Abstract
Background
The effects of the coronavirus disease 2019 (COVID‐19) pandemic on surgical oncology practice are not yet quantified. The aim of this study was to measure the immediate impact of COVID‐19 on surgical oncology practice volume.
Methods
A retrospective study of patients treated at an NCI‐Comprehensive Cancer Center was performed. “Pre‐COVID” era was defined as January–February 2020 and “COVID” as March–April 2020. Primary outcomes were clinic visits and operative volume by surgical oncology subspecialty.
Results
Abouyt 907 new patient visits, 3897 follow‐up visits, and 644 operations occurred during the study period. All subspecialties experienced significant decreases in new patient visits during COVID, though soft tissue oncology (Mel/Sarc), gynecologic oncology (Gyn/Onc), and endocrine were disproportionately affected. Telehealth visits increased to 11.4% of all visits by April. Mel/Sarc, Gyn/Onc, and Breast experienced significant operative volume decreases during COVID (25.8%, p = 0.012, 43.6% p < 0.001, and 41.9%, p < 0.001, respectively), while endocrine had no change and gastrointestinal oncology had a slight increase (p = 0.823) in the number of cases performed.
Conclusions
The effects of the COVID‐19 pandemic are wide‐ranging within surgical oncology subspecialties. The addition of telehealth is a viable avenue for cancer patient care and should be considered in surgical oncology practice.
Keywords: clinic volume, COVID‐19, operative volume, pandemic, telehealth
1. INTRODUCTION
The coronavirus disease 2019 (COVID‐19) was initially identified in Wuhan, China in December 2019 1 and the first reported case in the United States was January 22, 2020. 2 According to the Center for Disease Control (CDC), from January to December 2020 there were 16 519 668 new cases of COVID‐19 and 302, 992 deaths in the United States. 2 On March 9, 2020 the governor of New Jersey declared a state of emergency and on March 23, 2020 issued an executive order that as of March 27, 2020 all elective surgeries and invasive procedures were to be suspended based on the US Surgeon General's recommendations. 3 Our institution's peak census of patients with COVID‐19 was April 16, 2020. The profound impact of these restrictions, in addition to social distancing, created unprecedented challenges for clinicians to care for patients with cancer. Leading professional societies promptly released recommendations and guidelines for the triage and management of surgical patients with cancer. 4 , 5 , 6 , 7 , 8 , 9 , 10
A national physician cross sectional survey of 411 oncology physicians (58.6% surgeons) from March 27 to April 10, 2020 found that a majority of physicians had altered cancer treatment plans. 11 The primary reasons for surgeons altering care were conservation of personal protective equipment, institutional mandates, and professional society recommendations. Several retrospective studies of this initial period of the pandemic confirm alterations in treatment, a decrease or delay in oncologic surgical procedures, cancer screening, clinic visits, and a significant decline in newly identified patients with the six most common types of cancer (breast, colorectal, lung, pancreatic, gastric, and esophageal). 12 , 13 , 14 , 15 , 16 , 17 The prognostic outcomes of these alterations of cancer practice have yet to be determined.
The purpose of this study was to measure the immediate impact of the COVID‐19 pandemic on surgical oncology clinical practice and operative volume amongst subspecialties. We hypothesized that surgical subspecialties including a large proportion of outpatient or same‐day operations would be disproportionately affected.
2. MATERIALS AND METHODS
An IRB‐approved retrospective chart review was performed to include patients treated at Rutgers Cancer Institute of New Jersey—the only NCI‐Comprehensive Cancer Center in New Jersey—within the following surgical oncology subspecialties: Melanoma/Soft Tissue, Gastrointestinal (GI/Onc), Gynecology (Gyn/Onc), Breast, and Endocrine. “Pre‐COVID” era was defined as the months before the pandemic (January and February 2020) and “COVID” was defined as the initial months when COVID precautions were instituted (March and April 2020). Institutional guidelines for surgical deferment were created based on national surgical society guidelines. A virtual weekly conference call with hospital administration was initiated in March 2020 to prioritize operations from all surgical services.
The primary outcomes were clinic visits and operative volume by surgical oncology subspecialty. Secondary outcomes included proportion of in‐person versus telehealth clinic visits, patient demographics, benign versus malignant indications, hospital disposition, resident or fellow operative involvement, and inpatient length of stay. Descriptive statistics were performed and when appropriate, χ 2, Fisher's exact, and Student's t‐test were used to compare pre‐COVID and COVID cohorts.
3. RESULTS
Overall, 907 new patient visits, 3897 clinic visits and 644 operations were evaluated during the study period. The total number of clinic visits in the pre‐COVID period was 2292 versus 1695 in the COVID period (26.2% overall decrease). No telehealth visits were performed in the pre‐COVID era versus 193 visits in the COVID era (11.4% of all visits during that time). Trends in outpatient clinical encounters are shown in Figures 1, 2, and 3. The number of follow‐up visits (including both in‐person and telehealth encounters) were less affected in GI/Onc (390 pre‐COVID and 307 COVID, 21.3% reduction) and Gyn/Onc (776 pre‐COVID and 683 COVID, 12% reduction). Follow‐up visits in Melanoma/Soft Tissue (215 pre‐COVID and 122 COVID, 43.3% reduction), Breast (322 pre‐COVID and 178 COVID, 44.7% reduction) and Endocrine specialties (48 pre‐COVID and 33 COVID, 31.2% reduction) were more impacted (Figure 3). All specialties experienced statistically significant decreases in the number of new patient visits in the COVID period (ranging from 14.5% to 42.1%), with the greatest decreases seen in Breast, Melanoma/Soft Tissue, and Endocrine specialties (Figure 4).
Figure 1.
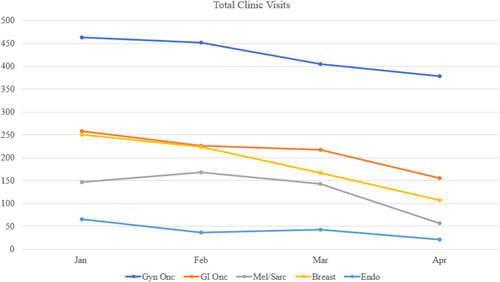
Trends in outpatient total clinic visits [Color figure can be viewed at wileyonlinelibrary.com]
Figure 2.
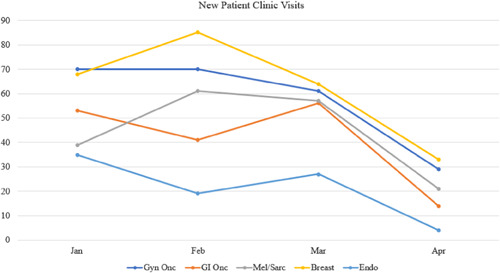
Trends in new patient clinic visits [Color figure can be viewed at wileyonlinelibrary.com]
Figure 3.

Trends in follow‐up visits [Color figure can be viewed at wileyonlinelibrary.com]
Figure 4.
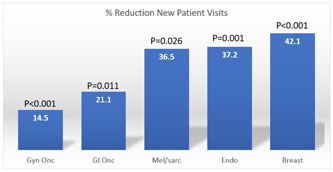
Reduction in new patient clinic visits, by specialty [Color figure can be viewed at wileyonlinelibrary.com]
Patient demographics and clinical variables for new patient clinic encounters are shown in Table 1. In the pre‐COVID period, 541 new patients were seen by the five subspecialties while 366 patients were seen in the COVID period (32.3% overall decrease). There were no major differences in age, sex, or ethnicity between the groups. The number of benign disease encounters decreased in the COVID period (31.2% vs. 21.9, p = 0.015) and there was an immediate 4.2% decrease in the number of surgeries scheduled at the initial visit evaluation in the COVID months (p < 0.001). New clinic patient demographics, diagnoses, and disposition within each subspecialty can be found in Table S1. The statistically significant decreases in the proportion of benign disease encounters were seen in Gyn/Onc and Breast. Only Melanoma/Soft Tissue experienced a significant decrease of 12.6% in the number of surgeries scheduled at the initial evaluation in the COVID period (p = 0.010).
Table 1.
Patient demographics and outpatient clinic visit variables for new patient clinic visits
| Pre‐COVID (n = 541) | COVID (n = 366) | p value | |
|---|---|---|---|
| Age, mean, years | 57.9 | 58.2 | 0.774 |
| Female sex | 409 (75.6) | 273 (74.6) | 0.729 |
| Ethnicity | 0.193 | ||
| White | 338 (71.7) | 238 (65.0) | |
| Black | 47 (8.7) | 40 (10.9) | |
| Hispanic | 48 (8.8) | 33 (9.0) | |
| Asian | 69 (12.8) | 29 (7.9) | |
| Other/declined | 39 (7.2) | 26 (7.1) | |
| Diagnosis | 0.015 | ||
| Benign | 169 (31.2) | 80 (21.9) | |
| Malignant | 233 (43.1) | 179 (48.9) | |
| Undetermined | 139 (25.7) | 107 (29.2) | |
| Disposition | < 0.001 | ||
| Follow‐up appointment | 336 (62.1) | 204 (55.7) | |
| Schedule for surgery | 190 (35.1) | 113 (30.9) | |
| Procedure in office | 15 (2.8) | 17 (4.6) | |
| Defer surgery due to COVID | N/A | 32 (8.7) | |
| By subspecialty | 0.703 | ||
| Mel/sarc | 100 (18.5) | 78 (21.3) | |
| Breast | 153 (28.3) | 97 (26.5) | |
| GI/Onc | 94 (17.4) | 70 (19.1) | |
| Gyn/Onc | 140 (25.8) | 90 (24.6) | |
| Endocrine | 54 (10.0) | 31 (8.5) |
Abbreviations: COVID, coronavirus disease; GI/Onc, Gastrointestinal/Oncology; Gyn/Onc, Gynecology/Oncology.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
New patient encounters with an indication for surgical intervention were evaluated. Reasons for deferral in the COVID period were outlined by specialty. Breast specialties deferred the greatest number of new patients (12 or 12.4%) with 50% of these patients receiving neoadjuvant endocrine therapy. Endocrine surgery deferred six (19.4%) patients to wait for surgical intervention, except one patient who was referred for genetic testing. Temporizing measures such as paracentesis were employed by Gyn/Onc and repeat imaging to monitor disease burden was recommended in Gyn/Onc and Melanoma/Soft Tissue specialties.
In the pre‐COVID period, 370 patients underwent surgery in all five subspecialties versus 274 patients in the COVID period (25.9% overall decrease). Trends in the number of operations performed by specialty are shown in Figures 5 and 6. The number of operations in the second month of the COVID period (April) experienced sharp decreases compared with the first month (March) (Figure 5). Breast, Melanoma/Soft Tissue, and Gyn/Onc subspecialties experienced large decreases in operative volume in the COVID period (range 25.8%–43.6%) while GI/Onc and Endocrine specialties had a small increase in operations (3.7%) and no change, respectively.
Figure 5.
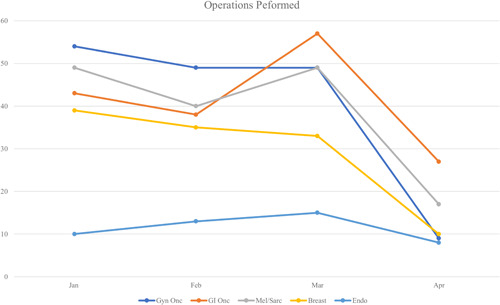
Trends in number of operations performed, by specialty [Color figure can be viewed at wileyonlinelibrary.com]
Figure 6.
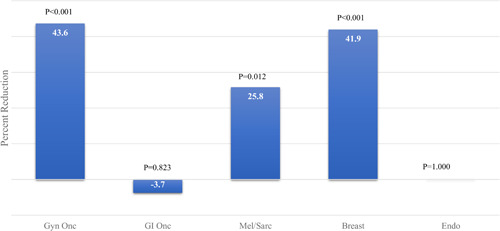
Reduction in number of operations, by specialty [Color figure can be viewed at wileyonlinelibrary.com]
Patient demographics and clinical variables for operative patient encounters are shown in Table 2. There were no major changes in age, sex, or ethnicity between the two groups. The number of cases performed for benign disease decreased significantly (13.9% from 20.0%; p = 0.049) as did the number of outpatient operations (63.1% from 71.9%; p = 0.018). There were no changes in resident or fellow surgeon involvement in cases in the COVID period.
Table 2.
Operations pre‐COVID versus COVID
| Pre‐COVID (n = 370) | COVID (n = 274) | p value | |
|---|---|---|---|
| Age, mean, years | 59.2 | 59.9 | 0.577 |
| Female sex | 257 (69.5) | 181 (66.0) | 0.359 |
| Ethnicity | 0.856 | ||
| White | 246 (66.5) | 187 (68.2) | |
| Black | 34 (9.2) | 19 (6.9) | |
| Hispanic | 27 (7.3) | 19 (6.9) | |
| Asian | 36 (9.7) | 26 (9.5) | |
| Other/declined | 27 (7.3) | 23 (8.4) | |
| Diagnosis type | 0.049 | ||
| Benign | 74 (20.0) | 38 (13.9) | |
| Malignant | 250 (67.6) | 209 (76.3) | |
| Undetermined | 46 (12.4) | 27 (9.8) | |
| Cases including trainee | 249 (67.3) | 187 (68.2) | 0.791 |
| Admission type | 0.018 | ||
| Inpatient | 104 (28.1) | 101 (36.9) | |
| Outpatient | 266 (71.9) | 173 (63.1) | |
| Hospital LOS, mean, days | 2.6 | 3.1 | 0.477 |
| ICU admission | 18 (4.9) | 10 (3.6) | 0.454 |
| COVID‐19 tested | N/A | 24 | |
| Number of operations | 0.038 | ||
| Gyn | 103 (27.8) | 58 (21.1) | |
| GI | 81 (21.9) | 84 (30.7) | |
| Mel/Sarc | 89 (24.0) | 66 (24.1) | |
| Breast | 74 (20.0) | 43 (15.7) | |
| Endocrine | 23 (6.2) | 23 (8.4) |
Abbreviations: COVID‐19, coronavirus disease‐2019; GI, Gastrointestinal; Gyn, Gynecology; ICU, intensive care unit; LOS, length of stay.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Operative patient demographics, diagnoses, operation type, and disposition within each subspecialty are documented in Table S2. The distribution of operation types did not change significantly within subspecialties during the COVID period, except within GI/Onc, where the number of staging procedures (exams under anesthesia and diagnostic laparoscopy) decreased from 16.0% to 5.9% in the COVID period. The number of excisional biopsy cases within Breast surgery decreased from 37.8% from 25.5% but the overall case distribution was not statistically different in the COVID period. All subspecialties demonstrated decreased hospital inpatient length of stay in the COVID period, though only GI/Onc had a statistically significant difference (12.4–8.0 mean days; p = 0.035)
4. DISCUSSION
During the peak of the COVID‐19 pandemic, healthcare providers experienced significant challenges in cancer patient management due to social distancing, depletion of healthcare resources, and lack of personal protective equipment. National, local, and institutional policies and societies dictated triaging of elective surgical cases, which influenced management and delivery of cancer care. Our findings demonstrate the immediate impact on surgical oncology practice volume at an NCI‐designated Comprehensive Cancer Center in the Northeastern United States during this time. Across all surgical oncology subspecialties at our institution, there was a significant decrease in new patient clinic visits, with Melanoma/Soft Tissue, Gyn/Onc, and Endocrine surgery being disproportionately affected. Melanoma/Soft Tissue, Gyn/Onc, and Breast surgery experienced significant and immediate operative volume decreases. Of note, although there was a decrease in overall new patient visits, telehealth clinic visits, which were nonexistent in the pre‐COVID era, were effectively implemented and increased to 11.4% of all visits by the second month of the pandemic.
4.1. Breast cancer
Both the Surgical Society of Oncology (SSO) and the COVID‐19 Pandemic Breast Cancer Consortium released guidelines for breast cancer management and triage. 5 , 7 Based on these recommendations, our institution implemented a protocol for breast cancer care. Recommendations were to proceed with cancer surgery as indicated, encouraging the following when appropriate: breast conservative therapy over mastectomy, same day discharge for mastectomy, neoadjuvant endocrine therapy, and to combine multidisciplinary visits with medical and radiation oncology. During the immediate COVID period, we found a decrease in total patient visits in Breast surgery, with a 42.1% decrease in new patient visits, as well as a decrease in follow‐up visits. There was a significant decrease in benign disease encounters (32.7% vs. 17.5%, p = 0.021) and 12 patients (12.4%) had their surgery deferred to wait for neoadjuvant endocrine therapy or repeat imaging. Overall, there was a reduction in breast operations by 41.9%. Data from the MD Anderson Cancer Center (MDACC) similarly demonstrated that median breast case volume decreased during the COVID‐19 pandemic from 40 to 11 cases per week (p = 0.010). 12 In addition, a study published by a group from Massachusetts General Hospital demonstrated that breast imaging, breast surgery, and genetic counseling experienced significant declines after the COVID‐19 outbreak. 18 In this study, the decline in breast surgery began first, with an average weekly decline of 20.5%, yet breast imaging experienced the most significant overall reduction. Consistent with these findings, our institution also saw large decreases in screening and diagnostic imaging by 48.4%, with decreases in screening and diagnostic mammograms by 52.1% and ultrasounds by 38.2%. Considering the reduction in breast imaging, this most likely led to delay in diagnosis and surgery as imaging is essential to breast cancer management. Though further studies are required, it appears that the COVID‐19 pandemic has significantly altered breast cancer management. The long‐term outcomes of these changes have yet to be elucidated.
4.2. Gynecologic cancer
The Society of Gynecologic Oncology (SGO) published recommendations during the COVID‐19 pandemic in response to the US Surgeon General's recommendations in March 2020 to triage elective surgical procedures based on acuity. 8 Most cancer cases were considered to be in the intermediate or high‐risk category, which influenced management of gynecologic oncology practice at our institution. Society guidelines also encouraged use of neoadjuvant, hormonal, or radiation therapy when appropriate to delay surgery and inpatient hospitalization. Our study demonstrated a decrease in total visits, reduction in new patient visits by 22.9%, and a decrease in follow‐up visits compared with the pre‐COVID time period. In addition, the gynecologic oncology service had the largest decrease in operations performed during the peak of the COVID‐19 pandemic with a reduction of 43.6%. As previously stated, a virtual weekly conference call with hospital administration prioritized cases from all surgical services based on acuity, those who met criteria underwent surgical intervention. Seventy‐eight percent on the patients referred to the gynecologic oncology service pre‐COVID were for benign or undetermined gynecologic conditions with a decrease during the post‐COVID period (66.7%), therefore elective surgery was appropriately delayed. Seven patients (7.8%) were deferred to wait for surgery, underwent paracentesis, or received hormonal therapy.
Initial data from MDACC demonstrated that median gynecologic oncology case volume per week decreased from 25 to 7 (p < 0.001) during the COVID‐19 pandemic. 12 In a retrospective study of three affiliated New York Presbyterian hospitals, 39% of patients with gynecologic cancer experienced modification (delay, change, or cancellation) to their treatment during the first 2 months of the pandemic. 13 Of the patients who received modifications to therapy, those scheduled for surgery were the largest group affected (67.4%), followed by those scheduled for systemic treatment (21.5%), and those scheduled for radiation (18.8%). In a survey of over 300 members of the SGO, practice volume reportedly dropped 61.6% since the beginning of the pandemic, with most cancellations being provider initiated. 19 It was also reported that more than 94% of responders proceeded with gynecologic cancer operations with the exception of Grade 1 endometrioid endometrial adenocarcinoma. When evaluating our practice patterns pre and post‐COVID restrictions, there was no difference in the percent of patients scheduled for surgery after initial clinic visit (38.6% vs. 40.0%). This most likely reflects those patients who met criteria for gynecologic cancer operations who proceeded to surgery. The significant decrease in total gynecologic oncology surgical volume is likely explained by the few high acuity patients who were able to undergo acceptable alternative oncologic treatment or delay referral and therefore surgery due to benign or undetermined disease.
4.3. Melanoma/Soft tissue
Our institution utilized resources from the SSO and a consortium of major US cancer centers to develop our protocol for melanoma, squamous cell carcinoma, basal cell carcinoma, and soft‐tissue sarcomas. 5 , 6 For low‐risk cutaneous malignancies, definitive procedures were generally deferred for at least 3 months. For indeterminate or high‐risk cutaneous lesions, in‐office procedures were advised when possible. Low‐grade sarcoma lesions were deferred 3 months as long as patients were asymptomatic. For intermediate and high‐grade sarcoma lesions, multidisciplinary consensus regarding neoadjuvant therapy was attained if possible. The Melanoma/Soft Tissue service had a decrease in total clinic visits. New patient visits decreased by 36.5%, and there was a decrease in follow‐up visits in the COVID period. Cases scheduled for surgery decreased from 69% to 56.4% and in‐office procedures increased from 2% to 12.8%. Four patients (8%) were deferred from surgery and total operations decreased by 25.8%. A possible explanation for the decreased volume in Melanoma/Soft Tissue during the pandemic could have resulted from the triaging of patients and closure of primary care and dermatology offices leading to a decrease in referrals. Additionally, considering that certain cutaneous malignancies can be managed surgically in the office under local anesthesia, this increase in office procedures could have partially compensated for the decrease in surgery scheduling and overall operations. Similar to our findings, the median case volume per week for melanoma and sarcoma in the MDACC cohort decreased during the COVID‐19 pandemic (18 vs. 9 cases per week, p = 0.005 and 3 vs. 0 cases per week, p = 0.002, respectively). 12 There remains limited literature published on management of cutaneous malignancy and soft tissue sarcoma during the COVID‐19 pandemic, and our current study confirms this subspecialty was initially disproportionately affected.
4.4. Endocrine surgery
The SSO and American Association of Endocrine Surgeons released recommendations and statements during the COVID‐19 pandemic for triaging elective endocrine surgery, which were utilized to create an institutional endocrine specific COVID protocol. 5 , 9 Guidelines for surgical management and clinic visits were instituted for malignant thyroid nodules, benign thyroid disease, primary hyperparathyroidism, adrenal masses, and functional pancreatic tumors. During the COVID‐19 pandemic in the current study, total outpatient visits, follow‐up visits, and new patient visits decreased by 37.3%, 31.2%, and 37.2%, respectively. Out of all specialties investigated, endocrine surgery had the highest percentage of deferred cases while counseling patients in clinic. There was no change in total operations performed in the first month of the pandemic, though a slight decrease in total operations was demonstrated in April which is expected to continue and increase, as these cases tend to get scheduled over a longer period of time. While not observed in the first month of the pandemic, we expect subsequent case numbers would be similar to the recent MDACC study, which demonstrated a decrease in median case volume from 10 to 1 case per week during the COVID‐19 pandemic (p = 0.001). 12
4.5. Gastrointestinal cancer
On April 8, 2020, SSO guidelines regarding gastrointestinal malignancies, including colorectal, gastric, esophageal, and hepato‐pancreato‐biliary (HPB) cancer were published. 5 These guidelines as well as virtual weekly conference calls with hospital administration influenced the triage of cases. Our GI surgical oncology outpatient clinic had a decrease in total visits, which included a 22.9% decrease in new visits, and a 21.3% decrease in follow‐up visits. Only 1.4% of cases were deferred, and there was a slight, nonsignificant increase (3.7%) in total cases performed in the initial months of the pandemic. There was a decrease in the amount of upper GI cases, port placements, and staging procedures, while there was no change in HPB cases and an increase in lower GI cases. This is a contrast to the MDACC data which demonstrated a decrease in median case volume for HPB and colorectal services during the COVID‐19 pandemic (11 vs. 1 case per week, p = 0.001 and 22 vs. 10 cases per week, p = 0.001, respectively). 12
Our operative GI findings differ from the other surgical oncology services and those reported by MDACC. There are several possibilities that could have accounted for this. Taking into account the time period, our data demonstrates there was a slight increase in GI oncology cases before the executive order to cease elective surgeries in New Jersey followed by drastic reduction in April. Most likely, with more available OR time and the executive order enforcement looming, more GI oncology cases were expedited in mid to late March. In addition, considering there was no change in HPB case volume, the fact that these malignancies tend to be more aggressive with limited data for neoadjuvant and targeted therapy, early surgical intervention is necessary and most likely were prioritized during this period. The observation that lower GI cases increased, can possibly be explained by this patient population being a higher risk for emergency cases (i.e., bleeding, obstruction, or perforation) than other services, yet such data was not available to confirm this hypothesis.
Additionally, an interesting observation was that inpatient LOS for GI surgical oncology was significantly decreased from 12.4 to 8 days. The length of stay could have decreased because of an increased focus on “enhanced recovery” methods during the height of the pandemic, with ancillary staff focusing on early ambulation, incentive spirometry, and also patient motivation for being discharged to home as soon as medically stable.
4.6. Telehealth services
Telehealth is defined as the use of electronic information and communications technologies to provide and support health care when distance separates participants. 20 To minimize interruption of essential clinical services, there was a rapid transition from in‐person to telemedicine encounters in the United States during the pandemic. Our institution followed this trend as telemedicine visits were quickly implemented by April 2020. Before the COVID‐19 pandemic, our institution had no infrastructure for telemedicine, yet during this time, we were able to efficiently implement the Doxy.me platform for patient care. This shift in practice throughout the United States was supported and enabled by federal agencies promoting telehealth through regulatory relaxation and increased funding. 21 , 22 Both Centers for Medicare and Medicaid Services and commercial payers expanded telemedicine benefits for patients and provided equivalent reimbursement for video telemedicine visits as traditional in‐person visits. Previous surgical literature has demonstrated that telehealth is safe but has primarily been performed in the postoperative setting focusing on low‐risk patients undergoing low‐risk procedures. 20 , 22 , 23 However, telemedicine may also be effective postoperatively in high‐risk procedures, such as in liver transplant and colectomy. 24 , 25 With the overwhelming support and expansion of telemedicine during the COVID‐19 pandemic, this service may remain an option for patients and surgeons going forward. Further studies are required to truly evaluate the use and efficacy of telemedicine in the realm of surgical oncology and to further evaluate its utility in the preoperative setting.
This project demonstrates our institution's experience and response to an unprecedented global pandemic. Although this presented an unpredictable and difficult challenge, especially for immunocompromised oncology patients, multidisciplinary communication and organization, implementation of institutional protocols, and supplementation of clinical care with telemedicine were essential tools in providing care to our patients. Such practices were key to the quick and efficient transitions that enabled us to provide optimal care to a vulnerable patient population. This provides a framework for cancer care during the continued COVID‐19 pandemic, possible variants, and other similar impediments to care in the future.
4.7. Limitations
There are several important limitations to our study. First, we are reporting retrospective data from a single institution during the initial months of the COVID‐19 pandemic in the United States, which may not be generalizable to all populations or to the subsequent months. Results were largely based on chart review of institutional data, so findings are influenced by the degree and accuracy of documentation by healthcare providers. Lastly, this is a descriptive report of initial trends in response to change in management and practice in surgical oncology at an NCI‐designated cancer center during the COVID‐19 pandemic, long term sequalae of these interventions are currently unknown.
5. CONCLUSION
The initial impact of the COVID‐19 pandemic on surgical oncology practice appears to be disproportionate within various surgical oncology subspecialties. Future studies should correlate these immediate effects with long‐term oncologic outcomes. National, local, and institutional guidelines have impacted standards of care and aided providers in the management of patients in both the inpatient and outpatient settings. Telehealth services are a feasible method to provide outpatient oncologic care and should be strongly considered in surgical oncology practice.
DISCLOSURES
The authors have no disclosures and there is no funding source.
SYNOPSIS
The COVID‐19 pandemic presented challenges for healthcare providers due to social distancing, depletion of healthcare resources, and lack of personal protective equipment. Our findings demonstrate immediate wide‐ranging effects within surgical oncology subspecialty practice volume during this time.
Supporting information
Supporting information.
Supporting information.
Gazivoda V, Greenbaum A, Roshal J, et al. Assessing the immediate impact of COVID‐19 on surgical oncology practice: Experience from an NCI‐designated Comprehensive Cancer Center in the Northeastern United States. J Surg Oncol. 2021;124:7‐15. 10.1002/jso.26475
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Center for Disease Control (CDC) . United States COVID‐19 Cases and Deaths by State. Published 2020. https://covid.cdc.gov/covid-data-tracker/#cases_casesper100klast7days. Accessed December 17, 2020.
- 3. ASCA Foundation. State Guidance on Elective Surgeries . Published 2020. https://www.ascassociation.org/asca/resourcecenter/latestnewsresourcecenter/covid-19/covid-19-state. Accessed December 17, 2020.
- 4. American College of Surgeons . COVID‐19: Recommendations for Management of Elective Surgical Procedures. Published 2020. https://www.facs.org/covid-19/clinical-guidance/elective-surgery. Accessed December 13, 2020.
- 5. Bartlett DL, Howe JR, Chang G, et al. Management of cancer surgery cases during the COVID‐19 pandemic: considerations. Ann Surg Oncol. 2020;27(6):1717‐1720. 10.1245/s10434-020-08461-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. National Comprehensive Cancer Network . Short‐Term Recommendations for Cutaneous Melanoma Management During COVID‐19 Pandemic. Published 2020. https://www.nccn.org/covid-19/pdf/Melanoma.pdf. Accessed December 13, 2020.
- 7. Dietz JR, Moran MS, Isakoff SJ, et al. Recommendations for prioritization, treatment, and triage of breast cancer patients during the COVID‐19 pandemic. the COVID‐19 pandemic breast cancer consortium. Breast Cancer Res Treat. 2020;181(3):487‐497. 10.1007/s10549-020-05644-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dowdy S, Nickles Fader A. 2020. Surgical Considerations for Gynecologic Oncologists During The COVID‐19 Pandemic. Published. https://www.sgo.org/resources/surgical-considerations-for-gynecologic-oncologists-during-the-covid-19-pandemic/. Accessed December 13, 2020.
- 9. American Association of Endocrine Surgeons . Thoughts on elective endocrine surgery from AAES members. Published 2020. https://www.endocrinesurgery.org/covid-19. Accessed Dec. 2020.
- 10. Antonoff M, Backhus L, Boffa DJ, et al. COVID‐19 guidance for triage of operations for thoracic malignancies: a consensus statement from Thoracic Surgery Outcomes Research Network. J Thorac Cardiovasc Surg. 2020;160(2):601‐605. 10.1016/j.jtcvs.2020.03.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hui JYC, Yuan J, Teoh D, et al. Cancer management during the COVID‐19 pandemic in the United States. Am J Clin Oncol. 2020;43(10):679‐684. 10.1097/COC.0000000000000757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chang EI, Liu JJ. Flattening the curve in oncologic surgery: impact of Covid‐19 on surgery at tertiary care cancer center. J Surg Oncol. 2020;122(4):602‐607. 10.1002/jso.26056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frey MK, Fowlkes RK, Badiner NM, et al. Gynecologic oncology care during the COVID‐19 pandemic at three affiliated New York City hospitals. Gynecol Oncol. 2020;159(2):470‐475. 10.1016/j.ygyno.2020.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kiong KL, Guo T, Yao CMKL, et al. Changing practice patterns in head and neck oncologic surgery in the early COVID‐19 era. Head Neck. 2020;42(6):1179‐1186. 10.1002/hed.26202 [DOI] [PubMed] [Google Scholar]
- 15. Morrison DR, Gentile C, McCammon S, Buczek E. Head and neck oncologic surgery in the COVID‐19 pandemic: our experience in a deep south tertiary care center. Head Neck. 2020;42(7):1471‐1476. 10.1002/hed.26262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Patt D, Gordan L, Diaz M, et al. Impact of COVID‐19 on cancer care: how the pandemic is delaying cancer diagnosis and treatment for American seniors. JCO Clin Cancer Informatics. 2020;(4):1059‐1071. 10.1200/CCI.20.00134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kaufman HW, Chen Z, Niles J, Fesko Y. Changes in the number of US patients with newly identified cancer before and during the coronavirus disease 2019 (COVID‐19) pandemic. JAMA Network Open. 2020;3(8):e2017267. 10.1001/jamanetworkopen.2020.17267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yin K, Singh P, Drohan B, Hughes KS. Breast imaging, breast surgery, and cancer genetics in the age of COVID‐19. Cancer. 2020;126(20):4466‐4472. 10.1002/cncr.33113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nakayama J, El‐Nashar SA, Waggoner S, Traughber B, Kesterson J. Adjusting to the new reality: evaluation of early practice pattern adaptations to the COVID‐19 pandemic. Gynecol Oncol. 2020;158(2):256‐261. 10.1016/j.ygyno.2020.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gunter RL, Chouinard S, Fernandes‐Taylor S, et al. Current use of telemedicine for post‐discharge surgical care: a systematic review. J Am Coll Surg. 2016;222(5):915‐927. 10.1016/j.jamcollsurg.2016.01.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Contreras CM, Metzger GA, Beane JD, Dedhia PH, Ejaz A, Pawlik TM. Telemedicine: patient‐provider clinical engagement during the COVID‐19 pandemic and beyond. J Gastrointest Surg. 2020;24(7):1692‐1697. 10.1007/s11605-020-04623-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Purnell S, Zheng F. Safety of surgical telehealth in the outpatient and inpatient setting. Surg Clin North Am. 2021;101(1):109‐119. 10.1016/j.suc.2020.09.003 [DOI] [PubMed] [Google Scholar]
- 23. Nandra K, Koenig G, Del Mastro A, Mishler EA, Hollander JE, Yeo CJ. Telehealth provides a comprehensive approach to the surgical patient. Am J Surg. 2019;218(3):476‐479. 10.1016/j.amjsurg.2018.09.020 [DOI] [PubMed] [Google Scholar]
- 24. Lee TC, Kaiser TE, Alloway R, Woodle ES, Edwards MJ, Shah SA. Telemedicine based remote home monitoring after liver transplantation. Ann Surg. 2019;270(3):564‐572. 10.1097/SLA.0000000000003425 [DOI] [PubMed] [Google Scholar]
- 25. Bednarski BK, Nickerson TP, You YN, et al. Randomized clinical trial of accelerated enhanced recovery after minimally invasive colorectal cancer surgery (RecoverMI trial). Br J Surg. 2019;106(10):1311‐1318. 10.1002/bjs.11223 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


