Abstract
Eukaryotic mRNAs are emerging modalities for protein replacement therapy and vaccination. Their 5′ cap is important for mRNA translation and immune response and can be naturally methylated at different positions by S‐adenosyl‐l‐methionine (AdoMet)‐dependent methyltransferases (MTases). We report on the cosubstrate scope of the MTase CAPAM responsible for methylation at the N 6‐position of adenosine start nucleotides using synthetic AdoMet analogs. The chemo‐enzymatic propargylation enabled production of site‐specifically modified reporter‐mRNAs. These cap‐propargylated mRNAs were efficiently translated and showed ≈3‐fold increased immune response in human cells. The same effects were observed when the receptor binding domain (RBD) of SARS‐CoV‐2—a currently tested epitope for mRNA vaccination—was used. Site‐specific chemo‐enzymatic modification of eukaryotic mRNA may thus be a suitable strategy to modulate translation and immune response of mRNAs for future therapeutic applications.
Keywords: non-natural modifications, RNA modification, RNA vaccine, translation
Natural modifications at the 5′ cap of mRNA impact translation and immunogenicity. Now, mRNAs with non‐natural post‐transcriptional modifications are tested regarding translation and immunogenicity, providing a new strategy to tailor mRNA for therapeutic applications.
Introduction
Eukaryotic mRNA has emerged as a new therapeutic modality for vaccination and protein replacement. An mRNA coding for the spike protein of SARS‐CoV‐2 has recently been admitted as first Covid‐19 (coronavirus disease 2019) vaccine and several clinical studies for cancer treatment are underway, demonstrating the potential of this technology for fast development and personalized medicine. [1] mRNA made by in vitro transcription (IVT) can stimulate the innate immune system and lead to potent antigen‐specific cellular and humoral immune response. [2] This intrinsic adjuvant activity is an added benefit for vaccination. [3] Overstimulation, however, triggers the cellular antiviral defense mechanism via type I interferon (IFN) and blocks translation of the mRNA vaccine. [4]
Consequently, multiple approaches to engineer mRNAs have been taken, including optimization of the codon usage and the sequence of the untranslated regions (UTRs), introduction of naturally modified nucleosides and advances in purification and delivery strategies.[ 4 , 5 ] Above all, the development of enzymatically synthesized long mRNAs with a 5′ cap is the prerequisite for mRNA‐based therapies. These mRNAs are made by IVT using the anti‐reverse‐cap‐analog (ARCA) (5′‐AC, Scheme 1) representing a cap0 structure or the Clean Cap (5′‐CC, Scheme 1), representing a cap1 structure. The cap0 structure protects mRNA from degradation, is required for eukaryotic translation [6] and reduces the innate immune response elicited by receptors recognizing the 5′ triphosphate. [7] The additional 2′‐O‐methylation at the transcription start nucleotide (TSN) in the cap1 structure increases translation and reduces the innate immune response, as shown by preventing recognition by RIG‐I (cytosolic retinoic‐acid inducible gene I) and IFIT1 (Interferon‐induced protein with tetratricopeptide repeats 1). [8]
Scheme 1.
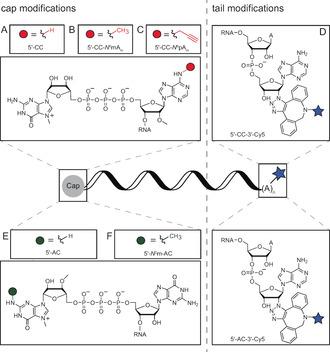
mRNAs and chemo‐enzymatic modifications used in this study. A) 5′‐Clean Cap (5′‐CC) mRNA is produced via IVT. B,C) N 6‐Modification of adenosine at the transcription start nucleotide using CAPAM and AdoMet (S‐adenosyl‐l‐methionine) or AdoMet analogs yields methylated Clean Cap (5′‐CC‐N 6mAm) or propargylated Clean Cap (5′‐CC‐N 6pAm), respectively. D) Modification of the poly(A) tail of 5′‐CC‐mRNA using poly(A) polymerase and 2′‐azido ATP followed by labeling with Cy5 yields 5′‐CC‐3′‐Cy5‐mRNA. E) 5′‐ARCA (5′‐AC) mRNA is produced via IVT. F) N 2‐Modification of m7G using GlaTgs and AdoMet results in N 2 methylated ARCA (5′‐N 2m‐AC)‐mRNA. G) Modification of the poly(A) tail of 5′‐AC‐mRNA using poly(A) polymerase and 2′‐azido ATP followed by labeling with Cy5 yields 5′‐AC‐3′‐Cy5‐mRNA.
However, recent studies revealed many more naturally occurring and potentially dynamic cap modifications, in particular at the TSN. [9] CAPAM was recently discovered as the enzyme responsible for N 6‐methylation of Am in cap1 to m6Am. [10] First studies indicate that m6Am could stabilize mRNA,[ 10a , 10c , 10d , 11 ] however the precise function of this methylation and its impact on translation are not fully understood.
Given the importance of cap modifications in distinguishing self from non‐self mRNA and the combinatorial possibilities in cap composition regarding nucleosides and methylation patterns, investigation of their respective translational and immunogenic properties is intriguing. [12] Moreover, the variation of the widely occurring methyl group by non‐natural alkyl or functional groups may provide new options to engineer the properties of 5′ caps for mRNA‐based therapies.
Several strategies for the modification of the mRNA cap with the aim to influence and tune its properties in cells have already been reported. Non‐natural cap‐analogs, containing for example, phosphorothioate, phosphorothiolate and/or methylenebisphosphonate moieties in the triphosphate have shown to improve RNA stability and translation. [13] Methyltransferase (MTase)‐based modification of a post‐transcriptionally added 5′ cap guanosine using AdoMet analogs decreased the translation efficiency of reporter mRNAs. [14] Also non‐natural modifications in the mRNA body and poly(A) tail have been investigated. Incorporation of phosphorothioate nucleosides (NTPαS) into the mRNA body improved translation, [15] while incorporation of ATPαS into the poly(A)‐tail stabilized the mRNA without impacting translation. [16] Click‐labeling at the poly(A) tail with fluorescent dyes increased translation. [17] However, while it is now clear that translation can be tailored by non‐natural enzymatic modifications of mRNA, their effect on immunogenicity is unknown. In this work, we explore the cosubstrate scope of the MTase CAPAM that gives access to enzymatic modification of adenosine as TSN in capped mRNA. We diversify the set of available cap modifications and investigate their effect on RNA translation and immunogenicity.
Results and Discussion
CAPAM is Active on AdoMet Analogs
First, we validated that recombinant CAPAM is active in vitro using the natural cosubstrate S‐adenosyl‐l‐methionine (AdoMet). To this end, a short model RNA (RNA 1, 26 nt long) was made by IVT with the Clean Cap (5′‐CC) and converted with AdoMet (Figure 1 A). For HPLC or LC‐MS analysis, the mRNA was digested to nucleosides using a combination of nuclease P1, snake venom phosphodiesterase and alkaline phosphatase (Figure 1 B). [18] RP‐HPLC analysis showed five peaks that could be assigned to the four canonical nucleosides and Am (Figure S8). In the presence of CAPAM, we observed conversion of Am and formation of a new peak at 4.5 min, which was absent in a negative control without enzyme (Figure 1 C). As expected, the new peak corresponded to m6Am according to LC‐MS analysis (Figure 1 D).
Figure 1.
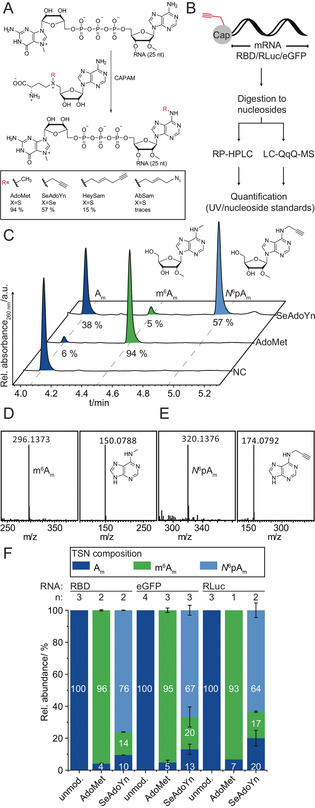
Chemo‐enzymatic preparation and analysis of mRNAs with modified 5′ caps. (A) CAPAM‐catalyzed reaction for site‐specific modification of capped RNAs using AdoMet or indicated analogs. Yields for biotransformation of short RNA are indicated. (B) Flow Scheme for analysis of cap modifications in mRNAs by RP‐HPLC or LC‐QqQ‐MS. The mRNAs (including their 5′ caps) are digested to nucleosides. For quantification by MS, N 6pAm, Am and m6Am were used as standards. (C) Analysis of CAPAM reactions using a short 5′‐CC‐RNA (26 nt) and AdoMet or SeAdoYn. RP‐HPLC after digestion is shown. Negative control (NC) was performed with AdoMet and w/o enzyme. (D,E) LC‐qTOF analysis of the methylation (D) and propargylation (E) reactions. m6Am: expected mass for [M+H]+ C12H18N5O4 +=296.1353. Analysis of N 6pAm (expected mass for [M+H]+ C14H18N5O4 +=320.1353). The right boxes in D/E show MS2 fragmentation of the modified nucleosides to the respective nucleobases N 6‐methyl‐adenine (expected mass for [M+H]+ C6H8N5 +=150.0774) or N 6‐propargyl‐adenine (expected mass for [M+H]+ C8H8N5 +=174.0774). (F) Analysis of long mRNAs (RBD, eGFP and RLuc) after modification. LC‐QqQ‐MS quantification of CAPAM reactions using AdoMet, SeAdoYn, or unmodified mRNA as control (for calibration see Figure S7). Data and error bars show average and standard deviation of n independent experiments.
MS2 fragmentation showed the mass of N 6‐methyl‐adenine, confirming that the modification is indeed installed at the nucleobase (Figure 1 D). The enzymatic conversion of RNA 1 with AdoMet using CAPAM (20 mol %) yielded 94 % methylation at the N 6‐position of the first transcribed adenosine, according to RP‐HPLC (Figure 1 C).
To assess the cosubstrate scope of CAPAM, we synthesized a set of AdoMet analogs with extended side chains, that is, propargyl (SeAdoYn), hexenynyl (HeySAM), azidobutenyl (AbSAM) and benzylic (AdoONB and AdoNP) residues instead of the methyl group (Figure 1 A, Figure S9–10). [19] Enzymatic conversion of RNA 1 with CAPAM and SeAdoYn led to efficient formation of N 6‐propargylated Am (N 6pAm), as confirmed by RP‐HPLC (Figure 1 C), LC‐MS and LC‐MS2 analysis (Figure 1 E). The conversion reached 57 %, which corresponds to 61 % relative to CAPAM methylation under otherwise identical conditions. For AdoMet analogs with longer side chains, we observed lower conversions, that is, 15 % for the hexenynyl group (from HeySAM) and only traces for the azidobutenyl group (from AbSAM), the ortho‐nitrobenzyl group (from AdoONB) or the 6‐nitropiperonyl group (from AdoNP). In all cases, minor amounts of m6Am were also formed, originating from copurified AdoMet bound to CAPAM. This background methylation was reduced by a LiCl washing step during CAPAM purification. These data indicate that CAPAM can accommodate AdoMet analogs with extended side chains, but that large groups are not as well accommodated, reflecting constraints in the active site, in line with the crystal structure (pdb 6IRW, Figure S1).
Cap Modification of mRNAs and LC‐QqQ‐MS Analysis
For subsequent functional studies of CAPAM‐modified mRNAs, we focused on propargylation. To investigate the effect of different cap modifications on translation and immunogenic properties, we turned our attention to representative long mRNAs. Specifically, we used RLuc and eGFP reporter‐mRNAs that result in an easily detectable protein output, as well as the RBD‐mRNA coding for the receptor binding domain of SARS‐CoV‐2 as a biologically relevant mRNA. This mRNA is currently investigated in clinical trials as a vaccination agent against Covid‐19. [20] These three mRNAs were made by IVT using the trinucleotide cap analog 5′‐CC and then subjected to CAPAM‐catalyzed methylation or propargylation.
To quantify the cap modification, we developed an LC‐QqQ‐MS based assay—as the RP‐HPLC analysis is not suitable for long RNAs—and prepared the required nucleoside standards. Here, quantification is based on detection using the dynamic multiple‐reaction monitoring (dMRM) mode, allowing for sensitive detection, similar to previously reported methods for the identification of different caps in the transcriptome.[ 9a , 9b ] While the standards Am and m6Am were obtained commercially, N 6pAm was synthesized in 4 steps from 6‐chloropurine riboside (Scheme S1). After protection of the 3′‐ and 5′‐OH groups using 1,3‐dichloro‐1,1,3,3‐tetraisopropyldisiloxane (TIPSDSiCl2), the 2′‐OH group was methylated using methyl iodide and NaH in DMF under argon. [21] Deprotection with tetra‐n‐butylammonium fluoride (TBAF) released 6‐chloropurine 2′‐O‐methyl riboside, which was treated with propargylamine and CaCO3 in ethanol. [22] The product N 6pAm was purified via preparative RP‐HPLC and analyzed by 2D‐NMR and LC‐MS (Figure S11, Figure S18–22). Using these standards, we could detect Am, m6Am and N 6pAm down to 4 amol via LC‐QqQ‐MS in dMRM mode (Figure 1 B, Figure S7).
With this method at hand, the enzymatic cap modification of long mRNAs using CAPAM was quantified and optimized. Compared to the bioconversions with short RNAs, we reduced the concentration of RNA and increased the mol % of enzyme. In vitro methylation of mRNAs by CAPAM was almost quantitative, yielding 93–96 % N 6‐methylation of 2′‐O‐methyl‐adenosines at the TSN. Importantly, we achieved also very good propargylation of mRNAs by CAPAM, namely 64–76 % conversion, depending on the mRNAs (Figure 1 F). The modified mRNAs were also analyzed via 7.5 % dPAGE to ensure integrity. Clear bands at the expected lengths can be seen for RBD (957 nt), RLuc (1179 nt) (Figure 2 B) and eGFP mRNA (963 nt) (Figure S4), indicating that CAPAM‐dependent propargylation of the cap did not cause degradation.
Figure 2.
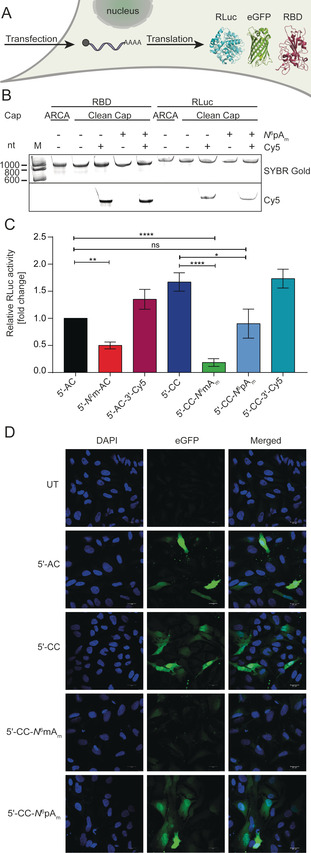
Translation of chemo‐enzymatically modified mRNAs in mammalian cells. (A) Scheme. HEK‐NF‐κB cells are transfected with modified mRNAs and reporter protein is measured. (B) Analysis of differently capped mRNAs used in this study. RBD (957 nt), RLuc (1179 nt). 7.5 % dPAGE, scanned in the SYBR Gold and Cy5 channel. (C) RLuc activity of HEK‐NF‐κB cells transfected with indicated modified RLuc‐mRNAs. The resulting data are normalized to the total cell count and to 5′‐AC‐mRNA. Average of N≥5 independent experiments and standard error of the mean (SEM) is shown. Statistical analysis: unpaired t‐test. p<0.05:*, p<0.01:**, p<0.001:***. (D) Microscopy of HeLa cells transfected with indicated modified eGFP‐mRNAs. Scale bar=20 μm. Abbreviations: compare Scheme 1; UT=untransfected control.
For subsequent studies, we prepared a panel of mRNAs distinguished only by modifications at the 5′ cap and/or the poly(A) tail (Scheme 1). In addition to 5′‐CC‐based and N 6‐modified caps (Scheme 1 A–C), three previously reported cap modifications and a modification at the poly(A) tail in the context of two different caps were tested (Scheme 1 D–G). ARCA (5′‐AC, Scheme 1 E) is widely used and prevents reverse incorporation of the cap, leading to a 2‐fold higher translation compared to the m7GpppG cap analog. [23] The additional N 2‐methylation of m7G (Scheme 1 F) slightly compromises translation, but its function and immunogenicity is unknown.[ 14 , 24 ] Modifications at the poly(A) tail by incorporation of azido‐A and click reaction with DBCO‐Cy5 (Scheme 1 D,G) were recently shown to increase translation, but not tested regarding immunogenicity. [17] The mRNAs with these cap or poly(A) tail modifications were prepared and analyzed on a polyacrylamide gel to confirm integrity and—in the case of Cy5 modifications—labeling (Figure 2 B).
Effect of N 6pAm on Translation in Mammalian Cells
After confirming that the modified mRNAs are intact, human HEK‐NF‐κB were transfected (Figure 2 A). To assess the effect of modifications on translation, we tested RLuc‐mRNAs with different modifications and measured luciferase activity. Readouts were normalized to the total cell count and shown in relation to RLuc activity from 5′‐AC‐mRNA in Figure 2 C.
Testing the effect of previously described mRNA modifications allowed us to benchmark our translation assays. This is important, because translation efficiency can not only depend on the cap modification but also on other factors, like the first transcribed nucleotide, the purity or the translation system or cell line used. [8d] In HEK‐NF‐κB cells, the 5′‐CC‐RLuc‐mRNA showed 1.7‐fold increased luciferase activity, in line with available data for other cell lines.[ 8d , 8e ] The 5′‐N 2m‐AC modified RLuc mRNA resulted in only 50 % luciferase activity, in line with previous reports where translation was assessed in rabbit reticulocyte lysate. [14]
Modifications at the poly(A) tail had only little effect on translation in HEK‐NF‐κB cells (Figure 2 C). Specifically, 5′‐AC‐RLuc‐3′‐Cy5‐mRNA increased the luciferase activity by 1.3‐fold, which is lower than the 3‐fold increase previously observed in HeLa cells. [17a] In summary, the effects of cap and poly(A) tail modifications observed in HEK‐NF‐κB cells are largely in line with previous reports in reticulocyte lysate or other cell lines, although the absolute numbers differ in some cases.
Next, we looked at the effect of modifications at the first transcribed A of 5′‐CC‐mRNA. Interestingly, we observed that 5′‐CC‐N 6mAm—a natural cap that is highly abundant in vertebrates—markedly decreased translation, yielding only 20 % of relative RLuc activity (Figure 2 C). In the literature, different effects on the translation efficiency were reported, depending on the cell line and transcript.[ 8d , 10c ] However, when we placed the bioorthogonal propargyl group instead of the methyl group at this position (5′‐CC‐N 6pAm), we observed luciferase activity similar (90 %) to 5′‐AC‐mRNA‐higher than with the natural methylation. This suggests that mRNA with the 5′‐CC‐N 6pAm cap is very efficiently translated.
To independently validate the effect of this non‐natural cap modification on protein production, we used eGFP‐mRNA, a different cell line and fluorescence microscopy as readout. We transfected HeLa cells with eGFP‐mRNAs distinguished by either a 5′‐CC‐N 6mAm, 5′‐CC‐N 6pAm, 5′‐AC or 5′‐CC cap. Images from confocal laser scanning microscopy showed green fluorescence in all cases, confirming that 5′‐CC‐N 6pAm‐eGFP‐mRNA is efficiently translated (Figure 2 D). Images obtained from cells transfected with 5′‐CC‐N 6mAm‐eGFP‐mRNA exhibited less green fluorescence compared to the 5′‐CC‐N 6pAm‐eGFP‐mRNA. We also performed Western blots from HeLa and HEK293T cells transfected with differently capped eGFP‐mRNAs and observed in all cases that eGFP protein is efficiently produced from 5′‐CC‐N 6pAm‐eGFP‐mRNA (Figure S5). This finding is remarkable, as molecular signatures marking mRNA as non‐self typically abrogate translation as response.[ 4b , 25 ] Our data suggests that non‐natural modifications at the N 6‐position of adenosine as TSN might be a way to exploit non‐natural modifications at the cap without abrogating translation.
Effect of N 6pAm on Immune Response in HEK‐NF‐κB Cells
We were therefore curious and went one step further and evaluated the effect of the non‐natural modification on immune response. To investigate if the cap and poly(A)‐tail modifications influence the activation of pathogen recognition receptors (PRRs), we again used the HEK‐NF‐κB cells. These cells express an NF‐κB driven firefly luciferase and provide a measure for activation of PRRs like MDA5, PKR and RIG‐I (Figure 3 A). Transfection of the mRNA‐constructs (Scheme 1) showed that almost all cap and poly(A) tail modifications caused immunogenicity in the same range as the control (5′‐AC‐RLuc‐mRNA) that was used as reference (Figure 3 B). This includes the fluorescently labeled RLuc‐mRNA‐constructs 5′‐AC‐3′‐Cy5 and 5′‐CC‐3′‐Cy5, suggesting that labeling at the poly(A) tail maintains translation and does not alter immunogenicity. These fluorescently labeled mRNAs may therefore be attractive to track mRNA uptake in cells and in vivo after vaccination, for example, in a lipid nanoparticle formulation. The N 2‐monomethylation of the m7G of 5′‐AC‐mRNA, whose biological function is unclear and which is found in Giardia lamblia, did not alter the immune response either. Untransfected cells served as negative control (≈0.35‐fold change compared to 5′‐AC; data not shown). These data suggest that the PRRs like MDA5, PKR and RIG‐I do not recognize the additional methylation.
Figure 3.
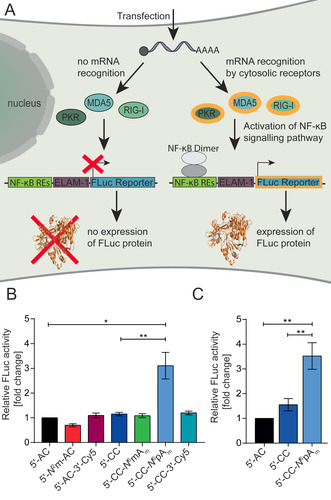
Immunogenicity of mRNAs after chemo‐enzymatic modification at the 5′ cap. (A) Scheme illustrating detection of immunogenicity using HEK‐NF‐κB cells. Recognition of the mRNA by cytosolic receptors like MDA5, PKR or RIG‐1 activates the NF‐κB signaling pathway. NF‐κB dimers bind to the transcription factor binding sites resulting in the expression of FLuc, driven by the ELAM‐1 minimal promoter and five NF‐κB response elements (REs). (B) FLuc activity of HEK‐NF‐κB cells transfected with indicated modified RLuc‐mRNAs. Data are normalized to the total cell count and to 5′‐AC‐mRNA. Average of N≥5 independent experiments and standard error of the mean (SEM) is shown. (C) Same as (B) but for RBD‐mRNA. Statistical analysis: unpaired t‐test. p<0.05:*, p<0.01:**, p<0.001:***.
The only modification that stands out is the N 6‐propargylated adenosine as TSN. The 5′‐CC‐N 6pAm‐RLuc‐mRNA increased the immune response ≥3‐fold (Figure 3 B). In combination with the maintained translational efficiency, this significant but moderate increase in immunogenicity may be an attractive feature for applications as mRNA vaccine, where both innate immune response and adaptive immunity are required.
To find out if this effect would be observed also with an mRNA coding for a biologically relevant protein for vaccination, we tested the RBD‐mRNA. RBD is the receptor binding domain located on the spike protein of the coronavirus. mRNA coding for the RBD was investigated as vaccination agent against SARS‐CoV‐2 and clinical trials confirmed that RBD is a suitable antigen to prime T‐lymphocytes to recognize the coronavirus.[ 20b , 26 ] For vaccination, two mRNA vaccines (Biontech/Pfizer, Moderna), both coding for the full length spike protein have now been authorized for emergency use by FDA. [27] We produced the RBD‐mRNA with different cap analogs, namely 5′‐CC‐N 6pAm and 5′‐AC and 5′‐CC cap as controls. All constructs were translated in HEK‐NF‐κB cells, as confirmed via Western blot (Figure S5) in line with the results we obtained with the luciferase mRNAs as reporters. When we tested the immunogenicity of 5′‐CC‐N 6pAm‐RBD‐mRNA in HEK‐NF‐κB cells, we observed a 3.5‐fold higher immunogenicity for 5′‐CC‐N 6pAm‐RBD‐mRNA compared to the same mRNA with 5′‐AC cap and a 2.3‐fold increase compared to the 5′‐CC cap (Figure 3 C). These data confirm the observations obtained from reporter mRNAs (Figure 3 B) and show that the N 6‐propargylation of adenosine as TSN leads to a ≈3‐fold increase in immunogenicity for different transcripts.
Conclusion
In summary, we characterized the cosubstrate scope of the recently described methyltransferase CAPAM and report an efficient strategy to make long mRNAs carrying a propargyl group at the N 6‐position of adenosine as TSN. We tested the effect of CAPAM‐dependent methylation and propargylation on translation in human cells and found that the methylation drastically reduced translation, whereas propargylation maintained translation. For the first time, we evaluate the immunogenic properties for a number of natural and non‐natural modifications at the 5′ cap and/or poly(A) tail. While most modifications did not alter the immune response in our NF‐κB‐responsive assay, the N 6‐propargylation of Am as TSN led to a significant ≈3‐fold increase, both for reporter mRNAs and for an mRNA coding for an epitope considered for Covid‐19 vaccination. In combination with the maintained translational efficiency, this moderate increase in immune response (higher compared to the ARCA cap but lower compared to the 5′ triphosphate)[ 8d , 28 ] might be an attractive approach to balance the immunogenic properties of mRNA by engineering the molecule itself and without relying on adjuvants. Our study also provides a proof of concept that exploring the chemical space of non‐natural higher cap modifications can be a promising strategy for engineering mRNA therapeutics.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
At the University of Münster, the authors thank S. Hüwel for assistance with microscopy, A.‐M. Lawrence‐Dörner for assistance with protein purification, A. M. Böttick for cloning, N. Klöcker and F. Weissenböck for chemical synthesis and enzymatic cap modifications and Dr. P. Špaček for assistance with LC‐QqQ‐MS. We thank Dr. W. Dörner and S. Wulff for assistance with mass spectrometry. The mass spectrometry and NMR facilities of the organic chemistry department are gratefully acknowledged for analytical services. We thank Dr. A. Oeckinghaus for advice on Western blotting. We thank Dr. Chengqi Yi (Peking University) for providing the plasmid pET28a‐PCIF1. Furthermore, we appreciate the fruitful discussions with Dr. Katalin Karikó and we thank TRON (Translational Oncology at the University Medical Center of the Johannes Gutenberg University Mainz) for providing us with the HEK‐NF‐κB cell line. This project has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (grant agreement No 772280) and the DFG (RE 2796/6‐1). Open access funding enabled and organized by Projekt DEAL.
M. van Dülmen, N. Muthmann, A. Rentmeister, Angew. Chem. Int. Ed. 2021, 60, 13280.
References
- 1.
- 1a. Walsh E. E., Frenck R. W., Falsey A. R., Kitchin N., Absalon J., Gurtman A., Lockhart S., Neuzil K., Mulligan M. J., Bailey R., Swanson K. A., Li P., Koury K., Kalina W., Cooper D., Fontes-Garfias C., Shi P.-Y., Türeci Ö., Tompkins K. R., Lyke K. E., Raabe V., Dormitzer P. R., Jansen K. U., Şahin U., Gruber W. C., N. Engl. J. Med. 2020, 383, 2439–2450; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1b. Jackson L. A., Anderson E. J., Rouphael N. G., Roberts P. C., Makhene M., Coler R. N., McCullough M. P., Chappell J. D., Denison M. R., Stevens L. J., Pruijssers A. J., McDermott A., Flach B., Doria-Rose N. A., Corbett K. S., Morabito K. M., O'Dell S., Schmidt S. D., Swanson P. A., Padilla M., Mascola J. R., Neuzil K. M., Bennett H., Sun W., Peters E., Makowski M., Albert J., Cross K., Buchanan W., Pikaart-Tautges R., Ledgerwood J. E., Graham B. S., Beigel J. H., N. Engl. J. Med. 2020, 383, 1920–1931; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1c. Krammer F., Nature 2020, 586, 516–527; [DOI] [PubMed] [Google Scholar]
- 1d. van Dülmen M., Rentmeister A., Biochemistry 2020, 59, 1650–1655. [DOI] [PubMed] [Google Scholar]
- 2.
- 2a. Weissman D., Ni H., Scales D., Dude A., Capodici J., McGibney K., Abdool A., Isaacs S. N., Cannon G., Karikó K., J. Immunol. 2000, 165, 4710–4717; [DOI] [PubMed] [Google Scholar]
- 2b. Hoerr I., Obst R., Rammensee H. G., Jung G., Eur. J. Immunol. 2000, 30, 1–7. [DOI] [PubMed] [Google Scholar]
- 3.
- 3a. Bourquin C., Schmidt L., Hornung V., Wurzenberger C., Anz D., Sandholzer N., Schreiber S., Voelkl A., Hartmann G., Endres S., Blood 2007, 109, 2953–2960; [DOI] [PubMed] [Google Scholar]
- 3b. Sander L. E., Davis M. J., Boekschoten M. V., Amsen D., Dascher C. C., Ryffel B., Swanson J. A., Müller M., Blander J. M., Nature 2011, 474, 385–389; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3c. Pardi N., Hogan M. J., Porter F. W., Weissman D., Nat. Rev. Drug Discovery 2018, 17, 261–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.
- 4a. Sahin U., Karikó K., Türeci Ö., Nat. Rev. Drug Discovery 2014, 13, 759–780; [DOI] [PubMed] [Google Scholar]
- 4b. Linares-Fernández S., Lacroix C., Exposito J.-Y., Verrier B., Trends Mol. Med. 2020, 26, 311–323. [DOI] [PubMed] [Google Scholar]
- 5. Karikó K., Buckstein M., Ni H., Weissman D., Immunity 2005, 23, 165–175. [DOI] [PubMed] [Google Scholar]
- 6.
- 6a. Müller-McNicoll M., Neugebauer K. M., Nat. Rev. Genet. 2013, 14, 275–287; [DOI] [PubMed] [Google Scholar]
- 6b. Sonenberg N., Hinnebusch A. G., Cell 2009, 136, 731–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hornung V., Ellegast J., Kim S., Brzozka K., Jung A., Kato H., Poeck H., Akira S., Conzelmann K.-K., Schlee M., Endres S., Hartmann G., Science 2006, 314, 994–997. [DOI] [PubMed] [Google Scholar]
- 8.
- 8a. Abbas Y. M., Laudenbach B. T., Martínez-Montero S., Cencic R., Habjan M., Pichlmair A., Damha M. J., Pelletier J., Nagar B., Proc. Natl. Acad. Sci. USA 2017, 114, E2106–E2115; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8b. Devarkar S. C., Wang C., Miller M. T., Ramanathan A., Jiang F., Khan A. G., Patel S. S., Marcotrigiano J., Proc. Natl. Acad. Sci. USA 2016, 113, 596–601; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8c. Furuichi Y., Shatkin A. J., Adv. Virus Res. 2000, 55, 135–184; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8d. Sikorski P. J., Warminski M., Kubacka D., Ratajczak T., Nowis D., Kowalska J., Jemielity J., Nucleic Acids Res. 2020, 48, 1607–1626; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8e.TrilinkBiotechnologies, “Clean Cap”, can be found under https://www.trilinkbiotech.com/cleancap, 2020;
- 8f. Williams G. D., Gokhale N. S., Snider D. L., Horner S. M., mSphere 2020, 5, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.
- 9a. Galloway A., Atrih A., Grzela R., Darzynkiewicz E., Ferguson M. A. J., Cowling V. H., Open Biol. 2020, 10, 190306; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9b. Wang J., Alvin Chew B. L., Lai Y., Dong H., Xu L., Balamkundu S., Cai W. M., Cui L., Liu C. F., Fu X.-Y., Lin Z., Shi P.-Y., Lu T. K., Luo D., Jaffrey S. R., Dedon P. C., Nucleic Acids Res. 2019, 47, e130; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9c. Mauer J., Sindelar M., Despic V., Guez T., Hawley B. R., Vasseur J.-J., Rentmeister A., Gross S. S., Pellizzoni L., Debart F., Goodarzi H., Jaffrey S. R., Nat. Chem. Biol. 2019, 15, 340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.
- 10a. Akichika S., Hirano S., Shichino Y., Suzuki T., Nishimasu H., Ishitani R., Sugita A., Hirose Y., Iwasaki S., Nureki O., Suzuki T., Science 2019, 363, eaav0080; [DOI] [PubMed] [Google Scholar]
- 10b. Sun H., Zhang M., Li K., Bai D., Yi C., Cell Res. 2019, 29, 80–82; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10c. Boulias K., Toczydłowska-Socha D., Hawley B. R., Liberman N., Takashima K., Zaccara S., Guez T., Vasseur J.-J., Debart F., Aravind L., Jaffrey S. R., Greer E. L., Mol. Cell 2019, 75, 631–643.e8; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10d. Sendinc E., Valle-Garcia D., Dhall A., Chen H., Henriques T., Navarrete-Perea J., Sheng W., Gygi S. P., Adelman K., Shi Y., Mol. Cell 2019, 75, 620–630.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pandey R. R., Delfino E., Homolka D., Roithova A., Chen K.-M., Li L., Franco G., Vågbø C. B., Taillebourg E., Fauvarque M.-O., Pillai R. S., Cell Rep. 2020, 32, 108038. [DOI] [PubMed] [Google Scholar]
- 12. Leung D. W., Amarasinghe G. K., Curr. Opin. Struct. Biol. 2016, 36, 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wojtczak B. A., Sikorski P. J., Fac-Dabrowska K., Nowicka A., Warminski M., Kubacka D., Nowak E., Nowotny M., Kowalska J., Jemielity J., J. Am. Chem. Soc. 2018, 140, 5987–5999. [DOI] [PubMed] [Google Scholar]
- 14. Holstein J. M., Anhäuser L., Rentmeister A., Angew. Chem. Int. Ed. 2016, 55, 10899–10903; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2016, 128, 11059–11063. [Google Scholar]
- 15. Kawaguchi D., Kodama A., Abe N., Takebuchi K., Hashiya F., Tomoike F., Nakamoto K., Kimura Y., Shimizu Y., Abe H., Angew. Chem. Int. Ed. 2020, 59, 17403–17407; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 17556–17560. [Google Scholar]
- 16. Strzelecka D., Smietanski M., Sikorski P. J., Warminski M., Kowalska J., Jemielity J., RNA 2020, 26, 1815–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.
- 17a. Anhäuser L., Hüwel S., Zobel T., Rentmeister A., Nucleic Acids Res. 2019, 47, e42; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17b. Westerich K. J., Chandrasekaran K. S., Gross-Thebing T., Kueck N., Raz E., Rentmeister A., Chem. Sci. 2020, 11, 3089–3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thüring K., Schmid K., Keller P., Helm M., Methods 2016, 107, 48–56. [DOI] [PubMed] [Google Scholar]
- 19.
- 19a. Willnow S., Martin M., Lüscher B., Weinhold E., ChemBioChem 2012, 13, 1167–1173; [DOI] [PubMed] [Google Scholar]
- 19b. Tomkuvienė M., Clouet-d'Orval B., Černiauskas I., Weinhold E., Klimašauskas S., Nucleic Acids Res. 2012, 40, 6765–6773; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19c. Bothwell I. R., Luo M., Org. Lett. 2014, 16, 3056–3059; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19d. Anhäuser L., Muttach F., Rentmeister A., Chem. Commun. 2018, 54, 449–451; [DOI] [PubMed] [Google Scholar]
- 19e. Ovcharenko A., Weissenboeck F. P., Rentmeister A., Angew. Chem. Int. Ed. 2021, 60, 4098–4103; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2021, 133, 4144–4149; [Google Scholar]
- 19f. Islam K., Bothwell I., Chen Y., Sengelaub C., Wang R., Deng H., Luo M., J. Am. Chem. Soc. 2012, 134, 5909–5915; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19g. Islam K., Zheng W., Yu H., Deng H., Luo M., ACS Chem. Biol. 2011, 6, 679–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.
- 20a.CureVacAG, “A Study to Evaluate the Safety and Immunogenicity of Vaccine CVnCoV in Healthy Adults in Germany”, can be found under https://clinicaltrials.gov/ct2/show/study/NCT04674189, 2021;
- 20b. Mulligan M. J., Lyke K. E., Kitchin N., Absalon J., Gurtman A., Lockhart S., Neuzil K., Raabe V., Bailey R., Swanson K. A., Li P., Koury K., Kalina W., Cooper D., Fontes-Garfias C., Shi P.-Y., Türeci Ö., Tompkins K. R., Walsh E. E., Frenck R., Falsey A. R., Dormitzer P. R., Gruber W. C., Şahin U., Jansen K. U., Nature 2020, 586, 589–593; [DOI] [PubMed] [Google Scholar]
- 20c. Zang J., Gu C., Zhou B., Zhang C., Yang Y., Xu S., Bai L., Zhang R., Deng Q., Yuan Z., Tang H., Qu D., Lavillette D., Xie Y., Huang Z., Cell Discovery 2020, 6, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Beigelman L., Haeberli P., Sweedler D., Karpeisky A., Tetrahedron 2000, 56, 1047–1056. [Google Scholar]
- 22. Jiang H., Congleton J., Liu Q., Merchant P., Malavasi F., Lee H. C., Hao Q., Yen A., Lin H., J. Am. Chem. Soc. 2009, 131, 1658–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.
- 23a. Stepinski J., Waddell C., Stolarski R., Darzynkiewicz E., Rhoads R. E., RNA 2001, 7, 1486–1495; [PMC free article] [PubMed] [Google Scholar]
- 23b. Peng Z.-H., Sharma V., Singleton S. F., Gershon P. D., Org. Lett. 2002, 4, 161–164; [DOI] [PubMed] [Google Scholar]
- 23c. Jemielity J., Fowler T., Zuberek J., Stepinski J., Lewdorowicz M., Niedzwiecka A., Stolarski R., Darzynkiewicz E., Rhoads R. E., RNA 2003, 9, 1108–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hausmann S., Shuman S., J. Biol. Chem. 2005, 280, 32101–32106. [DOI] [PubMed] [Google Scholar]
- 25.
- 25a. Bartok E., Hartmann G., Immunity 2020, 53, 54–77; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25b. Galloway A., Cowling V. H., Biochim. Biophys. Acta Gene Regul. Mech. 2019, 1862, 270–279; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25c. Nelson J., Sorensen E. W., Mintri S., Rabideau A. E., Zheng W., Besin G., Khatwani N., Su S. V., Miracco E. J., Issa W. J., Hoge S., Stanton M. G., Joyal J. L., Sci. Adv. 2020, 6, eaaz6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sahin U., Muik A., Derhovanessian E., Vogler I., Kranz L. M., Vormehr M., Baum A., Pascal K., Quandt J., Maurus D., Brachtendorf S., Lörks V., Sikorski J., Hilker R., Becker D., Eller A.-K., Grützner J., Boesler C., Rosenbaum C., Kühnle M.-C., Luxemburger U., Kemmer-Brück A., Langer D., Bexon M., Bolte S., Karikó K., Palanche T., Fischer B., Schultz A., Shi P.-Y., Fontes-Garfias C., Perez J. L., Swanson K. A., Loschko J., Scully I. L., Cutler M., Kalina W., Kyratsous C. A., Cooper D., Dormitzer P. R., Jansen K. U., Türeci Ö., Nature 2020, 586, 594–599. [DOI] [PubMed] [Google Scholar]
- 27.
- 27a. Baden L. R., El Sahly H. M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S. A., Rouphael N., Creech C. B., McGettigan J., Khetan S., Segall N., Solis J., Brosz A., Fierro C., Schwartz H., Neuzil K., Corey L., Gilbert P., Janes H., Follmann D., Marovich M., Mascola J., Polakowski L., Ledgerwood J., Graham B. S., Bennett H., Pajon R., Knightly C., Leav B., Deng W., Zhou H., Han S., Ivarsson M., Miller J., Zaks T., N. Engl. J. Med. 2021, 384, 403–416; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27b. Polack F. P., Thomas S. J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J. L., Pérez Marc G., Moreira E. D., Zerbini C., Bailey R., Swanson K. A., Roychoudhury S., Koury K., Li P., Kalina W. V., Cooper D., R. W. Frenck, Jr. , Hammitt L. L., Türeci Ö., Nell H., Schaefer A., Ünal S., Tresnan D. B., Mather S., Dormitzer P. R., Şahin U., Jansen K. U., Gruber W. C., N. Engl. J. Med. 2020, 383, 2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Durbin A. F., Wang C., Marcotrigiano J., Gehrke L., mBio 2016, 7, e00833-00816. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


