Graphical Abstract
Neutrophil dynamics in aging provide another key piece of the puzzle regarding the impact of aging and comorbid conditions on the severity of Covid-19.
Chronic inflammation associated with ageing, known also as inflammaging, contributes to and is associated with the pathogenesis of age-related diseases such as hypertension, arthritis, and diabetes.1
In the current issue of the Journal of Leukocyte Biology, He et al. report the observation that aged rhesus macaques contain fewer blood neutrophils despite higher levels of circulating Granulocyte colony stimulating factor (G-CSF), a major growth factor that drives neutrophil production in the bone marrow (BM).2 Elevated G-CSF in older macaques was also positively correlated with higher levels of other proinflammatory cytokines such as IL-1β and MIF-1α. Furthermore, the authors observed that older macaques release neutrophils from the BM earlier than their younger counterparts and that these neutrophils are less functional. Some of these cells also possess a phenotype similar to polymorphonuclear myeloid-derived suppressor cells. This paradoxical decline in blood neutrophils despite elevated G-CSF and the observation that blood neutrophils within aged rhesus macaques are less functional may shed some light on how ageing increases the risk of runaway innate immune activation resulting in poorer infection outcomes.
Senescence of hematopoietic stem cells resulting from biological aging may cause a decline in blood neutrophil numbers resulting in a homeostatic response increasing G-CSF production to compensate. Increased G-CSF leads to shortened neutrophil maturation time in the BM and faster release into circulation. These rapidly produced but still immature neutrophils were shown in the accompanying report to express lower levels of the antimicrobial myeloperoxidase and perhaps they are less functional in other aspects such as abnormal trafficking and clearance from tissues or even perhaps the aberrant production of neutrophil extracellular traps (NETs).
Decreased neutrophil maturation time resulting in an immature, less functional phenotype may reduce the abundance of good (functional) neutrophils and provide the host with a bad set of less functional innate immune warriors that result in an ugly outcome in the event of an infection. The observations reported by He et al. likely provide a key piece to the current puzzle: why diabetics, persons of advanced age, and even obese individuals are at risk for severe COVID-19.3 Both abnormalities in neutrophil function and dysregulation of a related cytokine, Granulocyte-macrophage colony stimulating factor (GM-CSF), appear to be common features in both diabetes and SARS-CoV-2 infection. Indeed, both impaired neutrophil function and increased GM-CSF have been associated with diabetes.4 Similar observations have been made with regard to both G-CSF and GM-CSF and obesity,5,6 and now in this report here, aging (ref). While it is already established that poor outcomes in SARS-CoV-2 infection involve excessive pulmonary neutrophil recruitment, accumulation, and formation of their extracellular traps in humans7,8 and nonhuman primate studies,9 the mechanism for the predisposition of persons with comorbid conditions associated with advanced age, diabetes, and obesity has not been adequately understood. Based on this new information, we propose a model (Figure 1) where under these conditions, SARS-CoV-2 infection results in recruitment of immature dysfunctional neutrophils. These cells are less able to clear debris while at the same time making the fight more difficult by aberrant NET production and accumulation due to a reduced capacity for proper trafficking. Patients with more severe COVID-19 exhibited elevated plasma levels of markers associated with NETs such as cell-free DNA.10 The infection persists causing more inflammation and emergency granulopoiesis11 further worsening the situation by flooding the lungs with even more immature dysfunctional neutrophils. Increased GM-CSF in the serum has been reported in type 2 diabetic subjects and is correlated with HbA1c levels.4 In mice, obesity has been associated with elevated levels of inflammatory cytokines in a GM-CSF-dependent manner5 and alter myeloid cell presence in the lung along with G-CSF.6 In conditions, where GM-CSF and G-CSF are elevated, susceptibility to SARS-CoV-2 severe disease would be predicted. For example, exogenous G-CSF provided to cancer patients to prevent neutropenia and infection has been associated with increased need for supplemental oxygen and death.12 This model emphasizes the role of GM-CSF and G-CSF as therapeutic targets for COVID-19 treatment. Indeed, blocking GM-CSF with a monoclonal antibody has been shown to improve severe COVID-19 outcomes in human subjects.13 Other studies have shown that targeting G-CSF and its receptor (G-CSFR) with monoclonal antibodies can also mitigate neutrophil-dependent inflammatory responses.14 A combinational approach in which both G-CSF and GM-CSF are targeted in severe COVID-19 may be even more effective. In our opinion, the findings reported by He and colleagues provide evidence for a link between aging and impaired neutrophil function that may help explained impaired innate immune cell function and resulting disease severity associated with severe COVID-19 infection.
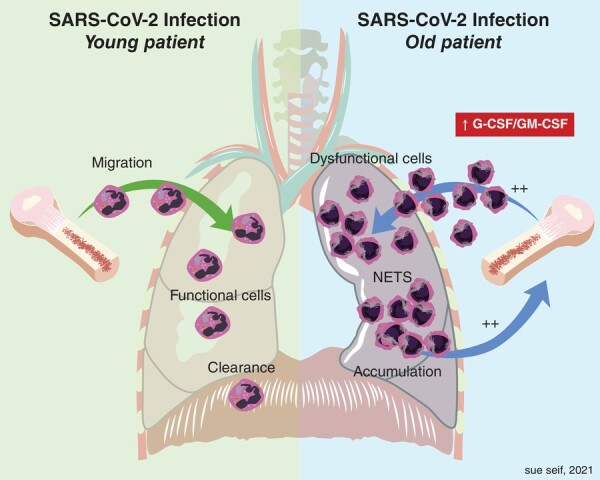
FIGURE 1.
SARS-CoV-2 infection in young versus old. Elevated G-CSF and earlier neutrophil release may drive neutrophil pathology in severe COVID-19. In younger individuals, infection recruits mature neutrophils that function and are cleared appropriately. In older individuals, elevated G-CSF results in immature neutrophil release. Infection recruits these cells that may be less functional and thus accumulate and cannot clear as effectively. Infection persists and may drive further neutrophil migration and accumulation
In summary, He et al. report increased G-CSF levels in aged rhesus macaques that is associated with earlier neutrophil release from the BM. High G-CSF in these animals is associated with higher levels of other proinflammatory cytokines suggesting a serum cytokine signature of inflammaging. These early released neutrophils are less mature, functional, and some possess a unique myeloid derived suppressor cell phenotype. These findings have implications for the underlying causes of severe SARS-CoV-2 infection and resulting COVID-19 in the older human population and provide more evidence supporting the targeting of G-CSF and GM-CSF in the clinic.
Footnotes
See corresponding article on R 779R
Contributor Information
Daniel L Moss, Tulane National Primate Research Center, Covington, LA, USA.
Jay Rappaport, Tulane National Primate Research Center, Covington, LA, USA.
References
- Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A. Inflammaging: a new immune–metabolic viewpoint for age-related diseases. Nat Rev Endocrinol. 2018;14:576-590. [DOI] [PubMed] [Google Scholar]
- Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of Apoptotic Neutrophils Regulates Granulopoiesis via IL-23 and IL-17. Immunity. 2005;22 (3):285-294. 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Ssentongo P, Ssentongo AE, Heilbrunn ES, Ba DM, Chinchilli VM. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: a systematic review and meta-analysis. PLoS One. 2020;15:e0238215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surendar J, Mohan V, Pavankumar N, Babu S, Aravindhan V. Increased levels of serum granulocyte-macrophage colony-stimulating factor is associated with activated peripheral dendritic cells in type 2 diabetes subjects (CURES-99). Diabetes Technol Ther. 2012;14:344-349. [DOI] [PubMed] [Google Scholar]
- Kim D-H, et al. The role of GM-CSF in adipose tissue inflammation. Am J Physiol-Endocrinol Metab. 2008;295:E1038-E1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quail DF, et al. Obesity alters the lung myeloid cell landscape to enhance breast cancer metastasis through IL5 and GM-CSF. Nat Cell Biol. 2017;19:974-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcanjo A, et al. The emerging role of neutrophil extracellular traps in severe acute respiratory syndrome coronavirus 2 (COVID-19). Sci Rep. 2020;10:19630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes BJ, et al. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J Exp Med. 2020;217(6):1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahlberg MD, et al. Cellular events of acute, resolving or progressive COVID-19 in SARS-CoV-2 infected non-human primates. Nat Commun. 2020;11:6078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry Ng, et al. Circulating markers of neutrophil extracellular traps are of prognostic value in patients with COVID-19. Arterioscler Thromb Vasc Biol. 2021;41:988-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manz MG, Boettcher S. Emergency granulopoiesis. Nat Rev Immunol. 2014;14:302-314. [DOI] [PubMed] [Google Scholar]
- Morjaria S, et al. The effect of neutropenia and filgrastim (G-CSF) in cancer patients with COVID-19 infection. medRxiv. 2020;2020. https://doi.org.10.1101/2020.08.13.20174565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temesgen Z, et al. GM-CSF neutralization with lenzilumab in severe COVID-19 pneumonia: a case-cohort study. Mayo Clin Proc. 2020;95:2382-2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalzo-Inguanti K, et al. A neutralizing anti–G-CSFR antibody blocks G-CSF–induced neutrophilia without inducing neutropenia in nonhuman primates. J Leukoc Biol. 2017;102:537-549. [DOI] [PubMed] [Google Scholar]


