Abstract
ObjectiveS
Due to the impact of COVID-19 epidemic, face-to-face follow-up treatments for patients with chronic pain and implanted spinal cord stimulation (SCS) devices are forced to be delayed or stopped. This has led to more follow ups being done remotely. Meanwhile, with the development of 4G/5G networks, smartphones, and novel devices, remote programming has become possible. Here, we investigated the demand and utility of remote follow-ups including remote programming for SCS for patients with chronic pain.
Materials and Methods
A questionnaire including questions on demographic characteristics, pain history, postimplantation life quality, standard follow-up experience, remote follow-up, and remote programming experience was sent to patients diagnosed as chronic intractable pain and treated with SCS during January 2019 to January 2020.
Results
A total of 64 participants completed the questionnaire. About 70% of participants expressed demands for remote follow-ups due to the inconvenience, high costs, and time consumption of traditional follow-up visits. Nearly 97% of participants have attempted remote follow-ups, and about 81% of participants have further tried remote programming. Approximately, 96% of them recognized the benefits.
Conclusions
The remote programming was in high demand among participants. Most of the participants have tried remote follow-ups or even remote programming. The remote programming appeared to be more efficient, economic and were widely recognized among participants.
Keywords: Chronic pain, COVID-19, remote programming, spinal cord stimulation, telemedicine
INTRODUCTION
Spinal cord stimulation (SCS) has been used to reduce chronic pain for decades (1,2). Although the SCS treatment can effectively improve pain relief and health-related quality of life instantly after the surgery (3), stable performance of SCS depends on regular professional programming (4). However, the frequent hospital visits for follow-ups are both time-consuming and costly (5). In China, the medical centers that are qualified for SCS implantation and subsequent programing are mostly located in limited number of front-line cities, most in the east coast of China. This leads to long journeys and high costs for those patients living in other provinces. The inconvenience of travel and unaffordable costs contribute to poor medical compliance (6) and further impede therapeutic effects of SCS in many cases.
With development of 4G/5G network and popularization of smartphones, and computers, telemedicine, which was popularized this century, has recently been increasingly recognized (7,8). Compared to traditional follow-ups, remote medical consultation is able to solve patients’ simple concerns and save them from unnecessary travels (7,9,10). However, for patients implanted with a neuromodulatory device, such as Parkinson’s disease (PD) patients with deep brain stimulation (DBS) or chronic pain patients with SCS, only postoperative consultation is not enough. Both of them require a regular postoperative programming to achieve the most suitable parameters and configuration of electric stimulation. However, remote programming system for SCS has been rarely studied. In this study, based on the development of DBS remote programming system, we introduced a novel wireless device which allows remote programming, making post-operative programming much easier for patients implanted with a SCS device.
After the outbreak of coronavirus, the demand for remote follow-ups markedly increased. Patients are suggested to stay at home to reduce risks of infection, and traveling across provinces or states are strictly restricted to limit the spread of the disease (11,12). Since hospitals are public places with high risks of exposure to the virus, patients are discouraged to seek medical care on-site, except for emergencies (13). Those conditions result in delayed or even lost follow-ups in patients implanted with SCS devices who often need regular post-operative programming to maintain the stable pain relief (14). Remote follow-ups, instead, can avoid social contacts and appear to be an ideal way for SCS post-operative care. Thus, to verify the demand and the effectiveness of remote programming among patients after SCS implantation, we performed an observational study on the demands and the experiences of the remote programming system we recently developed.
MATERIALS AND METHODS
Introduction of the System
The remote system ( Fig. 1) developed by Beijing PINS Medical Company enables physicians to implement the programming of the SCS system in patients whenever and wherever with network available. The PINS-App (PINS application) was designed as two versions: one for patients and the other for physicians. The version for patients was the patient’s client designed as a smartphone terminal to assist patients in contacting physicians and managing symptoms along the whole process of the treatment. Patients were allowed to choose a physician, make appointments for remote programming services, access medical history, and report daily outcomes via the App. Based on DBS remote programming system (15., 16., 17.), we previously developed, the implanted pulse generator (IPG) can wirelessly connect with participants’ cell phone via Bluetooth, which meanwhile connects with physicians’ computer to achieve remote control. The version for physicians was the physician’s client designed as a computer terminal, which could store patients’ medical records, images relative to electrodes implantation and previous programming records. In addition, the real-time video provides physicians with information sufficient for adjusting the configuration and parameters of the SCS system. Physicians were able to implement the adjustment of stimulation parameters, check battery status, check electrode impedance, and provide device troubleshooting on the tele-program module via the physician’s client. The programming records can be stored in the physician’s client, uploaded to the database, and shared with patients through the patient’s client. The studies involving human participants were reviewed and approved by Ethics committee of Beijing Tsinghua Changgung Hospital. All the participants provided their informed consent to participate in this study. Informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Figure 1.
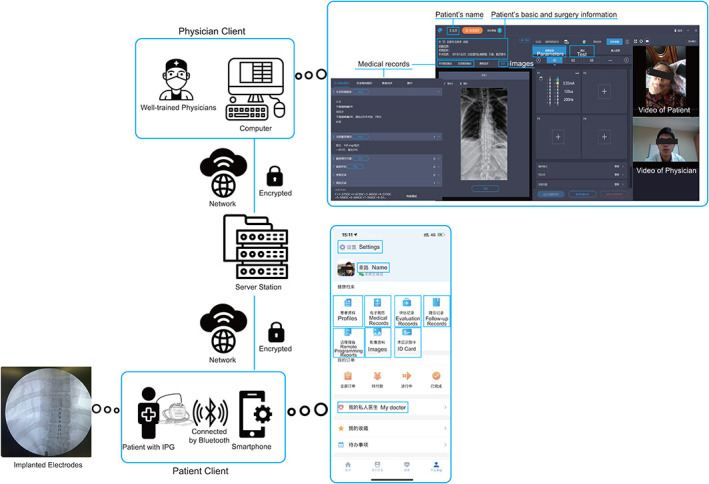
Introduction of the remote system. The remote system consists of three parts: the physician client, the server, and the patient client. A well-trained physician’s computer is connected to the server station, which is meanwhile connected to the patients smartphone. The IPG in the patient is externally activated and connected to the smartphone via Bluetooth. The physician interface is shown on the upper right side, and the patient interface on the PINS App is shown on the bottom right side. [Color figure can be viewed at wileyonlinelibrary.com]
Intervention
The percutaneous or paddle electrode was implanted and placed in the posterior spinal epidural space with the guidance of radiographic imaging under local anesthesia and sedatives (in certain cases, the general anesthesia was performed). The participant provided verbal feedback regarding paresthesia and electrodes were repositioned to achieve better paresthesia overlap of painful areas. An external pulse generator (T802; PINS, Inc., Beijing, BJ, China) was temporarily used to stimulate the spinal cord through electrodes in the trial period (seven days on average). Permanent implantation of the IPG was performed once at least 50% reduction of pain was attained during the trial period. A subcutaneous pocket was created in the chest, abdomen, or buttock for placement of the IPG based on participants and physician’s preference. The leads were anchored with a manufacturer-supplied anchor and connected to the IPG through a subcutaneous tunnel to the pocket site.
Participants
Participants in our study were recruited from multiple centers in China. Participants were diagnosed as chronic intractable pain and treated with SCS. Participants were selected only if they received implantation of an IPG (G122R; PINS, Inc) during January 2019 to January 2020. A total of 72 patients from 16 centers were invited to participate in the study. A questionnaire (Supplementary Material) consisting of 30 questions was sent to the participant via mobile Internet. Participants volunteered to participate in the study. Sixty-six patients completed the study and responded to the questionnaire. Eight patients lost contact and failed to respond to the questionnaire. Two patients who later had their IPG explanted were excluded. A total of 64 participants were included in the final analysis.
Data Collection
A questionnaire was designed to obtain information on demographic characteristics, pain history, post-implantation life quality, standard follow-up experience, remote follow-up experience, and remote programming experiences. Post implantation improvement score was a subjective evaluation scale for post implantation health (0 for completely no improvement with severe side effects and 10 for complete pain relief without any side effect). Daily life independency score was a subjective evaluation scale for post implantation life independency (0 for complete dependent and 24-hour care needed and 10 for complete independence and no care needed). Estimated costs were nonmedical costs including costs of travel and accommodation for follow-ups, which were calculated according to the location of hospitals and participants’ address, self-reported number of accompanying individuals, and self-reported total time consumed (medical costs in hospitals not included). For the question “Preferred ways of remote follow-ups,” the possible ways for participants to choose from were “1) emails, and local hospital accordingly,” which was explained as “communicate with your physicians through emails and go to local hospitals according to suggestions in emails. Pay for emails which means minimum costs,” “2) calls, and local hospital accordingly,” which was explained as “communicate with your physicians by calls and go to local hospitals according to suggestions in calls. Pay for calls which means slightly higher than national phone charges,” “3) video calls, and local hospital accordingly,” which was explained as “communicate with your physicians through video calls and go to local hospitals according to suggestions in video calls. Pay for video calls,” and “4) video calls, and remote programming directly,” which was explained as “communicate with your physicians through video calls, and they directly complete remote programming. Pay for video calls.”
Statistical Analysis
Continuous variables were summarized by the mean and standard deviation of mean (SEM), and categorial variables were summarized by frequencies. T-test and chi-square were used to compare traditional and remote follow-ups. The correlation between patients’ demands for remote programming and their demographic and pain characteristics was tested using t-test (sex, marital status, and employment status), analysis of variance (ANOVA) (education level) and Pearson’s correlation coefficient (age, pain history).
RESULTS
Demands for Remote Follow-Up
A total of 64 participants completed the questionnaire. On average, they have suffered chronic pain from various etiology for nearly ten years among whom the longest one is about 50 years ( Table 1). SCS relieved the pain to some extent. Visual analog scale (VAS) score indicated that the pain relief rates after IPG implantation were more than 50% for all participants and 46.9% of participants reported satisfactory health improvement with pain relief and negligible side effects. However, 40.6% of the participants live in rural area, and the mean home-hospital distance was about 800 km, which led to inconvenience of postoperative follow-up for IPG programming ( Table 2). Moreover, 48.4% of participants reported poor independence, and 84.4% of participants needed at least one accompanying individual for follow-up visits. Additionally, 76.6% of participants spent more than one day for each clinic visit, and 34.4% spent more than four days for each on-site clinic visit. Therefore, the traditional follow-up was both labor-consuming and time-consuming. The far distance, accompanying individuals and accommodation also contribute to high expenses. The average traveling costs including accommodation for each follow-up visit were estimated to be 1670 RMB (Chinese $). Considering average follow-up frequency as about three months, each participant spent 6680 RMB on follow-up related traveling and accommodation every year, accounting for 35.0% of family annual income in our study. For 10.9% of participants, this part of costs already exceeded their family annual income. The labor-consuming, time-consuming, and financial problems of traditional follow-ups thus result in inconvenience of postoperative programming. Meanwhile, remote follow-ups avoided the long journey related expenses and time consumption. Our survey showed that 39.1% of participants do not need to be accompanied for remote follow-ups, which would free their family members. Thus, those made remote follow-ups a helpful alternative for patients after SCS implantation. Not surprisingly, 68.8% of participants expressed demands for remote follow-ups (demands for remote programming score more than 5), and the high demands showed no difference among various indications. Participants who reported better post implantation health improvement showed higher demands for remote follow-ups. However, the self-reported demands showed no correlation with age (R 2 = 0.002, p = 0.70, Pearson’s correlation), sex (female: 6.6; male: 7.5, p = 0.26, t-test), education level (less than high school: 7.0; completed high school: 7.2; more than high school: 6.8, p = 0.95, ANOVA), marital status (married: 7.2; single/widowed/divorced: 5.9, p = 0.23, t-test), employment status (unemployed: 6.8; part-time/full-time job: 8.2, p = 0.22, t-test), or pain history (R 2 = 0.05, p = 0.09, Pearson’s correlation).
Table 1.
Characteristics of Study Participants (n = 64).
| Age (years), mean ± SEM | 58.6 ± 1.6 |
|---|---|
| Sex, n (%) | |
| Male | 36 (56.2) |
| Female | 28 (43.8) |
| Education status, n (%) | |
| Less than high school | 41 (64.1) |
| Completed high school | 12 (18.7) |
| More than high school | 11 (17.2) |
| Marital status, n (%) | |
| Married | 53 (82.8) |
| Single/widowed/divorced | 11 (17.2) |
| Employment status, n (%) | |
| Unemployed | 54 (84.4) |
| Part-time | 1 (1.6) |
| Full-time | 9 (14.1) |
| Annual household income, n (%) | |
| Less than ¥10,000 | 20 (31.3) |
| ¥10,000–¥50,000 | 29 (45.3) |
| ¥50,000–¥100,000 | 10 (15.6) |
| ¥100,000–¥200,000 | 2 (3.1) |
| More than ¥200,000 | 3 (4.7) |
| Health insurance, n (%) | |
| Available | 59 (92.2) |
| Unavailable | 5 (7.8) |
| Causes for chronic pain, n (%) | |
| Pain associated with spinal cord damage | 19 (29.7) |
| Failed back surgery syndrome (FBSS) | 16 (25.0) |
| Neuropathic pain secondary to peripheral nerve damage | 5 (7.8) |
| Brachial plexopathy | 5 (7.8) |
| Failed neck surgery syndrome (FNSS) | 4 (6.3) |
| Phantom limb pain | 4 (6.3) |
| Post-herpetic neuralgia (PHN) | 3 (4.7) |
| Amputation pain | 2 (3.1) |
| Post-syphilitic neuralgia | 2 (3.1) |
| Complex regional pain syndrome (CRPS) | 1 (1.6) |
| Painful diabetic neuropathy (PDN) | 1 (1.6) |
| Post-traumatic pain | 1 (1.6) |
| Perineal pain | 1 (1.6) |
| Pain history (years), mean ± SEM | 9.8 ± 1.2 |
| Post implantation improvement score (0–10), mean ± SEM | 5.1 ± 0.3 |
| Baseline VAS score (0–10), mean ± SEM | 8.2 ± 0.1 |
| Post implantation VAS score (0–10), mean ± SEM | 2.2 ± 0.1 |
| Pain relief, mean ± SEM | 72.8% ± 1.7% |
Table 2.
Demand for Remote Follow-ups (n = 64).
| Residence, n (%) | |
|---|---|
| Urban | 38 (59.4) |
| Rural | 26 (40.6) |
| Daily life independency score (0–10), mean ± SEM | 5.2 ± 0.4 |
| Standard follow-ups | |
| Total times so far, mean ± SEM | 1.8 ± 0.2 |
| Frequency (months), mean ± SEM | 2.2 ± 0.1 |
| Distance to hospital (km), mean ± SEM | 786.5 ± 178.1 |
| Traffic time (hours), mean ± SEM | 8.2 ± 1.2 |
| Estimated costs (¥), mean ± SEM | 1670.0 ± 155.5 |
| Estimated costs/year (¥), mean ± SEM | 6679.9 ± 621.9 |
| Cost/annual income ratio, mean ± SEM | 35.0% ± 5.0% |
| Number of accompanying individuals, n (%) | |
| 0 | 10 (15.6) |
| 1 | 46 (71.9) |
| ≥2 | 8 (12.5) |
| Total time consumed, n (%) | |
| Within 1 day | 15 (23.4) |
| 2 days | 14 (21.9) |
| 3 days | 8 (12.5) |
| 4 days | 5 (7.8) |
| ≥4 days | 22 (34.4) |
| Demands for remote programming (0–10), mean ± SEM | 7.0 ± 0.4 |
Estimated costs were nonmedical costs including costs of travel and accommodation for follow-ups, which were calculated according to the location of hospitals and participants’ address, self-reported number of accompanying individuals, and self-reported total time consumed (medical costs in hospitals not included). Estimate costs/year were calculated assuming four follow-up visits per year according to the average follow-up frequency.
Utility and Acceptability of Remote Follow-Ups
Almost all participants (63/64) in our study use either smartphones or computers in their daily lives, which indicates that there would be no extra costs on devices for remote programming. The familiarity with the devices were also related to less difficulties in process of remote programming. In addition, 82.8% of participants reported available assistance from family members for remote programming.
In total, 96.9% of participants have tried communicating with physicians remotely, and 96.7% of them were satisfied with the experience. Furthermore, 81.3% of participants have tried remote programming via PINS App more than twice on average ( Table 3). As a result, 96.7% of participants who underwent remote programming recognized the effect. On average, the remote follow-ups took only 0.8 hours in total, which was significantly decreased, compared to days spent on traditional follow-ups (0.8 vs. 52.7 hours, p < 0.001, t-test). Besides, fewer participants expressed demands for being accompanied during remote follow-ups than during traditional follow-ups (60.9% vs. 84.4%, p < 0.01, chi-square). The most worrying problem for remote programming, which is network or equipment failure, surprisingly, did not happen for most of our participants. Only 4.8% of participants experienced frequent network or equipment failure.
Table 3.
Practicability and Acceptability of Remote Follow-Ups.
| Use of mobile phones and computers in daily life, n (%) | ||
|---|---|---|
| None | 1 (1.6) | |
| Either | 37 (57.8) | |
| Both | 26 (40.6) | |
| Assistance availability for remote follow-ups, n (%) | ||
| Available | 53 (82.8) | |
| Unavailable | 11 (17.2) | |
| Experiences of remote communication with physicians | ||
| Frequency, n (%) | ||
| Never | 2 (3.1) | |
| Sometimes | 40 (62.5) | |
| Often | 22 (34.4) | |
| Satisfaction, n (%) | ||
| Excellent | 21 (35.0) | |
| Fair | 37 (61.7) | |
| Poor | 2 (3.3) | |
| Experiences of remote programming via App | ||
| Frequency, n (%) | ||
| Never | 12 (18.7) | |
| Sometimes | 43 (67.2) | |
| Often | 9 (14.1) | |
| Times, mean ± SEM | 2.5 ± 0.3 | |
| Satisfaction, n (%) | ||
| Excellent | 17 (32.7) | |
| Fair | 33 (63.5) | |
| Poor | 2 (3.8) | |
| Total time consumed (hours), mean ± SEM | 0.8 ± 0.1 | |
| Experiences of network/equipment failure, n (%) | ||
| Never | 38 (61.3) | |
| Sometimes | 21 (33.9) | |
| Often | 3 (4.8) | |
| Demands for being accompanied, n (%) | ||
| Necessary | 39 (60.9) | |
| Unnecessary | 25 (39.1) | |
| Preferred ways of remote follow-ups, n (%) | ||
| Emails and local hospital accordingly | 11 (17.2) | |
| Calls and local hospital accordingly | 30 (46.9) | |
| Video calls and local hospital accordingly | 14 (21.9) | |
| Video calls and remote programming directly | 28 (43.8) | |
| Preferred location for remote follow-ups, n (%) | ||
| Home | 51 (79.7) | |
| Community hospitals | 12 (18.8) | |
| Hospitals in capital cities (%) | 8 (12.5) | |
For more specific execution of remote follow-ups, compared to community hospitals or those in capital cities, most participants preferred remote communication at home, and nearly half of participants preferred direct remote programming during video calls. Tele consulting in a local hospital with another physician at the side was also a popular choice, which indicated that remote programming or remote follow-ups cannot solve all problems, and a considerable proportion of participants still preferred on-site follow-ups.
DISCUSSION
Our results indicated that the remote follow-ups were in high demands among participants due to the inconvenience, high costs, and time consumption of traditional follow-up visits. Given the popularity of smartphones and computers, most of the participants have tried remote follow-ups or even remote programming. The remote programming appeared to be more efficient, economic and were widely accepted among participants.
To solve the concerns on safety and confidentiality of remote programming raised up previously (18., 19., 20., 21., 22., 23.), multiple protection technologies during data transmission were adopted in our system. Hypertext transfer protocol secure (HTTPS), bidirectional identity authentication technology, and encrypted transmission between the authenticated servers and clients were implemented to ensure the confidentiality of communication and programming. Besides, back-up automatic instructions, emergency stimulator shutdown mechanism, and offline parameter recovery function were designed in our system to guarantee the safety of programming in case of network failure, power outage, or other potential emergencies. Additionally, patients or at least their caregivers need to be familiar with cell phones and the App. The necessary training and testing for remote programming were completed in hospital before discharge. The physicians were also well trained and qualified for remote programming and able to instruct patients to cooperate properly. During remote programming, the configuration and parameters always started with relatively safe and low level and gradually increased to optimal level to assure safety during remote programming. Those technologies and trainings contributed to a safe and confidential remote programming environment.
Telemedicine was initiated in 20th century, and WHO also addressed its value in 1997 (8). Realization of the importance and clinical demand of telemedicine has dramatically increased recently due to the COVID-19 outbreak. To reduce the risk of virus transmission, people were encouraged to stay at home and avoid personal contacts, which resulted in difficulty on face-to-face consultation. The development of telemedicine was thus largely increased. Remote consultation was applied in multiple countries (24., 25., 26.), including China (27). There have been reports of remote programming of DBS for neurological disorder including Parkinson’s disease (28., 29., 30., 31.), dystonia (32), and obsessive–compulsive disorder (OCD) (33). They confirmed that a remote system is in high demand due to the huge burden of DBS postoperative care (34), and that remote programming is able to effectively maintain the effectiveness of DBS treatment (28,30,32,33). Similar to DBS, in patients who just underwent SCS implantation, regular follow-ups were also affected by COVID-19 epidemic. In this study, we recruited those patients for remote follow-ups and collected their feedback for both traditional and remote follow-ups. Our survey confirmed that the remote follow-ups were satisfactory and efficient.
Previous studies have shown that compared with conventional therapy for chronic pain, SCS is more cost-effective (3., 35., 36., 37., 38., 39.). This study suggested that the costs of SCS could be further reduced by at least 7000 RMB per year by replacing traditional follow-up with remote follow-ups visits. Considering that 84.4% of participants were unemployed, this amount of expenses could be a tremendous burden. For some patients, the costs even exceeded their family income, considering the annual family income of 31.3% of participants is less than 10,000 RMB. The concern of life-long annual high costs could discourage patients from choosing SCS for pain control. Whereas the remote follow-ups avoided expenses on follow-up-related traveling and accommodation. In addition, the remote programming typically took less than an hour, and around 40% of participants did not need to be accompanied, which largely freed family members. Since the time constraint plays an important role in avoidance of medical care (40,41), this advantage of remote follow-ups will improve follow-up compliance for patients after SCS, which is important for stable pain relief (14). In addition, one of the most common complications of SCS is hardware dysfunctions (42., 43., 44.). However, some of the hardware issues do not require invasive treatment (42) and could be solved remotely with instructions or simple recommendations. Thus, patients are able to know if the situation must be solved in hospital by remote follow-ups to avoid unnecessary trips. In line with those strengths, remote follow-ups were in high demands among participants in our survey. Regardless of indications, most of participants were willing to use remote programming. Nonetheless, a considerable part of participants still preferred on-site follow-ups. Thus, face-to-face interactions are also important as a reassuring way.
Compared to DBS, the use of remote programming could be more feasible for SCS, due to the nature of their indications. The most popular indication of DBS is Parkinson’s disease. However, rigidity, which is one of its major symptoms, is hard to evaluate during video-based remote programming. The other symptoms such as dyskinesia and tremor, although can be followed through video, may respond to DBS parameter changes slowly (30). In contrast, for SCS remote programming for pain management, patients note real-time changes of their pain in response to the stimulation and easily convey the information to physicians. Since evaluating the dynamic changes of patients’ symptoms accounts for an important part in programming, the development of remote programming in SCS could be potentially even more promising than that in DBS.
There are several limitations in this study. First, we did not make a direct comparison of therapeutic effects between remote follow-ups and traditional follow-ups. Furthermore, a randomized controlled trial remains to be done to confirm the benefits of remote programming. Second, the size of our study was limited by the total SCS cases in China. In China, SCS as a therapy is still limited. Third, the study was done with a specific device, and due to different regulatory barriers, it is a challenge for the device system to be commercialized globally. Therefore, the feasibility of the remote system remains to be tested with other devices. Last, some patients with chronic pain who gave up SCS treatment because of financial difficulties maintaining follow up were not involved in this study. Our survey study suggested that the implement of remote follow-ups can benefit more patients and benefit the development of SCS in treating chronic pain.
Conflict of Interest: Dr. James Jin Wang and Dr. Yang Lu have received research support from Beijing PINS Medical Co. (donated SCS devices for pain). Luming Li reports personal fees from Beijing PINS Medical Co., outside the submitted work. The other authors report no conflict of interest.
Acknowledgments
Authorship Statements
Dr. James Jin Wang and Dr. Luming Li designed the study. Dr. Yang Lu, Dr. Yan Han, Dr. Dengyu Wang, Dr. Mingshan Ran, Dr. Qidong Ren, and Dr. Duo Xie conducted the study, including patient recruitment, data collection, and data analysis. Dr. Yan Han prepared the manuscript draft with important intellectual input from Dr. Yang Lu, Dr. Tipu Z. Aziz, Dr. Luming Li, and Dr. James Jin Wang. The National Key Research and Development Program of China, National Natural Science Foundation of China, Shenzhen International Cooperative Research Project, Beijing Science and Technology Project, and Tsinghua Precision Medicine Foundation provided funding for the study. All authors approved the final manuscript. Yan Han and Yang Lu contributed equally to this study.
Footnotes
For more information on author guidelines, an explanation of our peer review process, and conflict of interest informed consent policies, please go to http://www.wiley.com/WileyCDA/Section/id-301854.html
Source(s) of financial support: This study is supported by the National Key Research and Development Program of China (2016YFC0105502), National Natural Science Foundation of China (81527901), Shenzhen International Cooperative Research Project (GJHZ20180930110402104), Beijing Science and Technology Project (Z171100001017109), and a grant from the Tsinghua Precision Medicine Foundation (LC201906)
Supporting Information
Appendix S1. Supporting Information
REFERENCES
- 1.Schwartz S, Wingrove R. Implantable nerve stimulator and method of use. 1969; (US3522811A). https://patents.google.com/patent/US3522811A/en.
- 2.Geurts JW, Joosten EA, van Kleef M. Current status and future perspectives of spinal cord stimulation in treatment of chronic pain. Pain. 2017;158:771–774. doi: 10.1097/j.pain.0000000000000847. [DOI] [PubMed] [Google Scholar]
- 3.Deer TR, Mekhail N, Provenzano D, et al. The appropriate use of neurostimulation of the spinal cord and peripheral nervous system for the treatment of chronic pain and ischemic diseases: the neuromodulation appropriateness consensus committee. Neuromodulation. 2014;17:515–550. doi: 10.1111/ner.12208. [DOI] [PubMed] [Google Scholar]
- 4.Pain SA, Pain SA, Raff M, Melvill R, Coetzee G, Smuts J. Spinal cord stimulation for the management of pain: recommendations for best clinical practice. S Afr Med J. 2013;103:423–430. doi: 10.7196/samj.6323. [DOI] [PubMed] [Google Scholar]
- 5.Gray RT, Sut MK, Badger SA, Harvey CF. Post-operative telephone review is cost-effective and acceptable to patients. Ulster Med J. 2010;79:76–79. [PMC free article] [PubMed] [Google Scholar]
- 6.Darkins A, Dearden CH, Rocke LG, Martin JB, Sibson L, Wootton R. An evaluation of telemedical support for a minor treatment Centre. J Telemed Telecare. 1996;2:93–99. doi: 10.1177/1357633X9600200205. [DOI] [PubMed] [Google Scholar]
- 7.Craig J, Patterson V. Introduction to the practice of telemedicine. J Telemed Telecare. 2005;11:3–9. doi: 10.1177/1357633X0501100102. [DOI] [PubMed] [Google Scholar]
- 8.WHO Group Consultation on Health Telematics A health telematics policy in support of WHO’s health-for-all strategy for global health development: Report of the WHO Group consultation on health telematics, 11-16 December, Geneva, 1997. 1998.
- 9.Kumar K, Caraway DL, Rizvi S, Bishop S. Current challenges in spinal cord stimulation. Neuromodulation. 2014;17:22–35. doi: 10.1111/ner.12172. [DOI] [PubMed] [Google Scholar]
- 10.Deer TR, Krames E, Mekhail N, et al. The appropriate use of neurostimulation: new and evolving neurostimulation therapies and applicable treatment for chronic pain and selected disease states. Neuromodulation appropriateness consensus committee. Neuromodulation. 2014;17:599–615. doi: 10.1111/ner.12204. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Y, Xu R, Hu D, Yue Y, Li Q, Xia J. Effects of human mobility restrictions on the spread of COVID-19 in Shenzhen, China: a modelling study using mobile phone data. Lancet Digit Health. 2020;2:e417–e424. doi: 10.1016/S2589-7500(20)30165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Z, Chen Q, Feng L, et al. Active case finding with case management: the key to tackling the COVID-19 pandemic. Lancet. 2020;396:63–70. doi: 10.1016/S0140-6736(20)31278-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brooks SK, Webster RK, Smith LE, et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395:912–920. doi: 10.1016/S0140-6736(20)30460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharan A, Cameron T, Barolat G. Evolving patterns of spinal cord stimulation in patients implanted for intractable low back and leg pain. Neuromodulation. 2002;5:167–179. doi: 10.1046/j.1525-1403.2002.02027.x. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Hao H, Li L, Chen H, Lyu C, Ma B. Remote monitoring system for implanted medical equipment. 2015;(CN201510114358.1A). https://patents.google.com/patent/CN104683474A/en?q=(A+Remote+Monitoring+System+for+Implanted+Medical+Equipment)&assignee=Beijing+Pins+Medical+Co.%2c+Ltd&patents=false&oq=(A+Remote+Monitoring+System+for+Implanted+Medical+Equipment)+assignee:(Beijing+Pins+Medical+Co.%2c+Ltd).
- 16.Chen Y, Chen H, Ma B, Lyu C, Hao H, Li L. Patient terminal of remote monitoring system of implantable medical device. 2015; (CN201510114369.XA). https://patents.google.com/patent/CN104689475A/en?q=(Patient+Client+of+Remote+Monitoring+System+for+Implanted+Medical+Equipment)&assignee=Beijing+Pins+Medical+Co.%2c+Ltd&oq=(Patient+Client+of+Remote+Monitoring+System+for+Implanted+Medical+Equipment)+assignee:(Beijing+Pins+Medical+Co.%2c+Ltd)
- 17.Chen Y, Chen H, Ma B, Hao H, Li L. Working method of remote monitoring system of implantable medical device. 2015; (CN201510115286.2A). https://patents.google.com/patent/CN104660717A/en?q=A+Working+Method+of+Remote+Monitoring+System+for+Implanted+Medical+Equipment.&assignee=Beijing+Pins+Medical+Co.%2c+Ltd
- 18.Molfenter T, Boyle M, Holloway D, Zwick J. Trends in telemedicine use in addiction treatment. Addict Sci Clin Pract. 2015;10:14. doi: 10.1186/s13722-015-0035-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen C, DeMuro P. Legal and regulatory considerations associated with use of patient-generated health data from social media and mobile health (mHealth) devices. Appl Clin Inform. 2015;6:16–26. doi: 10.4338/ACI-2014-09-R-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LeRouge C, Garfield MJ. Crossing the telemedicine chasm: have the U.S. barriers to widespread adoption of telemedicine been significantly reduced? Int J Environ Res Public Health. 2013;10:6472–6484. doi: 10.3390/ijerph10126472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cherney LR, van Vuuren S. Telerehabilitation, virtual therapists, and acquired neurologic speech and language disorders. Semin Speech Lang. 2012;33:243–257. doi: 10.1055/s-0032-1320044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanders C, Rogers A, Bowen R, et al. Exploring barriers to participation and adoption of telehealth and telecare within the whole system demonstrator trial: a qualitative study. BMC Health Serv Res. 2012;12:220. doi: 10.1186/1472-6963-12-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rammo R, Gostkowski M, Rasmussen PA, Nagel S, Machado A. The need for digital health solutions in deep brain stimulation for Parkinson’s disease in the time of COVID-19 and beyond. Neuromodulation. 2020;24:331–336. doi: 10.1111/ner.13307. [DOI] [PubMed] [Google Scholar]
- 24.Ohannessian R, Duong TA, Odone A. Global telemedicine implementation and integration within health systems to fight the COVID-19 pandemic: a call to action. JMIR Public Health Surveill. 2020;6:e18810. doi: 10.2196/18810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jnr BA. Use of telemedicine and virtual care for remote treatment in response to COVID-19 pandemic. J Med Syst. 2020;44:132. doi: 10.1007/s10916-020-01596-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mann DM, Chen J, Chunara R, Testa PA, Nov O. COVID-19 transforms health care through telemedicine: Evidence from the field. J Am Med Inform Assoc. 2020;27:1132–1135. doi: 10.1093/jamia/ocaa072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu S, Yang L, Zhang C, et al. Online mental health services in China during the COVID-19 outbreak. Lancet Psychiatry. 2020;7:e17–e18. doi: 10.1016/S2215-0366(20)30077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J, Hu W, Chen H, Meng F, Li L, Okun MS. Implementation of a novel Bluetooth Technology for Remote Deep Brain Stimulation Programming: the pre- and post-COVID-19 Beijing experience. Mov Disord. 2020;35:909–910. doi: 10.1002/mds.28098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li D, Zhang C, Gault J, et al. Remotely programmed deep brain stimulation of the bilateral subthalamic nucleus for the treatment of primary parkinson disease: a randomized controlled trial investigating the safety and efficacy of a novel deep brain stimulation system. Stereotact Funct Neurosurg. 2017;95:174–182. doi: 10.1159/000475765. [DOI] [PubMed] [Google Scholar]
- 30.Xu J, Wang J, Keith S et al. Management of Parkinson’s disease patients after DBS by remote programming: preliminary application of single center during quarantine of 2019-nCoV. J Neurol 2020. e-pub ahead of print. 10.1007/s00415-020-10273-z. [DOI] [PMC free article] [PubMed]
- 31.Zhang C, Li D, Zeljic K, Tan H, Ning Y, Sun B. A remote and wireless deep brain stimulation programming system. Neuromodulation. 2016;19:437–439. doi: 10.1111/ner.12448. [DOI] [PubMed] [Google Scholar]
- 32.Zhang C, Zhu K, Lin Z, et al. Utility of deep brain stimulation telemedicine for patients with movement disorders during the COVID-19 outbreak in China. Neuromodulation. 2020;24:337–342. doi: 10.1111/ner.13274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang C, Zhu K, Li D, Voon V, Sun B. Deep brain stimulation telemedicine for psychiatric patients during the COVID-19 pandemic. Brain Stimul. 2020;13:1263–1264. doi: 10.1016/j.brs.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Y, Hao H, Chen H, Li L. The study on a telemedicine interaction mode for deep brain stimulation postoperative follow-up. Annu Int Conf IEEE Eng Med Biol Soc. 2015;2015:186–189. doi: 10.1109/EMBC.2015.7318331. [DOI] [PubMed] [Google Scholar]
- 35.Odonkor CA, Orman S, Orhurhu V, Stone ME, Ahmed S. Spinal cord stimulation vs conventional therapies for the treatment of chronic low back and leg pain: a systematic review of health care resource utilization and outcomes in the last decade. Pain Med. 2019;20:2479–2494. doi: 10.1093/pm/pnz185. [DOI] [PubMed] [Google Scholar]
- 36.Zucco F, Ciampichini R, Lavano A, et al. Cost-effectiveness and cost-utility analysis of spinal cord stimulation in patients with failed Back surgery syndrome: results from the PRECISE study. Neuromodulation. 2015;18:266–276. doi: 10.1111/ner.12292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kemler MA, Raphael JH, Bentley A, Taylor RS. The cost-effectiveness of spinal cord stimulation for complex regional pain syndrome. Value Health. 2010;13:735–742. doi: 10.1111/j.1524-4733.2010.00744.x. [DOI] [PubMed] [Google Scholar]
- 38.Hoelscher C, Riley J, Wu C, Sharan A. Cost-effectiveness data regarding spinal cord stimulation for low back pain. Spine. 2017;42:S72–S79. doi: 10.1097/BRS.0000000000002194. [DOI] [PubMed] [Google Scholar]
- 39.Niyomsri S, Duarte RV, Eldabe S, et al. A systematic review of economic evaluations reporting the cost-effectiveness of spinal cord stimulation. Value Health. 2020;23:656–665. doi: 10.1016/j.jval.2020.02.005. [DOI] [PubMed] [Google Scholar]
- 40.Taber JM, Leyva B, Persoskie A. Why do people avoid medical care? A qualitative study using national data. J Gen Intern Med. 2015;30:290–297. doi: 10.1007/s11606-014-3089-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu Y, Mao P, Wang G, et al. Spinal cord stimulation for chronic intractable trunk or limb pain: study protocol for a Chinese multicenter randomized withdrawal trial (CITRIP study) Trials. 2020;21:834. doi: 10.1186/s13063-020-04768-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kleiber JC, Marlier B, Bannwarth M, Theret E, Peruzzi P, Litre F. Is spinal cord stimulation safe? A review of 13 years of implantations and complications. Rev Neurol. 2016;172:689–695. doi: 10.1016/j.neurol.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 43.Kumar K, Hunter G, Demeria D. Spinal cord stimulation in treatment of chronic benign pain: challenges in treatment planning and present status, a 22-year experience. Neurosurgery. 2006;58:481–496. doi: 10.1227/01.NEU.0000192162.99567.96. [DOI] [PubMed] [Google Scholar]
- 44.Nissen M, Ikäheimo T-M, Huttunen J, Leinonen V, von Und Zu Fraunberg M. Long-term outcome of spinal cord stimulation in failed Back surgery syndrome: 20 years of experience with 224 consecutive patients. Neurosurgery. 2019;84:1011–1018. doi: 10.1093/neuros/nyy194. [DOI] [PubMed] [Google Scholar]
COMMENTARY
This can be an important work that will help boost companies develop tele-medicine initiatives for neuromodulation patients. This is an urgent need especially during pandemic times; however, this can be also a great solution to reduce the time spent in clinics and be more cost-effective
Pablo Rueda, PhD
Madrid, Spain
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information


