Abstract
Background
Neurological manifestations of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) are being widely documented. However, movement disorders in the setting of 2019 coronavirus infectious disease (COVID‐19) have been a strikingly less discussed topic.
Objectives
To summarize available pieces of evidence documenting de novo movement disorders in COVID‐19.
Methods
We used the existing PRISMA consensus statement. Data were collected from PubMed, EMBASE, Web of Science, and Scopus databases up to the 29th January, 2021, using pre‐specified searching strategies.
Results
Twenty‐two articles were selected for the qualitative synthesis. Among these, a total of 52 patients with de novo movement disorders were reported. Most of these had myoclonus, ataxia, tremor or a combination of these, while three had parkinsonism and one a functional disorder. In general, they were managed successfully by intravenous immunoglobulin or steroids. Some cases, primarily with myoclonus, could be ascribed to medication exposures, metabolic disturbances or severe hypoxia, meanwhile others to a post‐or para‐infectious immune‐mediated mechanism. SARS‐CoV‐2 could also invade the central nervous system, through vascular or retrograde axonal pathways, and cause movement disorders by two primary mechanisms. Firstly, through the downregulation of angiotensin‐converting enzyme 2 receptors, resulting in the imbalance of dopamine and norepinephrine; and secondly, the virus could cause cellular vacuolation, demyelination and gliosis, leading to encephalitis and associated movement disorders.
Conclusion
De novo movement disorders are scantly reported in COVID‐19. The links between SARS‐CoV‐2 and movement disorders are not yet established. However, we should closely monitor COVID‐19 survivors for the possibility of post‐COVID movement disorders.
Keywords: COVID‐19, SARS‐CoV‐2, myoclonus, tremor, ataxia
Neurological manifestations of a predominantly respiratory pathogen, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), are being widely documented. 1 These may include mild headache, hyposmia/anosmia, hypogeusia/ageusia, ischemic stroke, intracerebral hemorrhage, encephalitis, alterations in circadian rhythm, cognitive impairment, increased seizure frequency, myopathy, and Guillain‐Barré syndrome variants, among others 1 , 2 , 3 , 4 , 5 ; as such, the infection may affect almost all parts of the neural axis. 1 However, movement disorders preceded by coronavirus disease of 2019 (COVID‐19) have been a strikingly less discussed topic.
Infectious diseases are among the most common causes of neurological disability worldwide. A series of movement disorders can develop in isolation (i.e., direct neurovirulence) due to encephalopathy, or as part of a broader neurological dysfunction (i.e., indirect neurovirulence). 6 On most occasions, infection‐related movement disorders are the result of an active immune‐mediated process affecting the neural substrates through molecular mimicry and host susceptibility. 6 , 7 In some instances, this immunological mimicry may result in the development of targeted antibodies against cell surface dopaminergic receptors in the basal ganglia. 8 , 9 Similarly, anti‐neuronal antibodies directed against the cerebellar fastigial nucleus and omnipause neurons in the brainstem may give rise to an opsoclonus‐myoclonus syndrome, as reported in several infectious diseases. 10 , 11
Japanese encephalitis (JE) is one of the viral diseases with a major spectrum of movement disorders among its clinical manifestations. 12 In this infection, there is structural damage involving the thalamus, brainstem and basal ganglia, resulting in damage to the cortico‐basal ganglia‐thalamocortical loop with resultant movement disorders. 12 Norepinephrine and dopamine have important roles in movement disorders associated with JE. 13 Indeed, low levels of catecholamines in JE‐associated movement disorders compared to Parkinson's disease and other extrapyramidal symptoms have been reported and may be due to severe structural damage to the thalamus, basal ganglia and brainstem. 13 Analogous pathophysiological mechanisms are believed to be a feature of other flavivirus infections. 14 , 15 Similarly, anti‐basal ganglia antibodies targeted against large striatal neurons have been reported in infantile bilateral striatal necrosis after recent respiratory tract infection with Streptococcus spp, manifesting as dystonic movements. 16 In a significant number of cases, common herpetic encephalitis and JE have produced anti‐N‐methyl‐d‐aspartate receptor antibodies, resulting in diffuse encephalopathies and movement disorders. 17 , 18 Over the years, human immunodeficiency virus (HIV) infection has been associated with a multitude of movement disorders due to either direct or secondary damage to the basal ganglia or its connections (aberrant immunological response), or as a result of drug therapy. 19 Parkinsonism as a complication of viral infections is well documented and has been associated with influenza, JE, enterovirus, Epstein–Barr, western equine encephalitis, dengue, hepatitis E, measles, herpes simplex, HIV, coxsackie and many other viruses. 15 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 Further, acute post‐infectious anti‐neuronal antibody‐mediated cerebellar ataxia is seen in numerous cases following viral encephalitis in children. 29
The influenza pandemic of 1918 is famous for the post‐infectious complication known as encephalitis lethargica, 30 , 31 characterized by a myriad of abnormal movements, i.e., post‐encephalitic parkinsonism, tremor, dystonia, chorea, myoclonus, and oculo‐masticatorymyorhythmias. 32 Encephalitis and encephalopathies in COVID‐19 are being reported commonly, 1 but surprisingly, reports of movement disorders are scant. 33 During a time in which the medical literature is witnessing a deluge of COVID‐19 related papers, those that report COVID‐19 induced movement disorders are meager.
The purpose of this review was to summarize available pieces of evidence documenting de novo movement disorders in COVID‐19 patients. Indeed, the movement disorders that have been reported in those patients who have suffered from COVID‐19 rather than on the people already living with diagnosed or undiagnosed movement disorders. We also analyzed several putative pathological mechanisms of development of movement disorders in SARS‐CoV‐2 infection and finally, assessed why there is a scarcity of de novo movement disorders.
Methods
Search Strategy
We searched through the PubMed, Embase, Web of Science, and Scopus databases with the following keywords ([“Parkinsonism” OR “Ataxia” OR “myoclonus” OR “tremor” OR “dystonia” OR “chorea” OR “movement disorders”] AND [“SARS‐CoV‐2” OR “COVID‐19”]), up to 29th January 2021. The articles were imported into the Rayyan QCRI software. 34 We also hand‐searched additional COVID‐19 specific articles using the reference list of the selected studies and relevant journal websites from 2019 to the current date for literature inclusion. To decrease publication bias, we invigilated the references of all studies potentially missed in the electrical search. Content experts also searched the gray literature of any relevant articles.
Study Selection Criteria
All peer‐reviewed studies, including cohort, case–control studies, and case reports, which met the pre‐specified inclusion and exclusion criteria, were included in this study.
Inclusion Criteria
Our inclusion criteria were as follows: (1) studies reporting COVID‐19 associated movement disorders; (2) studies reporting of management and outcome of the cases; and (3) studies approved by an Ethics Committee or Institutional Review Board, along with mandatory written informed consent before inclusion. Only studies that were published in English were considered. Accordingly, we excluded the studies with the following criteria: (1) prior history of movement disorder; (2) insufficient data and, subsequently, failure to contact the authors; (3) non‐clinical research, animal studies and reviews; and (4) duplicate publications. The references of the original articles and reviews identified were manually searched further for any article that has been missed out.
Exclusion Criteria
We excluded studies if COVID‐19 had not been confirmed and those written in languages other than English. We also excluded review papers, viewpoints, commentaries, and studies where information related to neurological manifestations or movement disorders was not reported.
Study Selection and Evidence Synthesis
The titles and abstracts were studied by two reviewers independently, and selected studies underwent full‐text review. For the studies that were included for this review, the following information was extracted: demographics, disease semiology, time to diagnosis, clinical history, diagnostic methods employed, including neuroimaging, therapy, final outcome, cerebrospinal fluid (CSF) study, and autoimmune encephalitis panel. The extracted information was qualitatively synthesized. Because the number of studies and patients were limited, quantitative synthesis was not carried out.
Statistical Analysis
Qualitative data were expressed in percentages. Unit discordance among the variables was resolved by converting the variables to a standard unit of measurement. A P value <0.05 was considered statistically significant, but it could not be calculated due to insufficient data. A meta‐analysis was planned to analyze the association of the demographic findings, symptoms, biochemical and neuroimaging parameters and outcomes, but was later omitted due to lack of sufficient data.
Ethics
This is a review of published literature on de novo movement disorders in COVID‐19 and did not involve any human or animal subjects. Hence, approval from an Ethics Committee was not applicable.
Results
The selection process carried out according to the Preferred Reporting for Systematic Review and Meta‐Analysis (PRISMA) consensus statement is shown in Figure 1. The published cases are summarized in Table 1. 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 Twenty‐two articles were finally selected for the qualitative synthesis. Among these, a total of 52 patients with de novo movement disorders were reported. Most of these had myoclonus, ataxia, tremor or a combination of these, while three had parkinsonism and one a functional disorder. Cases of post‐infectious generalized myoclonus were firstly reported by Khoo et al. 35 and Rábano‐Suárez et al. 44 Anand et al. 38 in their case series, from three different centers, reported eight cases of myoclonus associated with SARS‐CoV‐2 infection; the cases had variable backgrounds, comorbidities and outcomes. 38 Seven of these eight patients had significant metabolic disturbances, hypoxemia and medication exposure, which may have contributed to the development of myoclonus. 38 Seven cases of opsoclonus‐myoclonus syndrome, presumably para‐infectious, were described by Emamikhah et al., 56 who inferred that COVID‐19 was probably the initial trigger infection. Six of these cases also had voice tremors. 56 Authors of all the four manuscripts concluded that myoclonus was probably secondary to immune‐mediated brain injury. 35 , 38 , 44 , 56
FIG. 1.
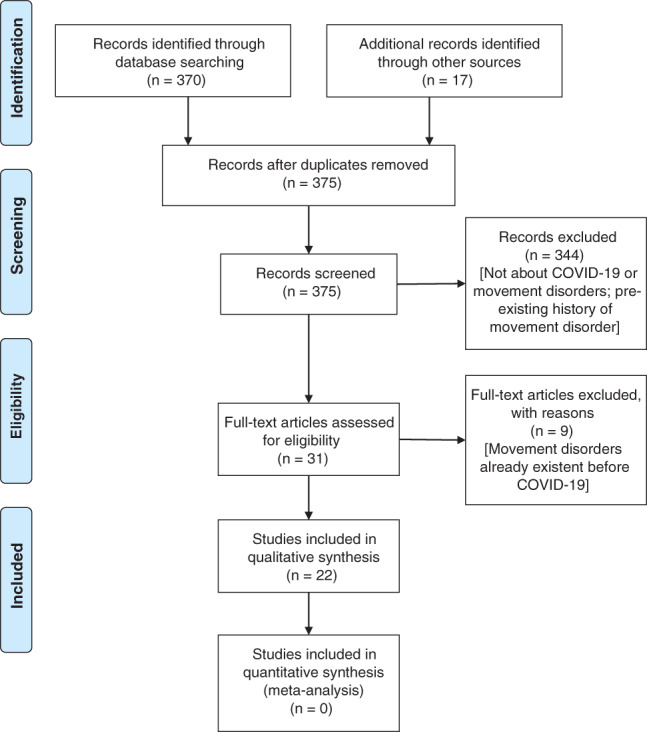
PRISMA 2009 flow diagram.
TABLE 1.
Clinical and radiological spectrum of COVID‐19 associated de novo movement disorders
| Author(s) | Age (y)/sex | Type of movement disorder(s) | Neuromaging/Electroencephalogram (EEG) | Therapy for movement disorder | Outcome | Time to movement disorder onset | CSF PCR for SARS‐CoV‐2 | Autoimmune encephalitis panel |
|---|---|---|---|---|---|---|---|---|
| Khoo et al. 35 | 65 / Woman | Generalized myoclonus, ocular flutter, ocular‐facial‐synkinesis, convergence spasm and acquired hyperekplexia | Normal / Normal EEG | Levetiracetam, clonazepam and corticosteroids | Improvement | Seven days | Negative | Negative |
| Lechien et al. 36 | 28 / Woman | Inspiratory and expiratory paradoxical movement of the vocal folds | Not performed | Speech therapy | Recovery after four months | Seven weeks | Not performed | Not performed |
| Dijkstra et al. 37 | 44 / Man | Generalized, stimulus‐sensitive myoclonus, transient ocular flutter and cerebellar ataxia | Normal / Not available | Intravenous corticosteroids and immunoglobulins | Full recovery after two months | Two weeks | Negative | Negative |
| Anand et al. 38 | Multicenter case series (eight patients) | Myoclonus (mostly stimulus sensitive; in one case it was spontaneous) | Normal in most cases/EEG was performed in most cases ranging from background slowing, transient bifrontal sharp waves to generalized dysfunction | Levetiracetam, clonazepam, valproate, dexmedetomidine (most cases), ketamine, lorazepam and primidone | One patient died. In five cases, myoclonus completely resolved while in three cases, it persisted for 10 days or longer. | Variable | Negative | Not performed |
| Cohen et al. 39 | 45 / Man | Asymmetric (right>left) tremor in legs, increased urinary frequency, micrography, hypomimia, bradykinesia and cogwheel rigidity | Normal brain magnetic resonance imaging. Positron emission tomography (PET) scan showed decreased 18F‐FDOPA uptake in both putamina, more apparent on the left side. Additionally, mild decreased uptake in the left caudate was also suspected / Normal EEG | Corticosteroids, pramipexole and biperidin | Persistence of symptoms | Three to four weeks | Negative | Negative |
| Faber et al. 40 | 35 / Woman | Generalized and asymmetric (right>left) bradykinesia, cogwheel rigidity, stooped posture, reduced arm swing, enbloc turning, and decreased stride length. | Normal magnetic resonance imaging and PET scans; decreased dopamine transporter (DAT) density on the left putamen (more evident in the mid‐putamen, different from the posterior involvement usual of idiopathic Parkinson's disease)/EEG not available | Levodopa/benserazide | Significant improvement with anti‐parkinsonian drugs | Two to four weeks | Negative | Negative |
| Cuhna P et al. 41 | Case series (five patients) | Upper limbs postural and action‐tremor in four patients; one of them had also irregular orthostatic tremor, another one bilateral upper limbs jerky/myoclonic abnormal movements at rest and during posture and action | Magnetic resonance imaging in four patients showed microbleeds and a bilateral frontotemporal hypoperfusion in one patient. Neuromelanin‐sensitive magnetic resonance imaging showed dorsal‐nigral hyperintensity bilaterally in all, but one. 123I‐FP‐CIT single photon emission computed tomography performed in four patients was normal / not performed | None | Not performed | Two to five weeks | Not performed | Not performed |
| Mas Serrano et al. 42 | Case series (two patients) | Myoclonus (multi‐focal) and ocular clonus | Normal neuroimaging in both cases/EEG showed diffuse encephalopathy in both cases | None | Improvement to baseline | One to two weeks | Not performed | Not performed |
| Diezma‐Martín et al. 43 | 70 / M | Tremor and ataxia | Normal / Not performed | Clonazepam | Improved after one moth of discharge | Two to five weeks | Negative | Negative |
| Rábano‐Suárez et al. 44 | Case series (three patients) | Stimulus sensitive generalized myoclonus | Normal / EEG showed diffuse background slowing | Dexmedetomidine, clonazepam, valproate, levetiracetam, propofol, corticosteroids and plasmapheresis | Improved | Two weeks | Negative | Negative |
| Balestrino et al. 45 | 73 / Man | Ataxia | Normal / EEG showed reactive, unstable, symmetrical background alpha activity in posterior regions; sporadic, low‐voltage, focal polymorph delta elements in the anterior‐frontal left cortex and sporadic spikes without clear epileptic correlate in the frontotemporal lobe, predominantly on the left | None | Improved after several weeks | One to two weeks | Not performed | Not performed |
| Méndez‐Guerrero et al. 46 | 57 / Man | Myoclonus, tremor, parkinsonism and vertical ocular movement abnormalities | DAT‐ single photon emission computed tomography showed bilateral decrease in presynaptic dopamine uptake asymmetrically involving both putamina / EEG showed diffuse mild and reactive slowing | No specific therapy | Improved to baseline within three weeks | 40 days | Negative | Negative |
| Chaumont et al. 47 | Case series (four patients) | Upper limbs myoclonus | Normal MRI except recent stroke in case 1 / EEG showed global slowing in patients 1, 2, and 3; and normal in patient 4 | Intravenous immunoglobulins and methylprednisolone | Some improvement | Variable | Negative | Not performed |
| Schellekens et al. 48 | 48/Man | Myoclonus and cerebellar ataxia | Normal / EEG was not performed | Levetiracetam | Improved | Variable | Negative | Not performed |
| Paterson et al. 49 | Case series (seven patients) | Ataxia and bilateral intention tremor in one patient | Normal in five; multiple large lesions in periventricular white matter of both cerebral hemispheres in one and mild to moderate small vessel disease in another one / EEG was not performed | Intravenous immunoglobulin and corticosteroids | Four cases recovered incompletely an three completely | Variable | Negative in four and not performed in three | Not performed in four; matched oligoclonal bands in serum in two; and negative in one |
| Piscitelli et al. 50 | 39/Woman | Lower limbs tremor and ataxia | Normal magnetic resonance imaging/EEG was not performed | Benzodiazepines | No improvement | 11 days | Not performed | Not performed |
| Grimaldi et al. 51 | 72/Woman | Cerebellar syndrome and myoclonus | 18F‐FDG‐PET showed diffuse cortical hypometabolism, associated with putaminal and cerebellum hypermetabolism / EEG showed symmetric bilateral background slowing | Intravenous corticosteroids | Rapid improvement | 12 days | Negative | High titers of autoantibodies directed against nuclei of Purkinje cells, as well as to striatal and hippocampal neurons were detected |
| Borroni B et al. 52 | Two cases (54 / Woman and 80 / Man) | Diaphragmatic myoclonus | Normal / EEG was normal in case 1 and lateralized periodic discharges synchronous and asynchronous with the diaphragmatic myoclonic movements in case 2 | Clonazepam in case 1 and levetiracetam in case 2 | Improvement | Two weeks in case 1 and one month in case 2 | Negative | Not performed |
| Shah et al. 53 | Middle aged male | Opsoclonus, cortical myoclonus, symmetric cerebellar ataxia of speech, limbs, trunk and gait | Normal / Not available | Corticosteroids, levetiracetam, valproate and clonazepam | Improvement | Three weeks | Negative | Negative |
| Muccioli et al. 54 | 58 / Man | Subcortical myoclonus | Chronic small vessel disease / Normal EEG | Corticosteroids, levetiracetam and clonazepam | Improvement | 12 days | Negative | Negative |
| Ros‐Castelló et al. 55 | 72 / Woman | Myoclonus | Cortical and brainstem small vessel disease / Normal EEG | Clonazepam | Improvement | Five days | Not performed | Not performed |
| Emamikhah et al. 56 | Case series (seven patients) | Opsoclonus‐myoclonus syndrome and voice tremor | Normal (in one, it was not performed) / EEG was normal in one patient and not performed in the rest | Levetiracetam, valproate, clonazepam, intravenous immunoglobulins and dexamethasone | Recovery (partial for three patients; complete for two patients); one lost to follow‐up; and one under treatment | Variable | Negative for two and not performed for rest | Negative for one and not performed for the rest |
Diezma‐Martín et al. 43 reported a case of tremor and ataxia in a 70‐year‐old man following COVID‐19. A similar case of gait ataxia was described by Balestrino et al. 45 in an aged patient with multiple comorbidities. Chaumont et al. 47 reported four cases of severe COVID‐19 in male patients aged 50–70 years after weaning of mechanical ventilation and extubation. Among a myriad of neurological manifestations, the patients had upper limb myoclonus, which persisted 3 weeks after discharge and an immunoglobulin regimen. 47 Méndez‐Guerrero et al. 46 reported a case of opsoclonus‐myoclonus complex in a 58‐year‐old man with an asymmetric hypokinetic‐rigid syndrome, in whom there was asymmetrically decreased presynaptic dopamine uptake in the putamen on I‐2β‐carbomethoxy‐3β‐(4‐iodophenyl)‐N‐(3‐fluoropropyl) nortropane (I‐FP‐CIT) dopamine transporter single photon emission computed tomography (DAT‐SPECT). Schellekens et al. 48 reported the case of a 48‐year‐old man with a history of asymptomatic HIV infection who had a partially reverted picture of myoclonus‐ataxia in the setting of COVID‐19. Paterson et al. 49 described a total of 43 COVID‐19 patients with neurological manifestations, among which seven had post‐COVID‐19 ataxia. Intriguingly, lower limb tremors were observed in a SARS‐CoV‐2 infected woman, which could neither be traced to neurological impairment on examination nor an infectious or hormonal etiology on serum testing. The authors categorized it as a “functional movement disorder threatening existential continuum”. 50
In general, the patients were managed successfully by intravenous immunoglobulin or steroids. In a few patients, a multimodal approach was employed. In the case of Cohen et al. 39 the symptoms persisted even after treatment with steroids, pramipexole, and biperidine. Clonazepam was used alone or in combination with other drugs in multiple patients where the condition improved. 35 , 38 , 43 , 44 , 52 , 53 , 55 , 56 In four cases, the patients improved without any specific treatment. 42 , 45 , 46 The positive effect of immunotherapy in many of the patients points towards that the pathogenetic mechanisms for the development of some movement disorders, following SARS‐CoV‐2 infection, may be mediated by aberrant immune‐mediated injury.
Discussion
During this ongoing pandemic, a plethora of neurological manifestations in COVID‐19 affecting both the central and the peripheral nervous systems are being reported. 1 However, surprisingly, data regarding movement disorders are scarce. Movement disorders in COVID‐19 might be an under‐recognized neurological complication. Thus, in the present review, we have only found 52 patients with de novo movement disorders.
Regarding the pathophysiology of movement disorders in COVID‐19 as a whole, there are currently three possible explanations. Firstly, many cases, mainly those with myoclonus, could be ascribed to drugs, metabolic disturbances, or simply severe hypoxia. 44 Further, cases of autoimmune encephalitis with subacute cerebellar syndrome and myoclonus, triggered by SARS‐CoV‐2 infection, have just started to surface, 51 as are therapy‐related complications, such as opsoclonus‐myoclonus in COVID‐19 therapy‐induced serotonin syndrome. 42 Secondly, a post‐ or para‐infectious immune‐mediated mechanism somehow related to the inflammatory phase of COVID‐19. In this sense, there are many reports in the literature of myoclonus or parkinsonism presenting shortly after the onset of infection. 15 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 57 Finally, the neuroinvasive potential of SARS‐CoV in humans is well known. 58 , 59 Notably, SARS‐CoV, 60 as well as the H1N1 influenza virus, 61 when they are inoculated intranasally, could spread through a transneuronal route to first‐ and second‐order structures connected with the olfactory bulb. SARS‐CoV‐2 is present in some COVID‐19 patients in their cerebrospinal fluid. 1 This agrees with previous studies that have found antibodies to coronaviruses in the cerebrospinal fluid of patients with Parkinson's disease, 62 suggesting the potential of SARS‐CoV‐2 for causing parkinsonism. Interestingly, striatal dopaminergic neurons, microglia, and astrocytes of basal ganglia bear angiotensin‐converting enzyme 2 (ACE‐2), the receptor for SARS‐CoV‐2, 63 , 64 which would create a pathway for the entry of this virus, affecting these structures. 65
The fact that hyposmia is one of the most prevalent symptoms of COVID‐19 66 and that the olfactory system is early affected in alpha‐synucleinopathies might be an intriguing coincidence. 67 However, it is notable that recent studies indicate that α‐synuclein may participate in the innate immune response to any viral infection. 68 Sadasivan et al. 69 demonstrated that multiple hits by H1N1 infection and exposure to environmental toxins simultaneously may damage the dopaminergic neurons in substantia nigra to a greater extent than a single hit by any single agent. This potentially keeps alive the threat of development of post‐COVID‐19 parkinsonism as a delayed and long‐term debility as it was in the pandemic of 1918. 70 , 71 , 72
The basal ganglia is involved in cases of encephalitis following infection with respiratory pathogens. 73 Fishman et al. 74 demonstrated that the murine coronavirus has neurotropic potential and particular predilection towards the involvement of the basal ganglia, resulting in extra‐ and intra‐cellular vacuolation, neuronal loss, chronic demyelination and gliosis. As suggested, 74 a virus capable of causing both encephalitis and persistent infection in several species may be related to post‐encephalitic parkinsonism. A study by Haddadi et al. 75 regarding a case of SARS‐CoV‐2 associated hemorrhagic encephalitis involving the basal ganglia bilaterally, further supports the affinity of coronaviruses by these structures. A cross‐sectional, retrospective, observational study by Chougar et al. 76 revealed two distinct patterns of brain parenchymal involvement in SARS‐CoV‐2 infection, including white matter enhancing lesions and basal ganglia abnormalities. Similarly, SARS‐CoV‐2 infection has been associated with immune‐mediated acute hemorrhagic encephalopathy involving brainstem and thalami 77 as well as with vasculopathic or demyelinating processes involving bilateral putamina. 78
Recently, both ACE‐2 and transmembrane serine protease 2 (TMPRSS2) have been found to be expressed in human corneal epithelium, suggesting that ocular surface cells could also be a potential viral entry point. 79 SARS‐CoV‐2 could invade the central nervous system, through vascular or retrograde axonal pathways, and infect the striatal neurons (Fig. 2). 80 From here onwards, there might be two primary mechanisms through which the virus may cause movement disorders (Fig. 2). Firstly, the downregulation of ACE‐2 receptors could result in an imbalance of neurotransmitter levels, 81 primarily dopamine and norepinephrine (Fig. 2), leading to dopamine deficiency. Secondly, the virus could cause cellular vacuolation, demyelination, and gliosis, leading to encephalitis and associated movement disorders (Fig. 2).
FIG. 2.
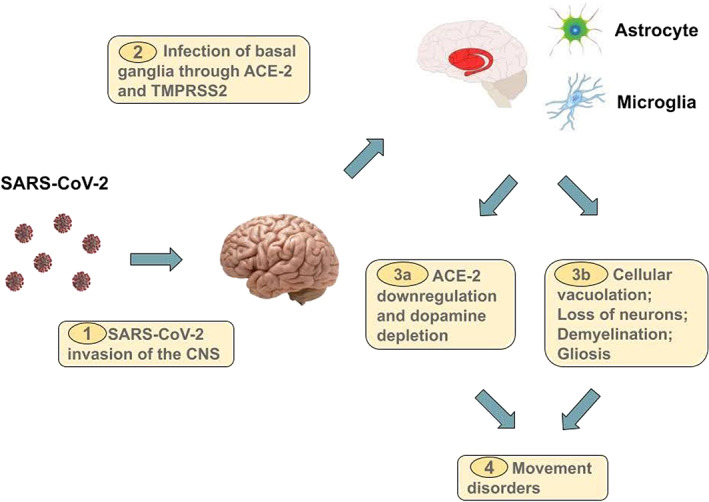
Proposed pathogenic pathway for the development of de novo movement disorders in COVID‐19.
Movement disorders are now sparse among COVID‐19 patients (Fig. 3). In most cases of neurological complications associated with SARS‐CoV‐2 infection, the neural substrates for movement disorders are spared; in fact, pure cortical, cortico‐subcortical interface and deep white matter involvement predominates, as evidenced by various imaging‐based studies. 76 , 82 , 83 , 84 , 85 Furthermore, movement disorders following an infectious‐autoimmune process typically develop after a period of weeks to months. Hence, there remains a possibility that expected parenchymal changes in the brain are just waiting to reach a threshold level to manifest as delayed movement disorders. 41 On the other hand, the differential distribution of ACE‐2 receptors and its ancillary protein co‐regulators among several brain cell types may be the cause of more strokes and fewer movement disorders. 86 Finally, human coronavirus can lie dormant in the leukocytes for a long period and may manifest as delayed or persistent central nervous system infection, later giving rise to movement disorders. 87 Indeed, immune dysregulation brought about by SARS‐CoV‐2 infection 88 may lead to neurodegeneration 89 and potentially neurodegenerative movement disorders might manifest later. Notwithstanding, movement disorders are being increasingly documented because of the higher reporting frequency of COVID‐19 cases worldwide.
FIG. 3.
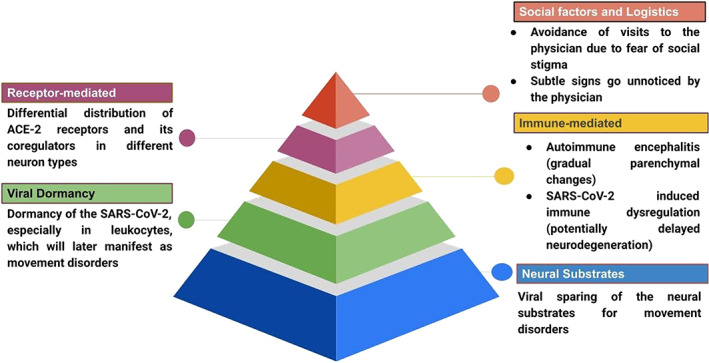
Possible factors responsible for the scarcity of reports of movement disorders in COVID‐19.
There are some limitations in the current review. Given the notable asymmetry between the total number of affected cases and reported cases of de novo movement disorders in COVID‐19, it can be assumed that cases are currently under‐reported. The current review is based on a small number of cases, even after an extensive search of available literature. Examination performed on in‐hospital patients wearing masks, mostly by videoconferencing, may perhaps explain the limited description of these conditions. Also, several of the available reports do not describe the timeline of events in an organized manner, making interpretation difficult. Laboratory, electroencephalography and neuroimaging features have also not been mentioned in detail in a few of the cases. In addition, there is considerable heterogeneity in the available data that may be considered a hindrance in advanced analysis. Finally, we have not included non‐English articles. Despite these shortcomings, the present organized review will act as a preliminary guide for clinicians while dealing with movement disorders that appear in the setting of COVID‐19.
In closing, only a few case reports have been published describing mainly patients suffering from myoclonus, parkinsonism, tremor, and ataxia. Some cases, mainly those with myoclonus, could be ascribed to medication exposures, metabolic disturbances, or by severe hypoxia, and others, to a post‐ or para‐infectious immune‐mediated mechanism somehow related to the inflammatory phase of COVID‐19. SARS‐CoV‐2 could also invade the central nervous system through vascular or retrograde axonal pathways and cause movement disorders. Finally, although SARS‐CoV‐2 may cause neurodegeneration, leading to movement disorders, this link is not yet established. However, we should closely monitor COVID‐19 survivors for the possibility of post‐COVID movement disorders. For now, we must patiently and vigilantly wait for time to unravel whether “post‐COVID movement disorder” will be an additional entity to add to the spectrum of movement disorders.
Author Roles
(1) Project: A. Conception, B. Organization, C. Execution; (2) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
R.G.: 1A, 1B, 1C, 2A
U.B.: 2A, 2B
D.R.: 1B, 1C, 2B
A.P.: 1C, 2B
D.L.: 1C, 2B
B.K.R.: 1C, 2B
J.B.L.: 1A, 1B, 1C, 2B
Disclosures
Ethical Compliance Statement
Informed patient consent was not necessary for this work. The authors confirm that the approval of an institutional review board was not required for this work. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding of Sources and Conflict of Interest
No specific funding was received for this work. The authors declare that there are no conflicts of interest relevant to this work.
Financial Disclosures for the Previous 12 Months
The authors declare that there are no additional disclosures to report.
Potential conflict of interest: The authors declare no competing financial interests.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Roy D, Ghosh R, Dubey S, Dubey MJ, Benito‐León J, Kanti RB. Neurological and neuropsychiatric impacts of COVID‐19 pandemic. Can J Neurol Sci 2021;48:9–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ghosh R, Dubey S, Kanti Ray B, Chatterjee S, Benito‐León J. COVID‐19 presenting with thalamic hemorrhage unmasking moyamoya angiopathy. Can J Neurol Sci 2020;47:849–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ghosh R, Lahiri D, Dubey S, Ray BK, Benito‐León J. Hallucinatory palinopsia in COVID‐19 induced posterior reversible encephalopathy syndrome. J Neuro‐Ophthalmol 2020;40:523–526. [DOI] [PubMed] [Google Scholar]
- 4. Ghosh R, Roy D, Sengupta S, Benito‐León J. Autonomic dysfunction heralding acute motor axonal neuropathy in COVID‐19. J Neurovirol 2020;26:964–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gutierrez‐Ortiz C, Mendez A, Rodrigo‐Rey S, et al. Miller fisher syndrome and polyneuritis cranialis in COVID‐19. Neurology 2020;95:e601–e605. [DOI] [PubMed] [Google Scholar]
- 6. Cucca A, Migdadi HA, Di Rocco A. Infection‐mediated autoimmune movement disorders. Parkinsonism Relat Disord 2018;46(Suppl 1):S83–S86. [DOI] [PubMed] [Google Scholar]
- 7. Kirvan CA, Swedo SE, Kurahara D, Cunningham MW. Streptococcal mimicry and antibody‐mediated cell signaling in the pathogenesis of Sydenham's chorea. Autoimmunity 2006;39:21–29. [DOI] [PubMed] [Google Scholar]
- 8. Sinmaz N, Tea F, Pilli D, et al. Dopamine‐2 receptor extracellular N‐terminus regulates receptor surface availability and is the target of human pathogenic antibodies from children with movement and psychiatric disorders. Acta Neuropathol Commun 2016;4:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dale RC, Merheb V, Pillai S, et al. Antibodies to surface dopamine‐2 receptor in autoimmune movement and psychiatric disorders. Brain 2012;135:3453–3468. [DOI] [PubMed] [Google Scholar]
- 10. Oh SY, Kim JS, Dieterich M. Update on opsoclonus‐myoclonus syndrome in adults. J Neurol 2019;266:1541–1548. [DOI] [PubMed] [Google Scholar]
- 11. Pike M. Opsoclonus‐myoclonus syndrome. Handb Clin Neurol 2013;112:1209–1211. [DOI] [PubMed] [Google Scholar]
- 12. Tiwari S, Singh RK, Tiwari R, Dhole TN. Japanese encephalitis: a review of the Indian perspective. Braz J Infect Dis 2012;16:564–573. [DOI] [PubMed] [Google Scholar]
- 13. Misra UK, Kalita J, Pandey S, Khanna VK, Babu GN. Cerebrospinal fluid catecholamine levels in Japanese encephalitis patients with movement disorders. Neurochem Res 2005;30:1075–1078. [DOI] [PubMed] [Google Scholar]
- 14. Solomon T, Fisher AF, Beasley DW, et al. Natural and nosocomial infection in a patient with West Nile encephalitis and extrapyramidal movement disorders. Clin Infect Dis 2003;36:E140–E145. [DOI] [PubMed] [Google Scholar]
- 15. Azmin S, Sahathevan R, Suehazlyn Z, et al. Post‐dengue parkinsonism. BMC Infect Dis 2013;13:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dale RC, Church AJ, Benton S, et al. Post‐streptococcal autoimmune dystonia with isolated bilateral striatal necrosis. Dev Med Child Neurol 2002;44:485–489. [DOI] [PubMed] [Google Scholar]
- 17. Schein F, Gagneux‐Brunon A, Antoine JC, et al. Anti‐N‐methyl‐D‐aspartate receptor encephalitis after herpes simplex virus‐associated encephalitis: An emerging disease with diagnosis and therapeutic challenges. Infection 2017;45:545–549. [DOI] [PubMed] [Google Scholar]
- 18. Ma J, Zhang T, Jiang L. Japanese encephalitis can trigger anti‐N‐methyl‐D‐aspartate receptor encephalitis. J Neurol 2017;264:1127–1131. [DOI] [PubMed] [Google Scholar]
- 19. Tse W, Cersosimo MG, Gracies JM, Morgello S, Olanow CW, Koller W. Movement disorders and AIDS: a review. Parkinsonism Relat Disord 2004;10:323–334. [DOI] [PubMed] [Google Scholar]
- 20. Misra UK, Kalita J. Spectrum of movement disorders in encephalitis. J Neurol 2010;257(12):2052–2058. [DOI] [PubMed] [Google Scholar]
- 21. Takahashi M, Yamada T. Viral etiology for Parkinson's disease–a possible role of influenza A virus infection. Jpn J Infect Dis 1999;52:89–98. [PubMed] [Google Scholar]
- 22. Bopeththa B, Ralapanawa U. Post encephalitic parkinsonism following dengue viral infection. BMC Res Notes 2017;10:655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dourmashkin RR, Dunn G, Castano V, McCall SA. Evidence for an enterovirus as the cause of encephalitis lethargica. BMC Infect Dis 2012;12:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guan J, Lu Z, Zhou Q. Reversible parkinsonism due to involvement of substantia nigra in Epstein‐Barr virus encephalitis. Mov Disord 2012;27:156–157. [DOI] [PubMed] [Google Scholar]
- 25. Pasha SA, Pasha SA, Suhasini T, Rao DA. Hepatitis E virus‐associated acute encephalitic parkinsonism. J Assoc Physicians India 2018;66:92–93. [PubMed] [Google Scholar]
- 26. Cubo E. [Movement disorders in adult‐onset measles encephalitis]. Neurologia 2003;18:30–33. [PubMed] [Google Scholar]
- 27. Liu X, Deng F, Chen L. Parkinsonism caused by viral encephalitis affecting the bilateral substantia Nigra. Clin Neuroradiol 2019;29:571–573. [DOI] [PubMed] [Google Scholar]
- 28. Jang H, Boltz DA, Webster RG, Smeyne RJ. Viral parkinsonism. Biochim Biophys Acta 2009;1792:714–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nussinovitch M, Prais D, Volovitz B, Shapiro R, Amir J. Post‐infectious acute cerebellar ataxia in children. Clin Pediatr 2003;42:581–584. [DOI] [PubMed] [Google Scholar]
- 30. Zandi MS. Encephalitis lethargica: a dying fall. Brain 2019;142:2888–2891.32445463 [Google Scholar]
- 31. Lutters B, Foley P, Koehler PJ. The centennial lesson of encephalitis lethargica. Neurology 2018;90:563–567. [DOI] [PubMed] [Google Scholar]
- 32. Vilensky JA, Goetz CG, Gilman S. Movement disorders associated with encephalitis lethargica: a video compilation. Mov Disord 2006;21:1–8. [DOI] [PubMed] [Google Scholar]
- 33. Geyer HL, Kaufman DM, Parihar RK, Mehler MF. Movement disorders in COVID‐19: Whither art thou? Tremor Other Hyperkinet Mov (N Y) 2020;10:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev 2016;5:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Khoo A, McLoughlin B, Cheema S, et al. Postinfectious brainstem encephalitis associated with SARS‐CoV‐2. J Neurol Neurosurg Psychiatry 2020;91:1013–1014. [DOI] [PubMed] [Google Scholar]
- 36. Lechien JR, Circiu MP, Crevier‐Buchman L, Hans S. Post‐COVID‐19 paradoxical vocal fold movement disorder. European archives of oto‐rhino‐laryngology 2021;278:845–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dijkstra F, Van den Bossche T, Willekens B, Cras P, Crosiers D. Myoclonus and cerebellar ataxia following coronavirus disease 2019 (COVID‐19). Mov Disorders Clin Pract 2020;7:974–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Anand P, Zakaria A, Benameur K, et al. Myoclonus in patients with coronavirus disease 2019: a multicenter case series. Crit Care Med 2020;48:1664–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cohen ME, Eichel R, Steiner‐Birmanns B, et al. A case of probable Parkinson's disease after SARS‐CoV‐2 infection. Lancet Neurol 2020;19:804–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Faber I, Brandão PRP, Menegatti F, de Carvalho Bispo DD, Maluf FB, Cardoso F. Coronavirus disease 2019 and parkinsonism: a non‐post‐encephalitic case. Mov Disord 2020;35:1721–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cuhna P, Herlin B, Vassilev K, et al. Movement disorders as a new neurological clinical picture in severe SARS‐CoV‐2 infection. Eur J Neurol 2020;27:e88–e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mas Serrano M, Pérez‐Sánchez JR, Portela Sánchez S, et al. Serotonin syndrome in two COVID‐19 patients treated with lopinavir/ritonavir. J Neurol Sci 2020;415:116944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Diezma‐Martín AM, Morales‐Casado MI, García‐Alvarado N, Vadillo Bermejo A, López‐Ariztegui N, Sepúlveda Berrocal MA. Tremor and ataxia in COVID‐19. Neurologia 2020;35:409–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rábano‐Suárez P, Bermejo‐Guerrero L, Méndez‐Guerrero A, et al. Generalized myoclonus in COVID‐19. Neurology 2020;95:e767–e772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Balestrino R, Rizzone M, Zibetti M, et al. Onset of Covid‐19 with impaired consciousness and ataxia: a case report. J Neurol 2020;267:2797–2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Méndez‐Guerrero A, Laespada‐García MI, Gómez‐Grande A, et al. Acute hypokinetic‐rigid syndrome following SARS‐CoV‐2 infection. Neurology 2020;95:e2109–e2118. [DOI] [PubMed] [Google Scholar]
- 47. Chaumont H, San‐Galli A, Martino F, et al. Mixed central and peripheral nervous system disorders in severe SARS‐CoV‐2 infection. J Neurol 2020;267:3121–3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schellekens MMI, Bleeker‐Rovers CP, Keurlings PAJ, Mummery CJ, Bloem BR. Reversible myoclonus‐ataxia as a postinfectious manifestation of COVID‐19. Mov Disord Clin Pract 2020;7:977–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Paterson RW, Brown RL, Benjamin L, et al. The emerging spectrum of COVID‐19 neurology: clinical, radiological and laboratory findings. Brain 2020;143:3104–3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Piscitelli D, Perin C, Tremolizzo L, Peroni F, Cerri CG, Cornaggia CM. Functional movement disorders in a patient with COVID‐19. Neurol Sci 2020;41:2343–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Grimaldi S, Lagarde S, Harle JR, Boucraut J, Guedj E. Autoimmune encephalitis concomitant with SARS‐CoV‐2 infection: insight from (18)F‐FDG PET imaging and neuronal autoantibodies. J Nucl Med 2020;61:1726–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Borroni B, Gazzina S, Dono F, et al. Diaphragmatic myoclonus due to SARS‐CoV‐2 infection. Neurol Sci 2020;41:3471–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shah PB, Desai SD. Opsoclonus myoclonus ataxia syndrome in the setting of COVID‐19 infection. Neurology 2021;96:33. [DOI] [PubMed] [Google Scholar]
- 54. Muccioli L, Rondelli F, Ferri L, Rossini G, Cortelli P, Guarino M. Subcortical myoclonus in COVID‐19: comprehensive evaluation of a patient. Mov Disord Clin Pract 2020;7:971–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ros‐Castelló V, Quereda C, López‐Sendón J, Corral I. Post‐hypoxic myoclonus after COVID‐19 infection recovery. Mov Disord Clin Pract 2020;7:983–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Emamikhah M, Babadi M, Mehrabani M, et al. Opsoclonus‐myoclonus syndrome, a post‐infectious neurologic complication of COVID‐19: case series and review of literature. J Neurovirol 2021;27:26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bhatia K, Thompson PD, Marsden CD. "Isolated" postinfectious myoclonus. J Neurol Neurosurg Psychiatry 1992;55:1089–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gu J, Gong E, Zhang B, et al. Multiple organ infection and the pathogenesis of SARS. J Exp Med 2005;202:415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Xu J, Zhong S, Liu J, et al. Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine mig in pathogenesis. Clin Infect Dis 2005;41:1089–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol 2008;82:7264–7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. de Wit E, Siegers JY, Cronin JM, et al. 1918 H1N1 influenza virus replicates and induces proinflammatory cytokine responses in extrarespiratory tissues of ferrets. J Infect Dis 2018;217:1237–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fazzini E, Fleming J, Fahn S. Cerebrospinal fluid antibodies to coronavirus in patients with Parkinson's disease. Mov Disord 1992;7:153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Joglar B, Rodriguez‐Pallares J, Rodriguez‐Perez AI, Rey P, Guerra MJ, Labandeira‐Garcia JL. The inflammatory response in the MPTP model of Parkinson's disease is mediated by brain angiotensin: relevance to progression of the disease. J Neurochem 2009;109:656–669. [DOI] [PubMed] [Google Scholar]
- 64. Rodriguez‐Perez AI, Garrido‐Gil P, Pedrosa MA, et al. Angiotensin type 2 receptors: role in aging and neuroinflammation in the substantia nigra. Brain Behav Immun 2020;87:256–271. [DOI] [PubMed] [Google Scholar]
- 65. Lan J, Ge J, Yu J, et al. Structure of the SARS‐CoV‐2 spike receptor‐binding domain bound to the ACE2 receptor. Nature 2020;581:215–220. [DOI] [PubMed] [Google Scholar]
- 66. Lechien JR, Chiesa‐Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild‐to‐moderate forms of the coronavirus disease (COVID‐19): A multicenter European study. Eur Arch Otorhinolaryngol 2020;277:2251–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rey NL, Wesson DW, Brundin P. The olfactory bulb as the entry site for prion‐like propagation in neurodegenerative diseases. Neurobiol Dis 2018;109(Pt B):226–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tulisiak CT, Mercado G, Peelaerts W, Brundin L, Brundin P. Can infections trigger alpha‐synucleinopathies? Prog Mol Biol Transl Sci 2019;168:299–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sadasivan S, Sharp B, Schultz‐Cherry S, Smeyne RJ. Synergistic effects of influenza and 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine (MPTP) can be eliminated by the use of influenza therapeutics: Experimental evidence for the multi‐hit hypothesis. NPJ Parkinsons Dis 2017;3:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Boika AV. A post‐COVID‐19 parkinsonism in the future? Mov Disord 2020;35:1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Helmich RC, Bloem BR. The impact of the COVID‐19 pandemic on Parkinson's disease: hidden sorrows and emerging opportunities. J Parkinsons Dis 2020;10:351–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Victorino DB, Guimarães‐Marques M, Nejm M, Scorza FA, Scorza CA. COVID‐19 and Parkinson's disease: are we dealing with short‐term impacts or something worse? J Parkinsons Dis 2020;10:899–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Beattie GC, Glaser CA, Sheriff H, et al. Encephalitis with thalamic and basal ganglia abnormalities: etiologies, neuroimaging, and potential role of respiratory viruses. Clin Infect Dis 2013;56:825–832. [DOI] [PubMed] [Google Scholar]
- 74. Fishman PS, Gass JS, Swoveland PT, Lavi E, Highkin MK, Weiss SR. Infection of the basal ganglia by a murine coronavirus. Science 1985;229:877–879. [DOI] [PubMed] [Google Scholar]
- 75. Haddadi K, Ghasemian R, Shafizad M. Basal ganglia involvement and altered mental status: a unique neurological manifestation of coronavirus disease 2019. Cureus 2020;12(4):e7869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chougar L, Shor N, Weiss N, et al. Retrospective observational study of brain magnetic resonance imaging findings in patients with acute SARS‐CoV‐2 infection and neurological manifestations. Radiology 2020;297:E313–E323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Dixon L, Varley J, Gontsarova A, et al. COVID‐19‐related acute necrotizing encephalopathy with brain stem involvement in a patient with aplastic anemia. Neurol Neuroimmunol Neuroinflamm 2020;7:e789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Brun G, Hak JF, Coze S, et al. COVID‐19‐white matter and globus pallidum lesions: demyelination or small‐vessel vasculitis? Neurol Neuroimmunol Neuroinflamm 2020;7:e777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhou L, Xu Z, Castiglione GM, Soiberman US, Eberhart CG, Duh EJ. ACE2 and TMPRSS2 are expressed on the human ocular surface, suggesting susceptibility to SARS‐CoV‐2 infection. Ocul Surf 2020;18:537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wambier CG, Goren A. Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection is likely to be androgen mediated. J Am Acad Dermatol 2020;83:308–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Nataf S. An alteration of the dopamine synthetic pathway is possibly involved in the pathophysiology of COVID‐19. J Med Virol 2020;92:1743–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kandemirli SG, Dogan L, Sarikaya ZT, et al. Brain MRI findings in patients in the intensive care unit with COVID‐19 infection. Radiology 2020;297:E232–e235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Klironomos S, Tzortzakakis A, Kits A, et al. Nervous system involvement in COVID‐19: results from a retrospective consecutive neuroimaging cohort. Radiology 2020;297:E324–E334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kremer S, Lersy F, de Sèze J, et al. Brain MRI findings in severe COVID‐19: a retrospective observational study. Radiology 2020;297:E242–e251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Coolen T, Lolli V, Sadeghi N, et al. Early postmortem brain MRI findings in COVID‐19 non‐survivors. Neurology 2020;95:e2016–e2027. [DOI] [PubMed] [Google Scholar]
- 86. Choi JY, Lee HK, Park JH, et al. Altered COVID‐19 receptor ACE2 expression in a higher risk group for cerebrovascular disease and ischemic stroke. Biochem Biophys Res Commun 2020;528:413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Desforges M, Le Coupanec A, Dubeau P, et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses 2019;12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kiselevskiy M, Shubina I, Chikileva I, et al. Immune pathogenesis of COVID‐19 intoxication: storm or silence? Pharmaceuticals (Basel, Switzerland) 2020;13:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lippi A, Domingues R, Setz C, Outeiro TF, Krisko A. SARS‐CoV‐2: at the crossroad between aging and neurodegeneration. Mov Disord 2020;35:716–720. [DOI] [PMC free article] [PubMed] [Google Scholar]


