Abstract
SARS‐CoV‐2 infection has emerged as not only a pulmonary but also potentially multi‐organ disease, which may cause long‐term structural damage of different organ systems including the lung, heart, vasculature, brain, liver, kidney, or intestine. As a result, the current SARS‐CoV‐2/COVID‐19 pandemic will eventually yield substantially increased numbers of chronically diseased patients worldwide, particularly suffering from pulmonary fibrosis, post‐myocarditis, chronic heart failure, or chronic kidney disease. Exercise recommendations for rehabilitation are complex in these patients and should follow current guidelines including standards for pre‐exercise medical examinations and individually tailored exercise prescription. It is of utmost importance to start exercise training at an early stage after COVID‐19 infection, but at the same time paying attention to the physical barriers to ensure safe return to exercise. For exercise recommendations beyond rehabilitation programs particularly for leisure time and elite athletes, more precise advice is required including assessment of sports eligibility and specific return‐to‐sports exercise programs. Because of the current uncertainty of long‐term course of SARS‐CoV‐2 infection or COVID disease, long‐term follow‐up seems to be necessary.
Keywords: chronic heart failure, chronic kidney disease, coronavirus disease‐19, individualized training program, lung fibrosis, sports eligibility
1. COVID‐19: A SYSTEMIC DISEASE
The view of SARS‐CoV‐2 as a primary respiratory pathogen has been challenged when increasing reports on multi‐organ manifestation involving lung, heart, kidney, intestine, and brain had been reported. 1 , 2 This multi‐organ manifestation of coronavirus disease‐19 (COVID‐19) can be explained by the different pathomechanisms that all contribute to the deleterious systemic effects of SARS‐CoV‐2 infection 3 , 4 , 5 , 6 , 7 , 8 :(a) Entry of SARS‐CoV‐2 into cells via binding to the ACE‐2 receptor, a ubiquitously present membrane receptor, (b) Down‐regulation of ACE‐2 inducing a decrease in anti‐fibrotic Ang1‐7 and an increase in pro‐fibrotic angiotensin, (c) Over‐activation of the innate immune system leading to a cytokine release syndrome (CRS), (d) Induction of a ubiquitously observed endothelialitis, accompanied by (e) Procoagulatory effects and induction of microthrombi. Overall, COVID‐19 disease, particularly in case of a severe course with long‐term bed rest, mechanical ventilation, and multi‐organ involvement will lead to significant physical and functional deconditioning affecting the whole organism (Figure 1). Moreover, organ tissue damage and degree of morphological as well as functional recovery after the acute phase of the lungs, myocardium, neurological system, kidneys, and peripheral musculature will result in reduced exercise capacity at short and long term. Exercise training has been shown to improve exercise capacity as well as quality of life during rehabilitation of patients with various chronic diseases, 9 and we hypothesize that also COVID‐19 patients will benefit from it, especially when organ‐specific exercise prescription is applied. Exercise tolerance is impaired in COVID‐19 patients particularly in case of pulmonary involvement, but involvement of other organs will have additional deleterious effects (Figure 1). Development of pulmonary fibrosis may reduce CO diffusing capacity. Moreover, pulmonary artery hypertension caused by distortion and destruction of the pulmonary vascular bed, 3 hypoxia‐induced vasoconstriction, and pulmonary artery thromboembolism will have a substantial impact on exercise tolerance. Furthermore, this can be potentiated by reduced ejection fraction when the heart is involved as in COVID‐19 myocarditis. 10 These cardio‐pulmonary impairments may be accompanied by thromboembolic complications of COVID‐19 presenting as ischemic and (less frequently) hemorrhagic stroke. 11 , 12 Like other infections, COVID‐19 may also induce encephalopathy 12 or peripheral neuropathy 11 including inflammation of peripheral myelin sheaths leading to Guillain‐Barre syndrome or even central variants like the Miller‐Fisher syndrome. 13 , 14 Whether these acute alterations persist in COVID‐19 patients after clinical recovery is rather likely, but remains to be seen.
FIGURE 1.
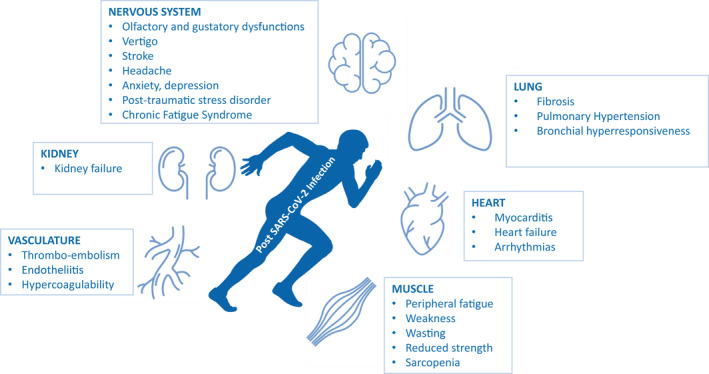
Organ dysfunction after SARS‐CoV‐2 infection limiting exercise performance and quality of life
2. RATIONALE FOR EXERCISE‐BASED REHABILITATION AFTER COVID‐19
Immobility particularly during severe courses of COVID‐19 with intensive care treatment and mechanical ventilation possibly for weeks will significantly lead to a substantial decline in physical functioning (Figure 1). Even milder forms of disease will have systemic symptoms such as fever and myalgia, which will also reduce physical activity for some time during the acute phase and reduce exercise capacity as well. The full clinical picture becomes even more complicated in patients with multi‐organ involvement (Figure 1).
As exercise training has shown to have substantial beneficial effects and is recommended as standard therapy in pulmonary disease, for example, fibrosis or pulmonary hypertension, 15 , 16 , 17 heart failure, 18 kidney disease 19 , 20 as well sarcopenia, 21 exercise programs for post‐COVID‐19 patients are absolutely mandatory. 22 , 23 These patients have to be integrated into general rehabilitation programs, which also consider COVID‐19‐specific comorbidities. Exercise training should be supervised during the early phase of rehabilitation, for example, during in‐hospital or in group exercises and can be added and later confined to non‐supervised training in a home‐based setting or in a fitness gym. Support by telemedicine should be considered as an additional tool to increase long‐term adherence to rehabilitation programs. 24 , 25
Beyond general COVID‐19 rehabilitation, young and previously physically active individuals or even athletes involved in competitive sports will have a special interest to return‐to‐sports and exercise on a leisure time or competition level. For them, specific recommendations for pre‐exercise examinations as well as exercise training are necessary 26 (Figure 2). Currently, it is unclear whether mild courses of SARS‐CoV‐2 infection have the potential to induce prolonged organ impairment, for example, subclinical reduction in lung diffusion capacity after pneumonia that may limit maximal exercise capacity. Therefore, a clinical examination has to be performed in all individuals after COVID‐19 before starting a rehabilitation program or an individualized training program in athletes (Figure 2).
FIGURE 2.
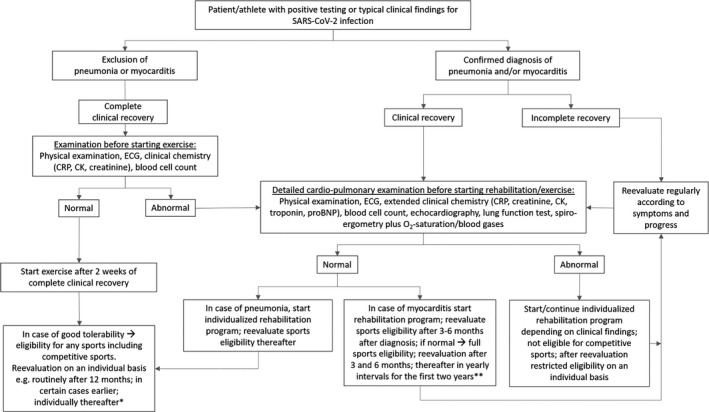
Algorithm for decision‐making on exercise recommendations in patients and athletes after SARS‐CoV‐2 infection and COVID‐19. *Recommendations for follow‐up intervals are based on authors’ consensus, due to the paucity of scientific evidence on long‐term impact of SARS‐CoV‐2 infection on cardio‐pulmonary function. **Recommendations are based on current guidelines 28
As pneumonia and myocarditis pose the potential for cardio‐pulmonary limitations, increased risk and reduced maximal exercise performance, symptoms as well as clinical examination, will have to focus on these entities (Figure 2). In leisure time and elite athletes, recommendations for return‐to‐sport and eligibility for competitive sports depend on clinical and functional recovery, the kind of organ involvement and the type of sport, for example, skill, power, mixed, or endurance. 27 , 28
From our perspective, in elite and recreational athletes, basic routine medical re‐evaluation including a resting ECG and blood analysis should be performed after 3‐6 months in order to assess currently unknown long‐term effects of SARS‐CoV‐2 infection. However, many especially young athletes have no or very few symptoms initially, so that in these cases individualized guidance based on initial symptoms may be more meaningful. Whether additional examinations are necessary beyond this period has to be decided on an individual basis. In case of pulmonary or myocardial disease, regular in‐depth cardiological examination including exercise testing with arterial blood gases and optimally spiroergometry are recommended in addition to ECG and echocardiography on an annual basis at least for the first two years (Figure 2). Leisure time and elite athletes will be followed on a routine basis at least in yearly intervals.
3. DIAGNOSTICS BEFORE STARTING EXERCISE IN COVID‐19 PATIENTS
As SARS‐CoV‐2 may affect multiple organ systems, exercise recommendations can only be applied after a sound clinical examination (Figure 2). These diagnostic measures should follow an algorithm that takes into consideration the severity of the disease course, age, as well as post‐disease exercise performance. 29 Besides detailed history taking and physical examination, a blood analysis should assess parameters of inflammation, blood cell count, kidney function, and cardiac biomarkers in case of previous myocarditis or pneumonia. Special focus should be laid on testing lung and myocardial function, which may be supplemental diagnostics, for example, neurological tests. Diagnostics are particularly not easy in case of suspected myocarditis, as this very much depends on the timing of initial evaluation from first signs/symptoms of infection as well as a combination of symptoms, cardiac markers, ECG changes, and structural changes examined by echocardiography and/or cardiac MR scan.
Moreover, as data for COVID‐19 and long‐term prognosis have not yet been established, recommendations are extrapolated from current understandings of other virus‐induced pathologies, but may change when more data become available. Because of the unknown long‐term prognosis of SARS‐CoV‐2 infection, we recommend follow‐up examinations in shorter intervals (Figure 2).
3.1. Pulmonary diagnostics
It is known from previous SARS epidemics that patients presenting during a 2‐year follow‐up revealed impaired diffusion capacity with overall decreased exercise performance capacity, 30 a finding that is also observed for COVID‐19. 31 Therefore, standard workup for athletes should include static and dynamic lung function testing for assessment of vital capacity and forced expiratory volumes. Because of the fibrotic features of COVID‐19, measurements of residual volume and total lung capacity by body plethysmography are recommended in those with priory proven pulmonary involvement or symptomatic athletes/patients with signs of persistent respiratory limitations like dyspnea or persistent bronchial hyperreactivity. In these patients, exercise testing including either oxygen saturation measurements during exercise or blood gas analysis before and after maximal exercise is advisable, as it will unmask impairment of diffusion capacity. The fall of oxygen saturation during exercise (<94%) is indicative of significant lung diffusion impairment, while mild forms of impairment may only be detected by measurements of arterial blood gases during cardiopulmonary exercise testing (CPET). 29 , 32 During CPET, special focus should additionally be laid on parameters of respiratory efficiency (ventilatory equivalents). Moreover, increased values for dead space at rest may resemble bronchial hyperreactivity and air trapping, a known finding after viral pneumonia. 33 In case of persistent dyspnoea or otherwise unexplained limitation of exercise capacity, measurement of inspiratory muscle strength capacity may also be included into the clinical workup. 34
3.2. Cardiovascular diagnostics
Since cardiovascular involvement including inflammation of the coronary endothelium and myocardium has been reported in COVID‐19, cardiovascular screening in recovered COVID‐19 patients is mandatory before starting exercise training (Figure 2). Therefore, a resting ECG is required in every person (even if asymptomatic) prior to return‐to‐sports, exercise, or physical activity (Figure 2). Even in patients with previous or ongoing mild symptoms, for example, palpitations or dyspnea on exertion or an abnormal resting ECG, echocardiography should be added. In those with myocardial involvement during the acute phase, echocardiography is obligatory. Particularly myocardial involvement in SARS‐CoV‐2‐infection, even in those with only mild symptoms, may be detected by specific speckle tracking deformation abnormalities. 35 Therefore, this sensitive echocardiographic procedure should optimally be included in those with inconclusive myocardial findings or clinical evidence for myocarditis even in the presence of normal systolic function. If clinical examination, ECG or echocardiography, is suggestive of myocarditis, which seems to be present in only about 1% of young student and professional athletes suffering from COVID‐19, 36 , 37 a cardiac MRI (CMR) has to be performed using all modern modalities (eg, late gadolinium enhancement, T2 imaging, T1 mapping) to assess possible myocardial involvement (Figure 2).
3.3. Cardio‐pulmonary exercise diagnostics
Before return‐to‐sport, the cardio‐pulmonary system has to be evaluated during maximal exercise (Figure 2). Therefore, even in patients with COVID‐19 and only light symptoms, an exercise ECG including measurement of O2‐saturation is advisable in order to detect subclinical impairment, for example, arrhythmias or premature dyspnoea as in impaired lung diffusion capacity. Patients with more severe symptoms and/or post‐pneumonia or myocarditis should undergo spiroergometry with blood gas analysis. 29 , 32 Then, exercise‐related parameters relevant for respiratory efficacy like the breathing equivalents and the O2‐pulse as global measures of dysfunction of the cardio‐pulmonary system are of particular interest. In case results from previous pre‐participation screenings exist, results should be compared and preferably, identical protocols should be applied for comparison.
Most importantly exercise prescription should be individually tailored, and precise advices concerning exercise duration and exercise intensity should be given. Therefore, a maximal CPET or exercise protocol including lactate testing should be performed. 38 These data will help guide training intensities particularly during early phases of return‐to‐play concepts. Increases of exercise intensity and volume should be introduced with caution and might take weeks to months until the pre‐infection status—if ever—is reached. 30
3.4. Muscular and neurological testing
Due to the high likelihood of neurological involvement even in mild forms of COVID‐19, every patient should undergo a clinical neurological exam before returning to exercise. Emphasis should be given to motor, sensory, and coordination testing, but cranial and peripheral nerves should be carefully evaluated as well. Olfactory and gustatory dysfunctions should be assessed, 39 but are per se no contraindication to prescribe moderate‐intensity exercise. Vertigo, however, needs to be evaluated carefully by examining the vestibulo‐ocular system, because its persistence will impair exercise performance and may also affect safety during sports and exercise. Continuation of vertiginous symptoms will prompt further testing. Particularly a history of thromboembolic complications in the context of neurological symptoms will make an MRI of the brain inevitable to screen for cerebral embolisms and to assess the cerebral vasculature that may be affected by COVID‐19. 39 Patients with a history of nerve entrapment may benefit from nerve conduction studies and electromyography and if dysexecutive syndromes are present, cognitive screening or possibly a formal neuropsychological exam might be helpful.
4. EXERCISE TRAINING AFTER COVID‐19
Despite the multi‐organ involvement (Figure 1), exercise training should be encouraged in all patients after discharge from hospital or after overall recovery from acute infection. In addition to the clinical severity and time course, performance will be lost due to the illness per se, but also due to bed rest and long periods of deconditioning. As different and unpredictable as the characteristics of the clinical symptoms can vary between individuals with COVID‐19, recommendations regarding “return‐to‐sport” have to be made based on analogies from similar viral infections and organ involvement, as controlled studies are currently lacking. However, it is unequivocal that exercise training has to be individually tailored in order to optimize the balance between strain and adaptation processes on the basis of disease state and exercise performance. With this in mind, the German Sports Medicine Association and American College of Cardiology's Sports & Exercise Cardiology Council have recently published expert consensus statements on this matter for post‐COVID‐19 patients and athletes. 29 , 40 If full eligibility is determined by the recommended diagnostics and no structural damage remains, athletes can gradually return to their specific training and to competitive activities (Figure 2). However, when myocarditis or pneumonia have been present, more detailed medical analyses have to be performed including spiroergometry, echocardiography, and preferably CMR. 41 In case of myocarditis, depending on clinical and diagnostic findings, exercise may earliest be resumed after 3 months, but in most cases not before after 6 months or longer. 27
In clinical practice, 2‐3 days of graded return should be considered for each training day lost due to the illness. This seemingly long duration is based on the fact that, regardless of the direct, for example, virus‐induced inflammation or indirect effects (eg, mechanical ventilation), recovery of pulmonary tissue and vasculature is prolonged even when virus load has resolved. With regard to training structure, first frequency, then duration, and finally intensity should be increased. Monitoring of oxygen saturation and heart rate during exercise is a viable and practical tool for guiding exercise training during recovery periods.
In the following chapter, exercise recommendations will be given based on organ involvement due to COVID‐19 based on current knowledge of rehabilitation concepts for primarily sedentary subjects, recreational as well as elite athletes competing at national or international level (Figure 2).
4.1. Exercise after COVID‐19: Lung fibrosis, pulmonary hypertension, and bronchial hyperreactivity
The length of recovery from pneumonia varies depending on the clinical severity during acute COVID‐19 infection. Therefore, a decisive clinical pulmonary workup has to be performed before resuming exercise and sports (Figure 2). However, it is undisputable that exercise is a hallmark during recovery periods from pulmonary disease, as there is clear evidence of significant clinical improvement in lung function by targeted respiratory rehabilitation (daily exercise program for at least 6 weeks, ≥10 min/d). 5 This should initially include training of the in‐ and expiratory muscles using specific respiratory muscle training equipment to avoid pulmonary overload, the former including diaphragmatic breathing. Sustained maximal inspiration training will also lead to an even distribution of ventilation and thus to a reduction in atelectases. Expiratory muscle training will promote clearance of secretion.
Endurance exercise and pulmonary function training are added by resistance and flexibility training to improve peripheral adaptations. Interval training seems to be superior to moderate continuous training regarding adherence. 17 Strength training should initially focus on a dynamic character, for example, 1‐3 sets of 15‐20 repetitions at moderate intensity (Borg CR10 scale: 4‐6). 16 Even in pulmonary artery hypertension, exercise training has been shown to be safe and feasible without negative sequelae of right ventricular overload. 17 , 42
In recreational or competitive athletes, impairment of pulmonary function is a key limiting factor in endurance sports, but may be of less significance in skill or power sports. If pulmonary impairment persists, athletes may never achieve pre‐COVID performance values. Nonetheless, a complex exercise training aiming at improvement in oxidative capacity of the peripheral musculature with steady increment will compensate for cardio‐pulmonary limitations and increase overall exercise performance.
4.2. Exercise after COVID‐19: Myocarditis
Exercise training after acute myocarditis should be resumed in a setting of supervised rehabilitation including a structured exercise program (Figure 2). As myocarditis ranges from those with complete recovery to those with persistent myocardial injury (positive LGE in CMR), training has to be individually adapted and eligibility for competitive sports has to be made on an individual basis.
Patients with an uncomplicated course of acute myocarditis and complete recovery, including normal LV function without late gadolinium enhancement in CMR, have a very good prognosis. 43 , 44 In these patients, exercise training of moderate intensity can be introduced 1 month after the acute phase, even if other limitations, for example, impaired lung function testing or gas exchange are lead findings, but should not be increased before three months 27 , 41 (Figure 2). In case of pericarditis without myocarditis, exercise may be resumed mostly after 6 weeks of acute phase recovery. 41
Patients and particularly athletes with recovered myocardial function after initially impaired ejection fraction should be advised to refrain from structured high‐intensity training or competitive sports for at least 3 but mostly 6 months. 27 , 41 Although the current course of myocardial disease in COVID‐19 is unclear, it is likely from previous experience with other SARS infections that viral load and chronic inflammation may persist even when clinical symptoms have subsided. A complete cardiac investigation (Figure 2) including CPET will have to be performed for assessment of pathologies, but also for exercise intensity prescription. 28 , 41 First endurance exercise should start at moderate intensity (50%‐70% VO2max) for 4‐6 weeks before higher intensity exercise is resumed. Thereafter, full eligibility for competitive sports can be approved in most cases (Figure 2). Patients and athletes who present with impaired myocardial function or myocardial fibrosis even after 6 months can resume moderate‐intensity exercise. 18 However, attesting eligibility for competitive sports in unresolved disease can likely not be attested particularly in endurance and mixed sports because of increased mortality rates 44 amplified by exercise.
4.3. Exercise after COVID‐19: Heart failure
Recently published recommendations authored by the Secondary Prevention and Rehabilitation Section of the European Association of Preventive Cardiology confirm the highest level of evidence for the effects of exercise‐based cardiac rehabilitation in all patients with heart failure, regardless of their left ventricular ejection fraction. 9 Enrollment should occur right after hospital discharge and long‐term aims should focus on a lifelong, heart healthy, and thus physically active lifestyle. Indeed, cardiac rehabilitation is well established in heart failure without COVID‐19 9 and there is no reason to believe that it was not effective in patients who recovered from COVID‐19. A recent systematic literature search summarized findings by stating that COVID‐19 patients should be granted access to early cardiac rehabilitation and that if face‐to‐face contact is not an option, then tele‐rehabilitation should be considered. 24
Since medical therapy is part of secondary prevention, it is noteworthy that treatment with ACE inhibitors and ARBs has been found not to elicit excess mortality in heart failure patients and standard heart failure medication should not be altered in fear of untoward effects caused by SARS‐CoV‐2. 45
In addition, exercise training has to be individually tailored, ideally during programs of cardiac rehabilitation where all types of endurance and resistance training may be considered, and individual choices can be made according to disease stage, comorbidities, and current physical fitness. No differentiation shall be made between HFpEF and HFrEF. 18 As in heart failure patients without COVID‐19, in case of NYHA III, exercise intensity should initially be <40% of VO2peak during the first few weeks and can then be cautiously up‐titrated to 50‐70% VO2peak for 30‐60 min/d. 9 Higher intensities up to 85% VO2peak even as high‐intensity interval training have been shown effective and can be chosen according to patients’ progress during exercise training and individual preference. 46 Resistance exercise training supplements aerobic exercise training and avoids or reverts loss of skeletal muscle and thus deconditioning. Weights should be chosen so that 2‐3 sets of 10‐15 repetitions can be performed. Respiratory training (15‐30 minutes, 30%‐60% of maximal inspiratory pressure) should be part of the training regime as in pulmonary disease to improve VO2peak, dyspnoea, and respiratory muscle competence.
4.4. Exercise after COVID‐19: Chronic kidney disease
The prevalence of chronic kidney disease (CKD) and long‐term hemodialysis will certainly increase with the SARS‐CoV‐2 pandemic, as the infection has shown to affect the kidneys inducing dysfunction or even kidney failure particularly in patients with multiple organ involvement. 1 , 47 Evidence from current statistics reveals that 75% of CKD patients are frail and spend a sedentary lifestyle, exercising less than once a week, which is directly correlated with adverse health outcomes. 48 Therefore, exercise training should be prescribed as early as possible, even during the acute phase, in order to maintain exercise capacity. Exercise should also be introduced in patients requiring hemodialysis. Then exercise can either be performed during hemodialysis (intradialytic exercise, eg, bed ergometry) or between dialysis sessions (interdialytic exercise), the former with superior adherence and preferred option to improve efficacy of hemodialysis, physical fitness, cardiovascular risk factors, quality of life, and other health‐related benefits. 49 There is only limited experience regarding the definition of optimal intensity or duration of exercise, which is currently investigated in large randomized trials (NCT03885102). In general, a low‐ to moderate‐intensity endurance exercise training on a bed ergometer is recommended in combination of resistance and coordination training at least twice or thrice a week. 49 Because of a high prevalence of polyneuropathy and sarcopenia, walking with the risk of falling is not recommended. To improve the latter, daily exercises for coordination should be included into daily life, optimally twice a day, combined with strength exercises. Intensity of exercise is mostly low, and adherence to the training protocol is the main target. If these regimens are followed, long‐term adherence even over five years is good. 50
If COVID‐19 leads to kidney function impairment or even kidney failure, kidney transplantation may become necessary. Full eligibility for non‐contact sports is usually attested in clinically stable athletes after transplantation.
4.5. Exercise after COVID‐19: Neurological disease
Similar to other organ manifestations of COVID‐19, no data on long‐term outcome or complications of neurological pathologies or effects of exercise on these are available so far. However, it can be expected that exercise will have positive effects on resaturation and plasticity of the nervous system like in other neurological disorders. Therefore, resuming or starting exercise is also highly desired from a neurological standpoint and should be encouraged in all patients. Patients with motor deficits, for example, COVID‐19‐associated strokes or remaining vertigo need to be assessed according to their functional state and most likely benefit from exercise and physical therapy under professional supervision.
If athletes are affected by neurological complications of COVID‐19, exercise should be adapted to neurological recovery. Competitive sports are not restricted by neurological diseases per se, but athletes may be limited due to neurological constraints.
5. PERSPECTIVE
In view of the systemic nature of COVID‐19, individualized training concepts for COVID‐19 patients in rehabilitation settings during the acute phase, early recovery phase, and beyond are mandatory. Patients intending to perform leisure time exercise training or even return to competitive sports will have to be specially guided. These recommendations should be differentiated in relation to level of exercise from ensuring independence in the fragile old patient to leisure time or elite athletes, pushing themselves to the limit in relation to exercise intensity and duration. For them a network of physicians specialized in sports medicine, sports cardiology, pulmonology as well as sports and exercise physiologists will have to be established to guarantee safety and efficacy of training at the same time.
CONFLICT OF INTEREST
All authors declare that they have no conflict of interest.
ACKNOWLEDGEMENT
This manuscript has been endorsed by the European Association of Preventive Cardiology of the European Society of Cardiology
Halle M, Bloch W, Niess AM, et al. Exercise and sports after COVID‐19—Guidance from a clinical perspective. Transl Sports Med. 2021;4:310–318. 10.1002/tsm2.247
Johannes Scherr and Josef Niebauer contributed equally to this article.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. JAMA. 2020;323:2052‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England). 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid‐19. N Engl J Med. 2020;383:120‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271‐280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu PP, Blet A, Smyth D, Li H. The science underlying COVID‐19: implications for the cardiovascular system. Circulation. 2020;142:68‐78. [DOI] [PubMed] [Google Scholar]
- 6. Bikdeli B, Madhavan MV, Jimenez D, et al. COVID‐19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow‐up: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2020;75:2950‐2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID‐19. Lancet (London, England). 2020;395:1417‐1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Merad M, Martin JC. Pathological inflammation in patients with COVID‐19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ambrosetti M, Abreu A, Corra U, et al. Secondary prevention through comprehensive cardiovascular rehabilitation: from knowledge to implementation. 2020 update. A position paper from the secondary prevention and Rehabilitation Section of the European Association of Preventive Cardiology. Eur J Prev Cardiol 2020:2047487320913379. [DOI] [PubMed] [Google Scholar]
- 10. Madjid M, Safavi‐Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5(7):831‐840. [DOI] [PubMed] [Google Scholar]
- 11. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS‐CoV‐2 infection. N Engl J Med. 2020;382:2268‐2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhao H, Shen D, Zhou H, Liu J, Chen S. Guillain‐Barre syndrome associated with SARS‐CoV‐2 infection: causality or coincidence? Lancet Neurol. 2020;19:383‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Toscano G, Palmerini F, Ravaglia S, et al. Guillain‐Barré Syndrome associated with SARS‐CoV‐2. N Engl J Med. 2020;382:2574‐2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kenn K, Gloeckl R, Behr J. Pulmonary rehabilitation in patients with idiopathic pulmonary fibrosis–a review. Respiration. 2013;86:89‐99. [DOI] [PubMed] [Google Scholar]
- 16. Vainshelboim B, Fox BD, Oliveira J, Kramer MR. Exercise training in idiopathic pulmonary fibrosis. Expert Rev Respir Med. 2016;10:69‐77. [DOI] [PubMed] [Google Scholar]
- 17. Gloeckl R, Schneeberger T, Jarosch I, Kenn K. Pulmonary rehabilitation and exercise training in chronic obstructive pulmonary disease. Deutsch Arztebl Int. 2018;115:117‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schindler MJ, Adams V, Halle M. Exercise in heart failure‐what is the optimal dose to improve pathophysiology and exercise capacity? Curr Heart Fail Rep. 2019;16:98‐107. [DOI] [PubMed] [Google Scholar]
- 19. Kirkman DL, Scott M, Kidd J, Macdonald JH. The effects of intradialytic exercise on hemodialysis adequacy: a systematic review. Semin Dial. 2019;32:368‐378. [DOI] [PubMed] [Google Scholar]
- 20. Barcellos FC, Santos IS, Umpierre D, Bohlke M, Hallal PC. Effects of exercise in the whole spectrum of chronic kidney disease: a systematic review. Clini Kidney J. 2015;8:753‐765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moore SA, Hrisos N, Errington L, et al. Exercise as a treatment for sarcopenia: an umbrella review of systematic review evidence. Physiotherapy. 2020;107:189‐201. [DOI] [PubMed] [Google Scholar]
- 22. Ambrosetti M, Abreu A, Cornelissen V, et al. Delphi consensus recommendations on how to provide cardiovascular rehabilitation in the COVID‐19 era. Eur J Prev Cardiol 2020:zwaa080. 10.1093/eurjpc/zwaa080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Faghy MA, Arena R, Stoner L, et al. The need for exercise sciences and an integrated response to COVID‐19: a position statement from the international HL‐PIVOT network. Prog Cardiovasc Dis. 2021:S0033‐0620(21)00013‐X. Online ahead of print. 10.1016/j.pcad.2021.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Scherrenberg M, Wilhelm M, Hansen D, et al. The future is now: a call for action for cardiac telerehabilitation in the COVID‐19 pandemic from the secondary prevention and rehabilitation section of the European Association of Preventive Cardiology. Eur J Prev Cardiol 2020:2047487320939671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Meinhart F, Stütz T, Sareban M, Kulnik ST, Niebauer J. Mobile technologies to promote physical activity during cardiac rehabilitation: a scoping review. Sensors. 2021;21(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bhatia RT, Marwaha S, Malhotra A, et al. Exercise in the Severe Acute Respiratory Syndrome coronavirus‐2 (SARS‐CoV‐2) era: a question and answer session with the experts endorsed by the section of sports cardiology & exercise of the European Association of Preventive Cardiology (EAPC). Eur J Prev Cardiol. 2020;27:1242‐1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pelliccia A, Solberg EE, Papadakis M, et al. Recommendations for participation in competitive and leisure time sport in athletes with cardiomyopathies, myocarditis, and pericarditis: position statement of the Sport Cardiology Section of the European Association of Preventive Cardiology (EAPC). Eur Heart J. 2019;40:19‐33. [DOI] [PubMed] [Google Scholar]
- 28. Pelliccia A, Sharma S, Gati S, et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J. 2021;42(1):17–96. 10.1093/eurheartj/ehaa605 [DOI] [PubMed] [Google Scholar]
- 29. Nieß AMBW, Friedmann‐Bette B, Grim C, et al. Position stand: return to sport in the current coronavirus pandemic (SARS‐CoV2 / COVID‐19). Dtsch Z Sportmed. 2020;E1‐E4. [Google Scholar]
- 30. Ngai JC, Ko FW, Ng SS, To KW, Tong M, Hui DS. The long‐term impact of severe acute respiratory syndrome on pulmonary function, exercise capacity and health status. Respirology. 2010;15:543‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Torres‐Castro R, Vasconcello‐Castillo L, Alsina‐Restoy X, et al. Respiratory function in patients post‐infection by COVID‐19: a systematic review and meta‐analysis. Pulmonology. 2020:S2531‐0437(20)30245‐2. Online ahead of print. 10.1016/j.pulmoe.2020.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guazzi M, Arena R, Halle M, Piepoli MF, Myers J, Lavie CJ. 2016 focused update: clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Eur Heart J. 2018;39:1144‐1161. [DOI] [PubMed] [Google Scholar]
- 33. Schwarze J, Hamelmann E, Bradley KL, Takeda K, Gelfand EW. Respiratory syncytial virus infection results in airway hyperresponsiveness and enhanced airway sensitization to allergen. J Clin Investig. 1997;100:226‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Severin R, Arena R, Lavie CJ, Bond S, Phillips SA. Respiratory muscle performance screening for infectious disease management following COVID‐19: a highly pressurized situation. Am J Med. 2020;133:1025‐1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stöbe S, Richter S, Seige M, Stehr S, Laufs U, Hagendorff A. Echocardiographic characteristics of patients with SARS‐CoV‐2 infection. Clin Res Cardiol. 2020;109:1549‐1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Starekova J, Bluemke DA, Bradham WS, et al. Evaluation for myocarditis in competitive student athletes recovering from coronavirus disease 2019 with cardiac magnetic resonance imaging. JAMA Cardiol. 2021:e207444. Online ahead of print. 10.1001/jamacardio.2020.7444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Martinez MW, Tucker AM, Bloom OJ, et al. Prevalence of inflammatory heart disease among professional athletes with prior covid‐19 infection who received systematic return‐to‐play cardiac screening. JAMA Cardiol. 2021:e210565. Online ahead of print. 10.1001/jamacardio.2021.0565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lollgen H, Leyk D. Exercise testing in sports medicine. Deutsch Arztebl Int. 2018;115:409‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Herman C, Mayer K, Sarwal A. Scoping review of prevalence of neurologic comorbidities in patients hospitalized for COVID‐19. Neurology. 2020;95:77‐84. [DOI] [PubMed] [Google Scholar]
- 40. Phelan D, Kim JH, Chung EH. A game plan for the resumption of sport and exercise after coronavirus disease 2019 (COVID‐19) infection. JAMA Cardiol. 2020;13(12):2635–2652. [DOI] [PubMed] [Google Scholar]
- 41. Halle M, Binzenhöfer L, Mahrholdt H, Schindler MJ, Esefeld K, Tschöpe C. Myocarditis in athletes: a clinical perspective. Eur J Prev Cardiol. 2020:2047487320909670. [DOI] [PubMed] [Google Scholar]
- 42. Glöckl R, Schneeberger T, Boeselt T, et al. Exercise training in patients with pulmonary hypertension: a systematic review and meta‐analysis. Pneumologie (Stuttgart, Germany). 2019;73:677‐685. [DOI] [PubMed] [Google Scholar]
- 43. Gannon MP, Schaub E, Grines CL, Saba SG. State of the art: evaluation and prognostication of myocarditis using cardiac MRI. J Magn Reson Imaging. 2019;49(7):e122‐e131. [DOI] [PubMed] [Google Scholar]
- 44. Eichhorn C, Biere L, Schnell F, et al. Myocarditis in athletes is a challenge: diagnosis, risk stratification, and uncertainties. JACC Cardiovasc Imaging. 2020;13(2 Pt 1):494–507. [DOI] [PubMed] [Google Scholar]
- 45. Guzik TJ, Mohiddin SA, Dimarco A, et al. COVID‐19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020;116(10):1666‐1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ellingsen O, Halle M, Conraads V, et al. High‐intensity interval training in patients with heart failure with reduced ejection fraction. Circulation. 2017;135:839‐849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Su H, Yang M, Wan C, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID‐19 in China. Kidney Int. 2020;98:219‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chowdhury R, Peel NM, Krosch M, Hubbard RE. Frailty and chronic kidney disease: a systematic review. Arch Gerontol Geriatr. 2017;68:135‐142. [DOI] [PubMed] [Google Scholar]
- 49. Sheng K, Zhang P, Chen L, Cheng J, Wu C, Chen J. Intradialytic exercise in hemodialysis patients: a systematic review and meta‐analysis. Am J Nephrol. 2014;40:478‐490. [DOI] [PubMed] [Google Scholar]
- 50. Anding K, Bär T, Trojniak‐Hennig J, et al. A structured exercise programme during haemodialysis for patients with chronic kidney disease: clinical benefit and long‐term adherence. BMJ open. 2015;5(8):e008709. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


