 (±18) | 88 (±15) | 88 (±14) | 88 (±16) | .748 |
| Blood pressure (mmHg) | ||||||
| Systolic | 126 (±17) | 126 (±17) | 126 (±17) | 127 (±17) | 126 (±16) | .574 |
| Diastolic | 77 (±10) | 77 (±10) | 77 (±10) | 78 (±10) | 78 (±10) | .788 |
| Temperature, °C | 37.0 (±0.5) | 37.0 (±0.5) | 37.0(±0.5) | 37.0 (±0.5) | 37.0 (±0.4) | .252 |
| Abnormal chest X‐ray n (%) | 1069 (32.3) | 380 (31.0) | 281 (33.3) | 325 (34.0) | 83 (29.5) | .313 |
| Laboratory values mean (±SD) | ||||||
| White blood cells, 109/L | 6.5 (±2.9) | 6.7 (±2.9) | 6.3 (±2.7) | 6.5 (±2.9) | 6.8 (±3.4) | <.011 |
| Neutrophil, % | 58.2 (±15.6) | 57.8 (±15.7) | 57.5 (±16.3) | 59.2 (±15.1) | 58.2 (±15.0) | .104 |
| Lymphocytes, % | 30.8 (±13.9) | 31.0 (±13.9) | 31.4 (±14.7) | 29.8 (±13.1) | 31.4 (±13.7) | .065 |
| Hemoglobin, g/L | 135 (±19) | 135 (±19) | 135 (±19) | 136 (±19) | 135 (±19) | .17 |
| Platelets, 109/L | 249.3 (±89.0) | 254.0 (±83.7) | 244.5 (±95.3) | 245.6 (±87.2) | 259.3 (±95.8) | <.007 |
| Prothrombin time, seconds | 13.5 (±2.1) | 13.6 (±2.5) | 13.6 (±2.3) | 13.4 (±1.4) | 13.5 (±1.4) | .703 |
| Activated partial thromboplastin time, seconds | 32.2 (±5.0) | 32.8 (±5.2) | 32.5 (±5.0) | 31.5 (±4.3) | 31.7 (±5.6) | <.001 |
| C‐reactive protein, mg/L | 34.3 (±67.5) | 33.2 (±68.6) | 38.5 (±78.4) | 33.7 (±62.6) | 28.2 (±40.0) | .311 |
| Procalcitonin ng/ml | 2.5 (±78.2) | 5.4 (±129.0) | 0.9 (±10.3) | 0.7 (±6.2) | 0.5 (±2.3) | .621 |
| Glucose, mmol/l | 7.1 (±12.6) | 7.3 (±20.4) | 7.0 (±3.6) | 6.9 (±3.5) | 7.1 (±3.6) | .884 |
| Estimated glomerular filtration rate, ml/min/1.73m2 | 97.6 (±25.1) | 97.6 (±26.0) | 96.2 (±26.0) | 98.7 (±23.4) | 98.4 (±23.6) | .242 |
| Albumin, g/L | 38.4 (±71.6) | 40.6 (±117.3) | 37.2 (±12.2) | 37.0 (±6.4) | 37.2 (±6.7) | .593 |
| Bilirubin, (μmol/L) | ||||||
| Total | 13.0 (±9.9) | 13.1 (±8.5) | 13.3 (±14.6) | 12.5 (±6.8) | 12.8 (±7.1) | .408 |
| Direct | 3.1 (±9.3) | 3.4 (±14.0) | 3.0 (±6.4) | 2.8 (±3.2) | 2.9 (±3.2) | .538 |
| Alkaline phosphatase, U/L | 74.9 (±45.8) | 77.5 (±50.4) | 70.7 (±40.4) | 74.7 (±41.3) | 77.1 (±54.0) | <.010 |
| Alanine aminotransferase, U/L | 35.9 (±97.2) | 37.5 (±149.0) | 35.3 (±42.1) | 34.7 (±52.0) | 34.4 (±28.2) | .902 |
Abbreviation: SD, standard deviation.
p‐value represents the result of a statistical comparison across all four blood groups for each outcome variable, using the statistical analyses described in the methods. p < .05 indicates a significant difference in the outcome variable among the blood groups.
Included the presence of any symptoms on presentation or up to 24 h within hospital admission.
A total of 1130 (34.2%) of SARS‐CoV‐2–positive patients were asymptomatic on presentation or within 24 h of hospital admission. A significant difference in the proportion of asymptomatic patients among the blood groups was found (p < .030), with the highest proportion in group O patients at 36.7%. Within symptomatic patients, sputum production was the only symptom that differed significantly among the blood groups (p < .002), with the highest frequency found in group A at 3.4%. No significant differences were found in presenting vital signs or chest X‐ray abnormalities on presentation across the different blood groups. Small but significant differences in specific presenting laboratory values were found: white blood cell count (p < .011), platelet count (p < .007), activated partial thromboplastin time (p < .001), and alkaline phosphatase (p < .010). The clinical significance of these differences is uncertain (Table 1).
3.2. Clinical outcomes of COVID‐19 patients stratified by ABO blood group
Among the blood groups, a significant difference was observed for the development of any adverse event (p < .006), with the highest frequency observed in group A at 26.2%. Similarly, pneumonia frequency was significantly different among the blood groups (p < .048) and highest in group A at 23.3%, consistent with the trend noted in sputum production. However, ABO blood groups were not associated with severe COVID‐19. The requirement for oxygen support (including noninvasive means, mechanical ventilation, or extracorporeal membrane oxygenation) was observed in 11.4% of the COVID‐19 cohort, without significant differences between patients by blood group (p = .160). In total, 261 (7.9%) patients were admitted to the intensive care unit (ICU) and 151 (4.6%) died. Neither outcome was found to be significantly associated with ABO blood groups (p = .621 and p = .799, respectively, Table 2).
TABLE 2.
Outcomes of COVID‐19 patients stratified by ABO blood group
| Total | O | A | B | AB | p‐value a | |
|---|---|---|---|---|---|---|
| (n = 1225, 37.1%) | (n = 843, 25.5%) | (n = 956, 28.9%) | (n = 281, 8.5%) | |||
| Adverse events, n (%) | ||||||
| Any b | 726 (22.0%) | 243 (19.8%) | 221 (26.2%) | 202 (21.1%) | 60 (21.4%) | <.006 |
| Septic shock | 75 (2.3%) | 32 (2.6%) | 17 (2.0%) | 20 (2.1%) | 6 (2.1%) | .789 |
| Acute respiratory distress syndrome | 207 (6.3%) | 75 (6.1%) | 55 (6.5%) | 60 (6.3%) | 17 (6.0%) | .984 |
| Acute kidney injury | 91 (2.8%) | 35 (2.9%) | 19 (2.3%) | 29 (3.0%) | 8 (2.8%) | .771 |
| Pneumonia | 661 (20.0%) | 225 (18.4%) | 196 (23.3%) | 186 (19.5%) | 54 (19.2%) | <.048 |
| Treatments, n (%) | ||||||
| Any c | 2068 (62.6%) | 738 (60.2%) | 535 (63.5%) | 624 (65.3%) | 171 (60.9%) | .092 |
| Oxygen/respiratory support d | 382 (11.4%) | 135 (11.0%) | 107 (12.7%) | 108 (11.3%) | 249 (11.4%) | .16 |
| Intubation | 130 (3.9%) | 47 (3.8%) | 31 (3.7%) | 38 (4.0%) | 14 (5.0%) | .802 |
| Vasopressors | 90 (2.7%) | 29 (2.7%) | 25 (3.0%) | 25 (2.6%) | 11 (3.9%) | .509 |
| Continuous renal replacement therapy | 36 (1.1%) | 15 (1.2%) | 8 (0.9%) | 12 (1.3%) | 1 (0.4%) | .571 |
| Admitted to ICU, | 261 (7.9%) | 99 (8.1%) | 70 (8.3%) | 73 (7.6%) | 19 (6.8%) | .841 |
| n (%) | ||||||
| ICU length of stay, days, mean (±SD) | 14.2 (±12.2) | 13.8 (±12.4) | 12.8 (±12.2) | 14.6 (±12.1) | 14.1 (±11.9) | .621 |
| Dead, n (%) | 151 (4.6%) | 58 (4.7%) | 42 (5.0%) | 40 (4.2%) | 11 (3.9%) | .799 |
Abbreviations: ICU, intensive care unit, SD, standard deviation.
p‐value represents the result of a statistical comparison across all four blood groups for each outcome variable, using the statistical analyses described in the methods. p < .05 indicates a significant difference in the outcome variable among the blood groups.
Definition of “Any adverse event” also included disseminated intravascular coagulation, rhabdomyolysis, myocarditis, myocardial infarction, stroke, encephalopathy, acute limb ischemia, and bowel ischemia.
Definition of “Any treatment” also included antimicrobials, anticoagulation, blood transfusion, convalescent plasma therapy, and immunomodulatory therapies including hydroxychloroquine, glucocorticoids, interferon, and intravenous immunoglobulin.
Oxygen/respiratory support encompassed patients requiring any method of respiratory support including supplemental oxygen, noninvasive ventilation, intubation with mechanical ventilation, or extracorporeal life support.
Multivariable analysis was performed to explore the relationship between ABO blood group and pneumonia, controlling for specific covariates that may cause potential confounding. These included clinically relevant variables with significant differences among blood groups on univariate analysis: sex and nationality. The analysis was further supplemented by variables putatively associated with more severe outcomes in COVID‐19: age > 50 years, obesity, diabetes mellitus, hypertension, cardiovascular disease, chronic obstructive pulmonary disease, and asthma. 2 Group A patients were found to have a higher risk of developing pneumonia, compared with non‐A blood groups with an adjusted odds ratio (OR) 1.324, 95% confidence interval (CI) 1.016–1.719 (p < .036). Other blood groups were not associated with an increased risk of pneumonia (Table 3).
TABLE 3.
Multivariable analysis a showing odd ratios for COVID‐19 patients developing pneumonia by blood group
| Blood group | Adjusted odds ratio | Lower CI b | Upper CI | p‐valuec |
|---|---|---|---|---|
| O | 0.9170 | 0.7120 | 1.177 | .4993 |
| A | 1.324 | 1.016 | 1.719 | <.0363 |
| B | 0.8533 | 0.6498 | 1.114 | .2486 |
| AB | 0.9327 | 0.589 | 1.434 | .7581 |
Adjusted for sex, nationality, age > 50, obesity, hypertension, diabetes mellitus, cardiovascular disease, chronic obstructive pulmonary disease, and asthma.
Denotes 95% confidence interval.
p < .05 denotes statistical significance.
3.3. Comparison of the ABO and RhD blood group distribution between COVID‐19 patients and the general population
Data regarding the blood group distribution were obtained for 3,730,027 people residing in Kuwait, representing 88% of the country's population of 4.2 million. 23 Blood group O was less frequently observed in the COVID‐19 hospital cohort (37.1%, CI = 35.4%–38.7%) compared with the general population (40.8%, CI = 40.8%–40.9%, p < .0001). Conversely, groups B (28.9%, CI = 27.4%–30.5%) and AB (8.5%, CI = 7.6%–9.5%) were observed more frequently in the COVID‐19 hospital cohort, compared to the general population (B: 26.6%, CI = 26.6%–26.6%, p < .0023, AB: 6.5%, CI = 6.5%–6.5%, p < .001). No significant difference in the proportion of group A individuals was found between the two populations (p = .4877) (Figure 1, Table S2). A similar relationship between the COVID‐19 hospital and general population with respect to ABO blood group frequencies was observed when the populations were divided into Kuwaiti nationals and non‐Kuwaitis (Figures S1 and S2, Tables S3 and S4) except for group B, the relationship of which was not found to be significant in the Kuwaiti population (Table S3).
FIGURE 1.
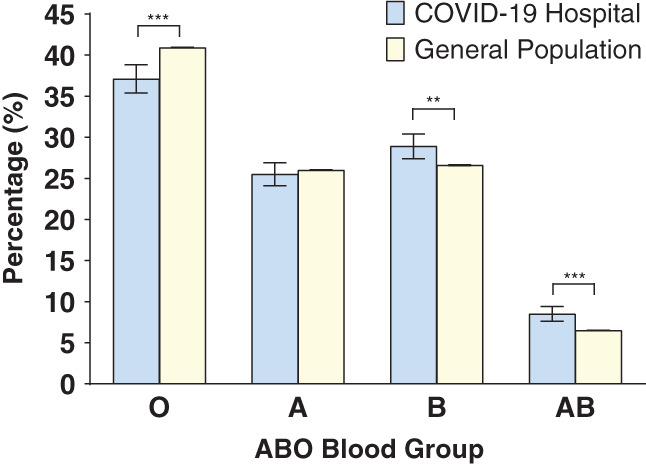
Comparison of ABO blood group distribution between the SARS‐CoV‐2–positive population in the designated COVID‐19 hospital (n = 3305) and Kuwait's general population (n = 3,730,037). The chi‐square test was used to compare the differences between each group. Error bars represent 95% confidence intervals. ***p < .0001 and **p < .005 [Color figure can be viewed at wileyonlinelibrary.com]
The ABO blood group distribution in the two populations was then examined with incorporation of RhD group. O+ represented a lower proportion in the COVID‐19 hospital population (34.8%, CI = 33.2%–36.4%), compared to the general population (38.2%, CI = 38.1%–38.2%) (p < .0001). B+, B‐ and AB+ were more frequently observed in the COVID‐19 hospital population (26.8%, CI = 25.3%–28.3%, 1.7%, CI = 1.7%–2.7%, 8.1%, CI = 7.2%–9.1%), compared to the general population (25.1%, CI = 25.0%–25.0% [p < .0268], 1.6%, CI = 1.6%–1.6% [p < .0040], 6.1%, CI = 6.1%–6.1% respectively, [p < .0001]). No significant differences were found between the COVID‐19 and general population in groups A+, A‐, O‐ and AB‐. (Figure 2(A), Table S5).
FIGURE 2.
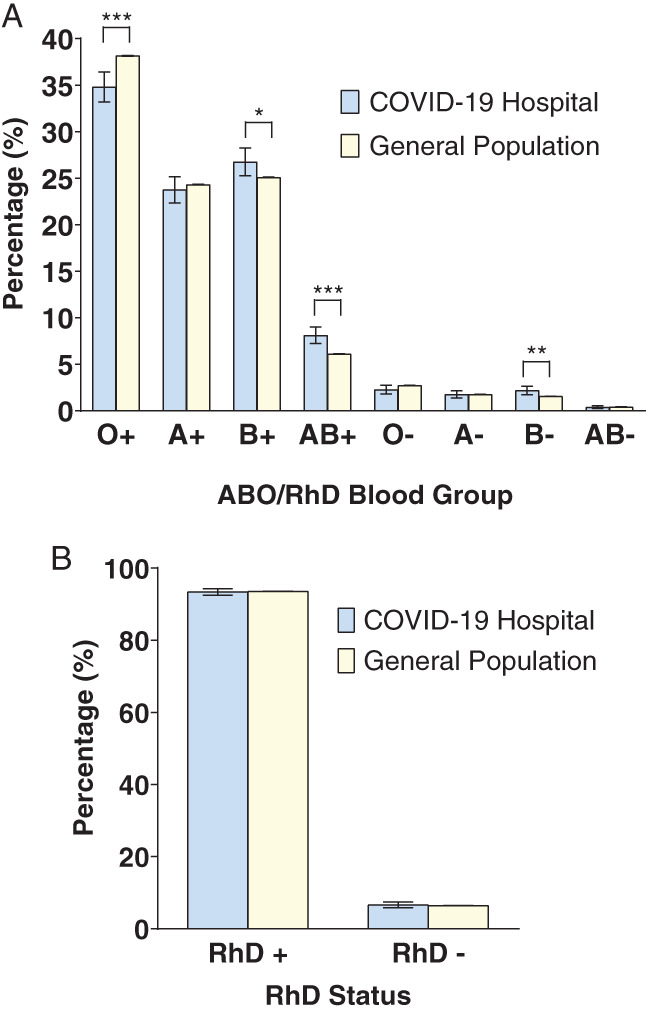
Comparison of blood group distribution between the SARS‐CoV‐2–positive COVID‐19 hospital cohort and the general population, with respect to both ABO and RhD status. (A) Distribution of ABO blood groups incorporating RhD status. (B) Distribution of RhD status alone. The chi‐square test was used to compare the differences between each group. Error bars represent 95% confidence intervals. ***p < .0001, **p < .005, and *p < .05 [Color figure can be viewed at wileyonlinelibrary.com]
Total RhD positive individuals were 93.4% (CI = 92.5%–94.2%) in the COVID‐19 hospital population and 93.6% (CI = 93.6%–93.6%) in the general population. The frequency of RhD negative subtype was low in both the COVID‐19 hospital and general populations at 6.6% (CI = 5.8%–7.5%) and 6.4% (CI = 6.4%–6.4%), respectively. No significant difference in RhD status was found between both populations (p = .9905 for RhD positive and p = .6904 for RhD negative) (Figure 2(B), Table S6).
4. DISCUSSION
This study was conducted to supplement the growing body of data evaluating the link between blood group and susceptibility to COVID‐19 in a large, unselected population. Several key findings emerged. First, in individuals testing positive for SARS‐CoV‐2, ABO blood group was not associated with severe clinical outcomes including mortality, intubation and ICU admission, but increased odds of developing pneumonia in group A was detected. Second, compared to Kuwait's general population, a lower prevalence of blood group O was found in the COVID‐19 cohort, whereas blood groups B and AB were more frequent in the COVID‐19 cohort. There was no significant difference in the prevalence of blood group A between the two groups. Third, no association between COVID‐19 and RhD status alone was detected.
Our data are consistent with the findings of Latz et al., who obtained a cohort of patients that underwent SARS‐CoV‐2 testing from a shared registry of hospital records in the United States and found no association between blood group and risk of intubation or death in patients with COVID‐19. 12 In contrast, Zhao et al. reported a higher risk of mortality in group A patients with COVID‐19 from three hospitals in China, 9 and Leaf et al. noted an increased proportion of critically ill COVID‐19 patients with blood group A, but this was limited to Caucasians only. 11 A genome‐wide association study of 1980 patients with severe COVID‐19 disease from the pandemic's European epicenter demonstrated a higher risk of respiratory failure in patients with blood group A genotypes compared with other blood groups. 8 One possible explanation for this difference is the lower proportion of severely ill patients in our cohort comprising 34.2% asymptomatic patients and 11.4% with severe COVID‐19 pragmatically defined by requiring supplemental oxygen or mechanical ventilation. 8 We hypothesize that the magnitude of the detrimental effect of group A on severe outcomes in COVID‐19 is relatively small and thus can only be detected with statistical significance in cohorts enriched for critically ill patients. The increased risk of pneumonia found on multivariable analysis in group A in our study may be an indication of this deleterious effect. Further studies of patients with severe COVID‐19 are required to confirm this hypothesis and establish whether ABO blood groups should be included in COVID‐19 risk stratification scores. 3 The mechanism underlying the potential difference in clinical outcomes among blood groups remains unknown. One hypothesis is that the higher levels of von Willebrand factor in non–group O individuals exacerbate pulmonary microvascular thrombosis, an increasingly recognized pathological consequence of COVID‐19 pneumonia. 24 , 25
The observation that infection with SARS coronaviruses is associated with a lower frequency of blood group O individuals is consistent across all studies examining the relationship between blood group and infection risk, 6 , 8 , 9 , 10 , 11 , 12 , 13 providing further credence to the notion that blood group O may have a protective effect against infection. One postulated mechanism is the ability of isohemagglutinins to block the interaction between coronavirus spike protein and the angiotensin‐converting enzyme‐2 receptor preventing virus attachment and entry, demonstrated in vitro with anti‐A. 26 However, the same phenomenon has not yet been shown with anti‐B. Although the underrepresentation of group O and overrepresentation of AB in our COVID‐19 cohort is congruent with this proposed mechanism, it is not supported by the increased prevalence of blood group B in COVID‐19 patients reported in this study and two others. 12 , 13 This suggests that the interaction between SARS‐CoV‐2 and anti‐A/B in vivo is complex and modulated by additional factors including antibody titers and secretor phenotype. Molecular investigation of this interaction may inform vaccine development and ongoing clinical trials of convalescent plasma as a therapeutic intervention for COVID‐19.
Unlike the ABO blood group, the association between the RhD group and predisposition to infection is less clear. One study reported a protective association between RhD‐negative individuals and SARS‐CoV‐2 infection and death after adjusting for ethnicity. 13 Two studies demonstrated a correlation between RhD‐positive status and an increased probability testing positive for SARS‐CoV‐2, 12 , 27 and that this correlation can be modulated by ethnicity. 27 The divergent relationships between RhD status and COVID‐19 reported may reflect the different populations studied or unaccounted for confounding variables. Notably, the proportion of RhD‐negative individuals in our population and the aforementioned studies 12 , 13 is less than 10%, which is inherent to the studied populations. Further studies in populations with a higher representation of RhD‐negative individuals, such as the Basques in Northern Spain, 28 can address this question more definitively.
Kuwait's population comprises Kuwaiti nationals and non‐Kuwaiti resident expatriates (38% vs. 62% in our population), with most expatriates from South Asian and other Arabic ethnicities. The association between blood group A and pneumonia maintained statistical significance after adjusting for nationality (Kuwaiti vs. non‐Kuwaiti) in the multivariable analysis, suggesting that this observation is consistent across different ethnicities. The relationships between blood groups and infection with SARS‐CoV‐2 remained consistent when stratified by nationality, except for the association between blood group B and the increased probability of infection, which was only significant in the non‐Kuwaiti population. This suggests that ethnicity‐related factors may modulate the association between blood group and infection risk. One example is blood group secretor status. This autosomal dominant trait is controlled by the FUT2 gene independent of ABO genotype. Secretor status varies by ethnicity, with only 22% of South Asians bearing the secretor phenotype compared with 80% of Caucasians. 5 Interestingly, a recent report showed that secretor status might influence clinical outcomes in COVID‐19. 29 Furthermore, ABO blood group distribution varies by ethnicity, partly due to selective genetic pressures exerted by malarial infection. 30 Compared with a Caucasian population, our cohort contained a lower frequency of group A individuals and a higher frequency of B, likely due to the higher prevalence of the B allele in the Asian subcontinent. 31 This study is the first to examine the relationship between blood group and COVID‐19 in Arabic and South Asian ethnicities, and more studies in different global populations will help elucidate the impact of ethnicity on this relationship.
Strengths of this study include a large, unselected cohort of infected individuals, all with documented blood groups, whereas the proportion of COVID‐19 patients with available blood group data in other studies was as low as 63%. 11 Moreover, a control group that included the majority of Kuwait's population with blood groups was used. This is in contrast to the control groups in other studies that used volunteer blood donors, 8 , 11 individuals testing negative for SARS‐CoV‐2 obtained from hospital records, or samples representative of the general population, 8 , 9 , 13 all of which subject to potential sampling bias.
Limitations of the study include its retrospective, correlative nature and the small proportion of patients with severe COVID‐19 compared with studies of hospitalized patients. 1 , 32 This is due to the widespread testing strategy implemented in Kuwait since the onset of the pandemic and hospitalization of all individuals testing positive irrespective of symptoms or disease severity. 16 As a result of this inclusive strategy, the prevalence of severe disease within our cohort is more comparable to that of general population studies 33 and is arguably more representative of true virulence. However, given the small fraction of patients with severe COVID‐19 requiring oxygen support in our cohort, it was not possible to further stratify patients by flow rate or administration route of oxygen as the low event rate precluded a meaningful subgroup analysis. Nevertheless, critically ill patients requiring intubation or intensive care were separately analyzed, with no significant blood group differences shown. Limitations in the control group include the possibility of contamination of the general population with SARS‐CoV‐2–positive patients who did not undergo testing. However, we predict that the number of unknown positive patients is low due to extensive testing and therefore represents a small fraction of the 3.7 million population unlikely to impact the overall results. Second, residual confounding due to the national database blood group data set lacking 12% of Kuwait's population cannot be excluded. The 12% unaccounted for in the national database likely represent individuals residing in Kuwait without a valid national identity card and consequently no registered blood group. Nevertheless, given the challenges of acquiring blood group data on a national level, our study still represents the largest data set examining the relationship between blood group and COVID‐19 to date.
In conclusion, we found no association between severe COVID‐19 and ABO blood group in a unique, unselected population but identified an increased risk of pneumonia in group A. We detected a lower prevalence of blood group O in individuals infected with SARS‐CoV‐2 and a higher prevalence of B and AB in agreement with studies in other populations. No association between SARS‐CoV‐2 infection rates with blood group A or RhD group was found. Further examination of the mechanistic link between ABO antigens, antibodies, and SARS‐CoV‐2 and its implications on controlling the current pandemic is warranted.
CONFLICT OF INTERESTS
The authors declare no relevant conflicting financial interests or disclosures.
AUTHOR CONTRIBUTIONS
Sarah A. Al‐Youha, Ahmad Al‐Serri, and Salman K. Al‐Sabah conceived the study. Sarah A. Al‐Youha and Ahmad Al‐Serri collected data and performed the statistical analysis. Waleed Alduaij and Sarah A. Al‐Youha wrote the manuscript. Sulaiman M. Almazeedi, Mohannad Al‐Haddad, Mohammad H. Jamal, and Salman K. Al‐Sabah established the COVID‐19 patient registry, which allowed data collection. Sarah A. Al‐Youha, Waleed Alduaij, Andrew W. Shih, Ahmad Al‐Serri, Sulaiman M. Almazeedi, Mohannad Al‐Haddad, Mohammad H. Jamal, and Salman K. Al‐Sabah analyzed data and edited the manuscript. All coauthors approved the final version of the manuscript.
Supporting information
Appendix S1: Supporting Information
ACKNOWLEDGMENTS
This work has been funded by a grant from the Kuwait Foundation for the Advancement of Science, grant code: Cor‐prop‐35. We are grateful to the Kuwait National Centre for Health Information for assistance with data collection and analysis.
Al‐Youha SA, Alduaij W, Al‐Serri A, et al. The impact of ABO blood groups on clinical outcomes and susceptibility to COVID‐19: A retrospective study in an unselected population. Transfusion. 2021;61:1631–1641. 10.1111/trf.16365
Contributor Information
Waleed Alduaij, Email: waleed.alduaij@bccancer.bc.ca.
Salman K. Al‐Sabah, Email: salman.k.alsabah@gmail.com.
REFERENCES
- 1. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. JAMA. 2020;323(20):2052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Scientific evidence for conditions that increase risk of severe illness | COVID‐19 | CDC . [cited 2020 Jul 25]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/evidence-table.html
- 3. Wynants L, Van Calster B, Collins GS, Riley RD, Heinze G, Schuit E, et al. Prediction models for diagnosis and prognosis of covid‐19 infection: Systematic review and critical appraisal. BMJ. 2020;369:m1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID‐19: Immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cooling L. Blood groups in infection and host susceptibility. Clin Microbiol Rev. 2015;28(3):801–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cheng Y, Cheng Y, Cheng G, Chui CH, Lau FY, Chan PKS, et al. ABO blood group and susceptibility to severe acute respiratory syndrome. JAMA. 2005;293(12):1450–1. [DOI] [PubMed] [Google Scholar]
- 7. 23andMe finds evidence that blood type plays a role in COVID‐19. 23andMe Blog. 2020. [cited 2020 Jul 25]. Available from: http://blog.23andme.com/23andme‐research/blood‐type‐and‐covid‐19/
- 8. Ellinghaus D, Degenhardt F, Bujanda L, Buti M, Albillos A, Invernizzi P, et al. Genomewide association study of severe Covid‐19 with respiratory failure. N Engl J Med. 2020;383(16):1522–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhao J, Yang Y, Huang H, Li D, Gu D, Lu X, et al. Relationship between the ABO blood group and the COVID‐19 susceptibility. Clin Infect Dis. 2020;ciaa1150. 10.1093/cid/ciaa1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li J, Wang X, Chen J, Cai Y, Deng A, Yang M. Association between ABO blood groups and risk of SARS‐CoV‐2 pneumonia. Br J Haematol. 2020;190(1):24–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leaf RK, Al‐Samkari H, Brenner SK, Gupta S, Leaf DE. ABO phenotype and death in critically ill patients with COVID‐19. Br J Haematol. 2020;190(4):e204–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Latz CA, DeCarlo C, Boitano L, Png CYM, Patell R, Conrad MF, et al. Blood type and outcomes in patients with COVID‐19. Ann Hematol. 2020;99(9):2113–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zietz M, Zucker J, Tatonetti NP. Associations between blood type and COVID‐19 infection, intubation, and death. Nat Commun. 2020;11(1):5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boudin L, Janvier F, Bylicki O, Dutasta F. ABO blood groups are not associated with risk of acquiring the SARS‐CoV‐2 infection in young adults. Haematologica. 2020;105(12):2841–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dzik S, Eliason K, Morris EB, Kaufman RM, North CM. COVID‐19 and ABO blood groups. Transfusion. 2020;60(8):1883–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Almazeedi S, Al‐Youha S, Jamal MH, Al‐Haddad M, Al‐Muhaini A, Al‐Ghimlas F, et al. Characteristics, risk factors and outcomes among the first consecutive 1096 patients diagnosed with COVID‐19 in Kuwait. EClinicalMedicine. 2020;24:100448. 10.1016/j.eclinm.2020.100448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alghounaim M, Almazeedi S, Al Youha S, Papenburg J, Alowaish O, AbdulHussain G, et al. Low‐cost polyester‐tipped 3‐dimensionally‐printed nasopharyngeal swab for the diagnosis of severe acute respiratory syndrome‐related coronavirus 2 (SARS‐CoV‐2). J Clin Microbiol. 2020;58(11):e01668–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K, et al. Diagnosis and treatment of adults with community‐acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Singer M, Deutschman CS, Seymour CW, Shankar‐Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (sepsis‐3). JAMA. 2016;315(8):801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. World Health Organization . Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: Interim guidance. Geneva: World Health Organization; 2020, 2020. Available from: https://apps.who.int/iris/handle/10665/332299. [Google Scholar]
- 21. KDIGO . Clinical practice guidelines for acute kidney injury 2012, Section 2: AKI definition. Kidney Int Suppl. 2012;2(1):19–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weiderpass E, Botteri E, Longenecker JC, Alkandari A, al‐Wotayan R, al Duwairi Q, et al. The prevalence of overweight and obesity in an adult Kuwaiti population in 2014. Front Endocrinol. 2019;10:449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Main page ‐ Central Statistical Bureau . [cited 2020 Aug 3]. Available from: https://www.csb.gov.kw/Pages/Statistics_en?ID=67&ParentCatID=1.
- 24. McGonagle D, O'Donnell JS, Sharif K, Emery P, Bridgewood C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID‐19 pneumonia. Lancet Rheumatol. 2020;2(7):e437–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. O'Sullivan JM, Ward S, Fogarty H, Cai Y, Deng A, Yang M. More on “Association between ABO blood groups and risk of SARS‐CoV‐2 pneumonia”. Br J Haematol. 2020;190(1):27–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guillon P, Clément M, Sébille V, Rivain JG, Chou CF, Ruvoën‐Clouet N, et al. Inhibition of the interaction between the SARS‐CoV spike protein and its cellular receptor by anti‐histo‐blood group antibodies. Glycobiology. 2008;18(12):1085–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Niles JK, Karnes HE, Dlott JS, Kaufman HW. Association of ABO/Rh with SARS‐CoV‐2 positivity: The role of race/ethnicity in a female cohort. Am J Hematol. 2020;96(1):E23–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Anstee DJ. The relationship between blood groups and disease. Blood. 2010;115(23):4635–43. [DOI] [PubMed] [Google Scholar]
- 29. Valenti L, Villa S, Baselli G, Temporiti R, Bandera A, Scudeller L, et al. Association of ABO blood group and secretor phenotype with severe COVID‐19. Transfusion. 2020;60(12):3067–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cserti CM, Dzik WH. The ABO blood group system and Plasmodium falciparum malaria. Blood. 2007;110(7):2250–8. [DOI] [PubMed] [Google Scholar]
- 31. Racial and ethnic distribution of ABO blood types ‐ BloodBook.com, blood information for life. [cited 2020 Aug 4]. Available from: http://www.bloodbook.com/world-abo.html.
- 32. Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, et al. Features of 20 133 UK patients in hospital with covid‐19 using the ISARIC WHO clinical characterisation protocol: Prospective observational cohort study. BMJ. 2020;m1985:369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: Summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supporting Information


