Abstract
Introduction
The phosphodiesterase inhibitors theophylline and pentoxifylline have anti‐inflammatory properties that may make them useful in COVID‐19 pneumonia. We conducted a retrospective review of hospitalized COVID‐19 patients requiring oxygen who received these drugs.
Objectives
To examine the potential efficacy and safety of theophylline and pentoxifylline in COVID‐19 pneumonia patients.
Methods
Adults with a positive test for SARS‐COV2 and were hospitalized due to pneumonia requiring either high flow nasal cannula or mechanical ventilation were included. Patients with a history of asthma or chronic obstructive pulmonary disease were preferentially given theophylline. All other patients received pentoxifylline 400 mg orally TID. A group of hospitalized COVID‐19 patients receiving standard of care acted as a comparison group. The coprimary outcomes were a change in C‐reactive protein (CRP) and ROX score between groups from day 1 to day 4 of therapy.
Results
Two hundred and nine inpatients were reviewed. Fifty‐eight patients received pentoxifylline/theophylline, with 151 patients serving as the comparison group. Active therapy was associated with an increase in the ROX score (mean: 2.9 (95% CI: 0.6, 5.1)) and decrease in CRP (mean: −0.7 (95% CI: −4.7, 3.2). Mortality rates were theophylline/pentoxifylline 24% and comparison group had a 26%, respectively.
Conclusion
In this retrospective study, theophylline and pentoxifylline were associated with an increase in ROX score and nominal decreases in CRP and mortality. Treatment was safe with few adverse reactions documented. We believe that this study could the basis for randomized‐controlled trials to further explore these drugs’ role in COVID‐19.
Keywords: COVID‐19, pentoxifylline, pneumonia, theophylline
1. BRIEF REPORT
Patients with severe COVID‐19 disease can develop acute respiratory distress syndrome (ARDS), which can lead to profound refractory hypoxia, organ failure, and death. At the center of this, pathophysiologic reaction is the theory that a massive proinflammatory reaction to the viral infection, termed cytokine storm, is the trigger for this process. 1 Clinicians have proposed a variety of methods to stem or ameliorate the cytokine storm including the IL‐6 blocker tocilizumab. 2 The phosphodiesterase inhibitors theophylline and pentoxifylline are medications that have been approved by U.S. Food & Drug Administration (FDA) for over 40 years. Theophylline is used for the treatment of asthma and chronic obstructive pulmonary disease (COPD) and has many pharmacologic attributes that may be beneficial in COVID‐19 respiratory failure—including its potent bronchodilatory effects, improved diaphragmatic muscle function, and its anti‐inflammatory properties. 3 Pentoxifylline is indicated for intermittent claudication, raises intracellular cAMP, and inhibits numerous proinflammatory chemicals including IL‐6. In addition, pentoxifylline improves red blood cell deformability, known as a hemorrheologic effect, and may have antithrombotic properties. 4 Pentoxifylline has a very favorable adverse effect profile and is inexpensive. Due to the rapidity and severity of the COVID ‐19 crisis, we developed a pilot protocol to test the use of these drugs in COVID‐19 patients with severe respiratory failure. We included patients 18 years or older with a positive real‐time polymerase chain reaction test for SARS‐COV2 and were hospitalized due to pneumonia requiring either high flow nasal cannula or mechanical ventilation. For markers of inflammation, patients needed to have a serum C‐reactive protein (CRP) > 15 mg/dl or serum Ferritin > 900 ng/ml. 5 Patients were excluded if they had a known hypersensitivity to either drug, had a severe bleeding episode (ie, defined as >2 g/L drop in hemoglobin with signs of clinical bleeding), serum ALT/AST > 5 times the upper limit of normal or a high risk of adverse effects or drug interactions in the opinion of the ordering physician. Our system IRB approved data collection and analysis for this study.
2. METHODS/OUTCOMES
Patients with a history of asthma or COPD were preferentially given theophylline as the drug is FDA approved for both disease states. Intravenous infusion or oral tablets/elixir was given with a dose targeted to reach a serum drug level of 5‐12 mg/dl. All other patients received pentoxifylline 400 mg orally three times daily. Despite manufacturer information, this drug can be crushed and given via a feeding tube with no significant change to its pharmacokinetic properties. 6
Both drugs were continued for a maximum of 7 days or until discharge from the hospital, death, or transition to comfort care. A group of hospitalized COVID‐19 patients meeting the above criteria and not on either drug served as a comparison group. The coprimary outcomes were a change in CRP between groups from day 1 to day 4 of therapy and similar change in their ROX score. 7 In‐hospital mortality was also examined. Safety was assessed via chart review and included reports of stomach upset, bleeding, tachycardia (ie, >130 bpm), and tremor. Study outcomes were modeled based on patients’ binary drug exposure status (ie, theophylline or pentoxifylline versus neither drug) using linear and survival regression models. Potential confounding variables in the sample were addressed using stabilized inverse treatment weights. Results are presented as means and hazard ratio with 95% confidence intervals (CI).
3. RESULTS
Two hundred and nine inpatients records were collected from March 15, 2020, to June 19, 2020, with a positive COVID‐19 test. Fifty‐eight patients (28%) received pentoxifylline (20%) or theophylline (8%), with 151 patients serving as the comparison group. Baseline patient characteristics are presented in Table 1. Patients in the cohort were approximately 60 years old with a slight majority being male. Initial CRP and ROX scores were similar between groups (CRP was 16 vs. 15 mg/L and ROX Score 9 vs. 10 in active vs. control arms, respectively). Overall 59% of the patients required mechanical ventilation with the remainder all requiring some form of oxygen support. Of the group that received theophylline 8 had asthma and 9 had COPD. No patients in the control arm had these diseases. At day 4 of therapy, patients receiving theophylline/pentoxifylline had a mean 1.7 (95% CI: −0.3, 3.8 [ie, 12.2 vs. 10.5]) higher ROX score and a mean −0.6 (95% CI: −3.8, 2.6 [ie, 13.4 vs. 12.8) lower CRP than the comparison group. Looking at changes from baseline over time, theophylline and pentoxifylline therapy were associated with an increase in ROX score (mean: 2.9 (95% CI: 0.6, 5.1)) and decrease in CRP (mean: −0.7 (95% CI: −4.7, 3.2)) from day 1 to day 4 of therapy. Inpatients receiving theophylline/pentoxifylline had a 24% (14/58) mortality rate, while the comparison group had a 26% (39/151) mortality rate (Figure 1). There was a 1.69 (95% CI: 0.85, 3.4) times greater hazard for mortality in the comparison group. The 75% survival for patients receiving theophylline/pentoxifylline was 21 (95% CI: 2, 30) days and 11 (95% CI: 8, 14) days in the control group. No reports of gastrointestinal or other major bleeding were reported in the treatment group. Three episodes of nursing reported stomach upset with pentoxifylline and one probable adverse event of tachycardia and nausea was reported in a theophylline patient with a level of 18.5 mg/L.
TABLE 1.
Baseline patient characteristics for inpatients with COVID‐19 requiring oxygen support stratified based on theophylline/pentoxifylline status, n = 209 a
| Characteristic (mean) | Theophylline/pentoxifylline | Comparison group |
|---|---|---|
| n = 58 | n = 151 | |
| Age (years) | 60 (±15) | 62 (±14) |
| Male gender | 53% | 59% |
| Body mass index | 34 (±12) | 32 (±10) |
| History of diabetes | 42% | 39% |
| C‐reactive protein day 1 (mg/L) | 16 (±10) | 15 (±11) |
| ROX value day 1 | 9 (±4) | 10 (±7) |
| Mechanical ventilation | 57% | 60% |
| Remdesivir | 6% | 4% |
| Convalescent plasma | 40% | 37% |
| Tocilizumab | 17% | 24% |
All descriptive statistics were based on the use of stabilized inverse treatment weights modeled using all of the variables listed in this table.
FIGURE 1.
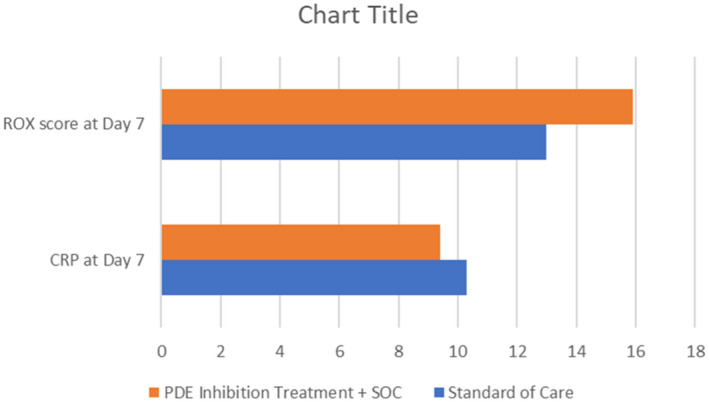
Differences in C‐reactive Protein and ROX scores at day seven between patients who received theophylline and pentoxifylline compared to standard of care
4. DISCUSSION
In this retrospective study, theophylline and pentoxifylline appeared to be associated with an increase in ROX score and nominal decreases in CRP and mortality. Treatment was safe with few minor and no major adverse reactions documented. The patients were balanced for suspected confounding variables in the analyses, but due to the non‐randomized study design, the presence of an unknown confounder(s) cannot be ruled out. The overall use of remdesivir in this study was low. More patients in the theophylline and pentoxifylline group received convalescent plasma and fewer received tocilizumab. Also, the study time period was before the widespread use of dexamethasone stemming from the RECOVERY study, 8 which may affect related outcomes. Although not conclusive, we believe this pilot project does not show a signal for harm for theophylline and pentoxifylline treatment and the drugs could be associated with benefits. We feel our findings support the possible consideration of a randomized clinical trial of these treatments to assess the true potential benefits and harms.
CONFLICT OF INTEREST
No real or potential conflicts of interest exist for any of the authors and this manuscript.
AUTHOR CONTRIBUTIONS
All authors contributed substantially to the study design, data analysis and interpretation, and writing of the manuscript.
Wall GC, Smith HL, Trump MW, et al. Pentoxifylline or theophylline use in hospitalized COVID‐19 patients requiring oxygen support. Clin Respir J. 2021;15:843–846. 10.1111/crj.13363
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Henderson LA, Canna SW, Schulert GS, et al. On the alert for cytokine storm: immunopathology in COVID‐19. Arthritis Rheumatol. 2020;72(7):1059‐1063. 10.1002/art.41285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu B, Li M, Zhou Z, Guan X, Xiang Y. Can we use interleukin‐6 (IL‐6) blockade for coronavirus disease 2019 (COVID‐19)‐induced cytokine release syndrome (CRS)? J Autoimmun. 2020;111:102452. 10.1016/j.jaut.2020.102452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Theophylline: Mechanism of action and general monograph. In: Micromedex (Electronic version). Greenwood Village, CO: Truven Health Analytics. Accessed April 17, 2020. [Google Scholar]
- 4. Pentoxifylline: Mechanism of action and general monograph. In: Micromedex (Electronic version). Greenwood Village, CO: Truven Health Analytics. Accessed April 17, 2020. [Google Scholar]
- 5. Fardet L, Galicier L, Lambotte O, et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66(9):2613‐2620. 10.1002/art.38690 [DOI] [PubMed] [Google Scholar]
- 6. Cleary JD, Evans PC, Hikal AH, Chapman SW. Administration of crushed extended‐release pentoxifylline tablets: bioavailability and adverse effects. Am J Health Syst Pharm. 1999;56(15):1529‐1534. [DOI] [PubMed] [Google Scholar]
- 7. Roca O, Caralt B, Messika J, et al. An index combining respiratory rate and oxygenation to predict outcome of nasal high‐flow therapy. Am J Respir Crit Care Med. 2019;199(11):1368‐1376. 10.1164/rccm.201803-0589OC [DOI] [PubMed] [Google Scholar]
- 8. RECOVERY Collaborative Group , Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid‐19—preliminary report. N Engl J Med. 2020. Jul 17. 10.1056/NEJMoa2021436. [Epub ahead of print] [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


