Abstract
COVID‐19‐related mortality in high‐risk individuals is substantial and current treatment options are limited. There is convincing evidence that the COVID‐19 vaccines reduce the severity of infection and prevent deaths. Three COVID‐19 vaccines are approved in the United Kingdom with many more in development. There are limited data on the triggers and mechanisms of anaphylaxis to these vaccines. We review the potential allergenic compounds in the COVID‐19 vaccines and describe an innovative allergy support model for the vaccination hubs that allows most patients with severe allergy be immunized. Finally, we propose a practical algorithm for the investigations of anaphylaxis to these vaccines.
Key messages.
COVID‐19 vaccines can reduce the severity of infection and prevent deaths.
Three COVID‐19 vaccines are approved in the United Kingdom but there is limited data on the mechanisms of anaphylaxis to these vaccines.
Potential allergenic compounds include polyethylene glycol, polysorbate 80 and tromethamine but a role has only been proven for polyethylene glycol.
Both IgE and non IgE mechanism(s) may be responsible for hypersensitivity to these vaccines.
1. BACKGROUND
According to the World Health Organization by the 27 March 2021, there have been 125 781 957 confirmed cases of COVID‐19 (SARS‐CoV‐2), including 2,759,432 deaths. 1 COVID‐19‐related mortality is substantial (2% overall) and higher in high‐risk individuals (>10%). Current treatment options are limited. Trials and real‐life experience confirm that COVID‐19 vaccines drastically reduce the severity of COVID infections, prevent deaths and curb the spread of the pandemic. 2 As of 27 March 2021, there are 3 vaccines approved in the United Kingdom (UK) (Table 1): Pfizer‐BioNTech BNT162B2, Moderna mRNA‐1237 and Oxford/Astra Zeneca (ChAdOx1/AD1222) with many more in development. The UK Medicines and Healthcare Products Regulatory Agency (MHRA) was the first to approve the Pfizer vaccine on 02/12/2020, and the first dose was administered on 08/12/2020. 3 The public health imperative is to vaccinate as many people as possible.
TABLE 1.
UK MHRA approved COVID 19 vaccines as of 27/03/2021 a
| Pfizer |
Astra Zeneca ChAdOx1 |
Moderna | |
|---|---|---|---|
| Active ingredient | Nucleoside‐modified mRNA for SARS‐CoV‐2 viral spike (S) glycoprotein | Recombinant, replication‐deficient chimpanzee adenovirus vector encoding the SARS‐CoV‐2 spike (S) glycoprotein produced in genetically modified human embryonic kidney (HEK) 293 cells. |
Nucleoside‐modified mRNA for SARS‐CoV‐2 viral spike (S) glycoprotein |
| Potential allergen | 2‐[(polyethylene glycol [PEG])‐2000]‐N,N‐ditetradecylacetamide | polysorbate 80 (Tween 80) |
Polyethylene glycol (PEG) 2000 dimyristoyl glycerol Tromethamine, Tromethamine hydrochloride SM‐102 (proprietary to Moderna) |
| Other substances |
1,2‐distearoyl‐sn‐glycero‐3‐phosphocholine (4‐hydroxybutyl)azanediyl) bis(hexane‐6,1‐diyl)bis(2‐hexyl decanoate) cholesterol, potassium chloride, monobasic potassium phosphate, sodium chloride, dibasic sodium phosphate dehydrate, sucrose; NaCl (diluent) |
L‐histidine, L‐histidine hydrochloride monohydrate, magnesium chloride hexahydrate, ethanol, sucrose, sodium chloride, disodium edetate dehydrate, water for injections |
1,2‐distearoyl‐sn‐glycero‐3‐phosphocholine cholesterol, acetid acid, sodium acetate, sucrose |
| Recommended dosing protocol |
Two 0.3 ml doses IM 21 days apart |
Two 0.5 ml doses IM 4‐12 weeks apart |
Two 0.5 ml doses IM 28 days apart |
Janssen's single‐dose COVID‐19 vaccine, Ad26.COV2.S was approved in the United States on 28/2/2021 (excipient: 0.16 mg polysorbate 80); UK approval process due to start soon; Novavax NVX‐CoV2373 (adjuvant from Quillaja saponaria Molina, polysorbate 80, no other excipients mentioned) likely to be approved in the UK soon.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Not unexpectedly, adverse reactions, including anaphylaxis have been reported within 24 hours of the start of the rollout of vaccination, causing major concerns.
We describe an innovative allergy support model for the COVID vaccination hubs (Figure 1). Immediate remote access to an allergist helps non‐allergists running the hubs manage (vaccine) allergy concerns, minimizes the risk of patients unnecessarily being denied vaccination and protects those patients at high risk of an allergic reaction, particularly to the excipients in the vaccines, from inadvertent exposure. We propose a practical algorithm for the investigations of patients with a history of allergy to an excipient in the currently approved COVID vaccines as well patients who experienced hypersensitivity reaction to the first dose of the vaccine (Figure 2). As detailed allergy workup data emerges, these algorithms are likely to be simplified. This will aid the medical community reach the ultimate goal of vaccinating the entire population in a safe and time and cost‐efficient manner.
FIGURE 1.
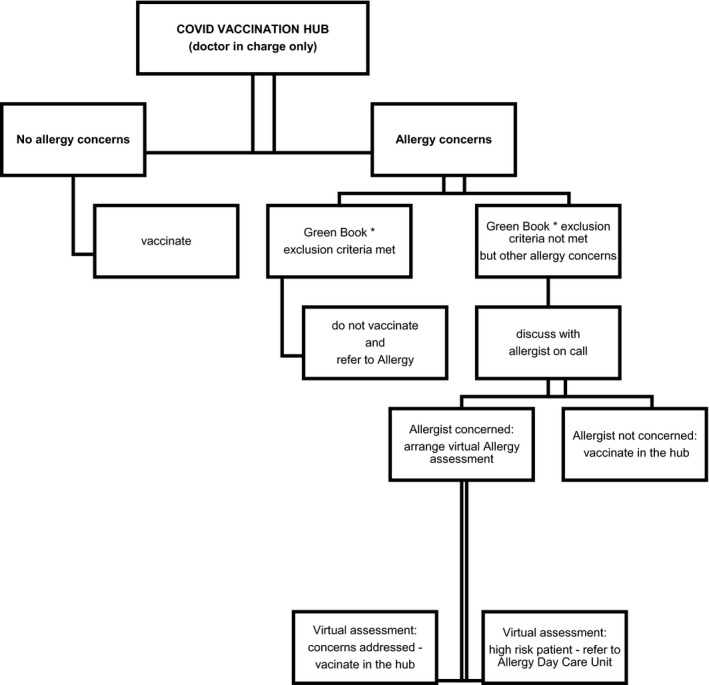
Allergy support model for COVID vaccination hubs
FIGURE 2.
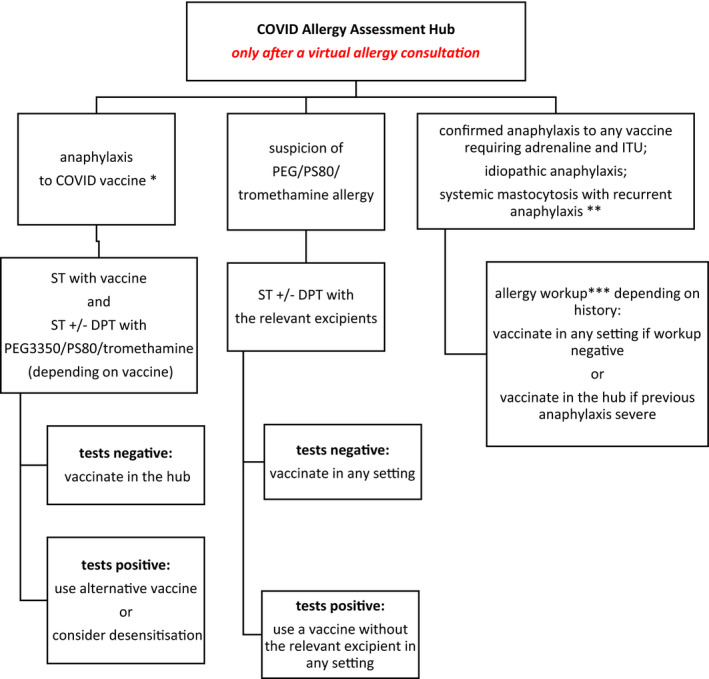
COVID Allergy Assessment Hub. *Patient seen around the time of the 2nd dose of the relevant COVID vaccine; **This condition is not a contraindication sensu stricto but patients’ and health care professionals’ fear of anaphylaxis often leads to refusal to vaccinate in the community; stable mastocytosis does not warrant any special precautions. ***Allergy workup can include ST, DPT, relevant blood tests and bone marrow tests. ST: skin tests (skin prick tests and intradermal tests); DPT: drug provocation test (if skin tests negative)
2. VACCINE COMPOSITION
Two of the approved vaccines (Pfizer and Moderna) use a novel technology based on viral spike protein mRNA delivered in lipid nanoparticles. 4 , 5 The clinical trials with both vaccines have shown their safety and efficacy with a low incidence of adverse events. It is important to note that Pfizer and Moderna excluded individuals with a history of allergic reaction to vaccines or components of their vaccines from the phase 3 trials. Individuals with previous allergic reactions to food or medications were not excluded. 4 , 5 The ChAdOx1 vaccine uses a more conventional, chimpanzee modified Adenovirus vector which also contains the genetic sequence of the surface spike protein. The trial exclusion criteria included history of allergic disease or reactions likely to be exacerbated by any component of ChAdOx1 or MenACWY (Meningococcal group A, C, W‐135 and Y conjugate vaccine) and any history of angioedema and/or of anaphylaxis. 6
3. POTENTIAL ALLERGENS IN COVID‐19 VACCINES
Table 1 lists the ingredients of the currently approved COVID vaccines and potential allergens. The Pfizer‐BioNTech vaccine contains 4 lipids including ALC‐0159 which is a PEG/lipid conjugate (PEGylated lipid), specifically, the N,N‐dimyristylamide of 2‐hydroxyacetic acid, O‐pegylated to a PEG chain mass of about 2 kilodaltons (corresponding to about 45–46 ethylene oxide units per molecule of N,N‐dimyristyl hydroxyacetamide). 4 The Moderna vaccine also contains 4 lipids including PEG2000 dimyristoyl glycerol. 5 However, it is important to emphasize that there is as yet only limited evidence about which component was responsible for any of the reported reactions to the COVID‐19 vaccines. So far only a single case of confirmed IgE mediated allergy to PEG in the Pfizer vaccine has been reported. 7 As we gain more insight into potential mechanisms of hypersensitivity reactions to these vaccines, non‐IgE mechanisms might become more relevant.
4. POLYETHYLENE GLYCOL: BACKGROUND
Documented hypersensitivity to polyethylene glycol (PEG) is listed as a contraindication to COVID‐19 the Pfizer and Moderna vaccines. PEG‐2000 has not been previously used in a vaccine but there are lessons to be learnt from our current understanding of PEG allergy. PEGs are a family of hydrophilic polymers of ethylene oxide. Case reports of PEG anaphylaxis first appeared in the early 1990s. Only small case series of PEG allergic patents have been reported despite the widespread presence of these compounds in common toiletries, household chemical agents and drugs. 8 , 9 , 10 The seminal paper by Wenande reported 37 patients with PEG hypersensitivity and brought this rare allergy to the attention of a wider audience. 8 No large epidemiological studies have been carried out to ascertain the precise incidence of PEG allergy in the general population. In the context of mass vaccination, the evidence that PEG molecules and their covalently bound (PEGylated) products/drugs (In the US, PEG 3350 is contained in 1155 FDA approved medications) can cause IgE mediated hypersensitivity, including anaphylaxis has been a cause for concern. 11
5. PEG ALLERGY
5.1. Allergenicity of PEG: importance of molecular weight
The molecular weight of PEGs varies from a few hundreds to more than 30 000 g/mol and beyond. Allergenicity correlates with an increase in MW. PEGs of low molecular weight (LMW PEG) below 1000 g/mol are liquid/viscous and mainly used in household chemicals and toiletries; the high molecular weight PEGs (HWM PEG) exist mainly in powder form. The LMW PEG can cause delayed hypersensitivity reactions: contact dermatitis/rash on repeated exposure. It is not clear whether repeated cutaneous/mucosal exposure contributes to IgE sensitization and predisposes to severe allergic reactions in response to HMW PEGs. 8 , 9 , 10 Gastrointestinal absorption of PEG above MW 1000 g/mol is poor and a large amount (grams) must be ingested (eg macrogol laxative [PEG3350]) to trigger a reaction. A much smaller amount (micrograms) suffices if a PEG‐containing drug is administered parenterally. 9 , 10 However, if the gut mucosal barrier is compromised, such as in inflammatory bowel disease, a small amount of an orally ingested PEG‐containing drug (especially with a HMW PEG) can trigger a systemic reaction. 12 , 13 The individual thresholds of reactivity to PEGs of different molecular weight and at different concentrations in vivo and even during diagnostic skin testing varies. Patients sensitized to a LMW PEG can react to a PEG of higher molecular weight. 14 The reverse possibility, that is that patients sensitized to HMW PEGs react to a small amount of lower MWPEG such as PEG2000 in the Pfizer and Moderna vaccines, requires further study. 15
5.2. Cross‐reactivity between PEG and polysorbates
Polysorbate 80 (PS80; Tween 80) is derived from polyethoxylated sorbitan and oleic acid. PS80 is used as a common emulsifier and stabilizer in the food industry and excipient in medications and vaccines such as influenza, hepatitis B and human papillomavirus (HPV) vaccines. In the United States, PS80 is an excipient in 6821 FDA approved medications. 11 It is one of the excipients in the Astra Zeneca vaccine. The hydrophilic groups in PS80 are polyethers also known as polyoxyethylene groups, which are polymers of ethylene oxide. PEG and PS80 share an allergenic epitope, that is the repeating polyether domain. Immediate hypersensitivity to PEG 3350 with skin test cross‐reactivity to PS80 has been reported but its wider clinical relevance is unclear. 11
5.3. Epidemiology of anaphylaxis to COVID‐19 vaccines
Within 24 hours of the administration of the first dose of the Pfizer vaccine (authorized in the UK on 02/12/2020), 3 cases of suspected anaphylaxis were reported in the UK. Two occurred in healthcare workers with a history of anaphylaxis and were treated with adrenaline. Formal allergy workup has not been published for these cases yet and it is unknown at present how many further cases of anaphylaxis to this vaccine have been reported in the UK, but a single case report has confirmed that PEG can cause severe anaphylaxis to the Pfizer vaccine. 7 The Pfizer vaccine was given an emergency use authorization by the United States Federal Drug Administration (US FDA) on 11/12/2020. Following the introduction of the Pfizer vaccine in the United States by December 2020 more than 1.8 M first doses were administered and 4393 adverse drug reactions had been submitted to the Vaccine Adverse Event Reporting System. Twenty‐one reactions were classified as anaphylaxis according to Brighton Collaboration criteria for reactions to vaccines. Six were limited to skin only. 16 , 17 This would represent an estimated rate of 11.1 cases per million (0.001%) doses administered. The European Medicines Agency (EMA) approved the Pfizer vaccine on 21/12/2020 but no European Union (EU) cases of anaphylaxis have been officially reported until now.
The FDA issued an emergency use authorization for the Moderna vaccine on the 18/12/2020. Early safety monitoring reported 10 cases of anaphylaxis for 4.04 million (0.00025%) of first doses administered. 18 This vaccine was authorized in the EU on 06/1/2021 and in UK on 08/1/2021 but its rollout has not started yet.
The Oxford vaccine was approved in the UK on 30/12/2020 and the EU on 30/1/2021 but no official cases of anaphylaxis have been as yet reported. It has not been licensed in the United States yet.
Any drug can cause hypersensitivity reactions: immune and nonimmune mediated; acute and delayed. Immune reactions may also occur on first exposure to the drug due to pre‐existing sensitization to the active ingredient or excipient, or alternatively possibly due to non‐adaptive immune cell activation. The prevalence of anaphylaxis to vaccines based on the flu vaccine is reported at a rate of 1.31 (0.90‐1.84) cases per million with very few deaths. 19 , 20 In the UK, the voluntary MHRA Yellow Card scheme collects and monitors information on suspected side effects of vaccination reported by health professionals and the public and is a key data resource (www.yellowcard.mhra.gov.uk).
The active ingredient is seldom responsible for hypersensitivity reaction to vaccines; the seemingly innocuous and inert excipients caused most reactions. Moreover, many reported cases of vaccine anaphylaxis are not subsequently confirmed. Given the very limited available clinical data, the possibility remains that some reactions might have actually represented non‐specific acute urticaria, exacerbation of chronic urticaria or anxiety‐related events. 16 , 17 , 18 After a thorough allergy workup many patients can safely receive the same vaccine again. 21 Three patients with suspected hypersensitivity to the Pfizer‐BioNTech vaccine have been reported to have successfully received the 2nd dose of the same vaccine after negative skin tests with the vaccine. 22
Following the 3 cases of anaphylaxis to the Pfizer vaccine in the UK, on 09/12/2020 the MHRA recommended that ‘Any person with a history of immediate‐onset anaphylaxis to a vaccine, medicine or food should not receive the Pfizer/BioNTech vaccine’. However, as the prevalence of anaphylaxis to any allergen is 3%‐5% in the West and the prevalence of self‐reported food and drug allergy is significantly higher, the initial MHRA recommendations would inevitably have excluded a significant number of low‐risk individuals from the vaccination campaign. 23
The guidance changed on 30/12/2020 to ‘only people with a history of allergic reactions to the ingredients of the vaccine should not receive it’. This was broadly welcomed by the allergy community and supported by what is known about risk factors for vaccine allergy. EMA and FDA also agree that only patients with previously documented hypersensitivity reactions to the COVID vaccine or any of its ingredients/excipients should not be vaccinated.
6. PEG ALLERGY: DIAGNOSTIC TOOLS AVAILABLE
6.1. Clinical history
Particular attention should be paid to patients with a history of anaphylaxis to the most common drug culprits: depo medroxyprogesterone, depo methylprednisolone acetate and macrogol laxatives and severe allergic reactions to multiple (seemingly unrelated) drugs, where routine allergy tests do not confirm the specific drug as the culprit. 9 , 10 , 11 , 14
6.2. Tests
All tests should be performed by an allergist/immunologist experienced in the diagnosis of PEG allergy. Standardized ST protocols and in vitro tests are a work in progress and individual patients may need tailored testing regimes, 9 , 10
Skin testing should be tailored to the suspected mechanism of the reaction, for example SPT and IDT with immediate reading for immediate reactions and IDT with late reading and patch test for non‐immediate reactions.
There are no licensed PEG testing solutions for human use in the UK. Various laboratory‐grade PEGs of different MW have been previously used with some success, but use of such reagents presents regulatory challenges. The injectable steroid preparations containing PEG might not be a suitable diagnostic tool as they contain relatively low amounts of PEG. The steroid content does not interfere with immediate reading of skin tests to PEG. Currently, the only pure clinical grade of PEG routinely available for testing is 10% Macrogol solution (PEG 3350) in the form of commonly used laxatives. 9 , 10 , 12 , 14
Drug provocation test (DPT) with the relevant PEG‐containing drug might be considered in case of a discrepancy between history and skin tests but only if ST are completely negative. Oral DPT can produce false‐negative results due to poor absorption.
New (experimental) in vitro methods include basophil activation test and specific IgE to PEG but there is not enough published data yet to recommend their routine use. 24 , 25
6.3. Differential diagnosis
A detailed clinical description and allergy workup of all cases of COVID vaccine‐related anaphylaxis are crucial to avoid making erroneous assumptions as to aetiology. Differential diagnoses of anaphylaxis must be carefully considered. PEG/PS80 allergy is rare and it would be surprising if all reported cases of COVID vaccine allergy were due to PEG/PS80 allergy. Indeed, one group reported negative PEG 3350 skin tests in 8 patients with a history of hypersensitivity to Pfizer‐BioNTech and Moderna vaccines and successful administration of the 2nd dose of the same vaccine in 7/8 (8th patient not vaccinated yet). 26 There is only a single report of confirmed PEG allergy in a patient with a history of anaphylaxis to the Pfizer vaccine. 7 Other mechanisms might also be relevant. IgG and IgM anti‐PEG antibodies have been detected in up to 50% of healthy individuals.
There is experimental evidence that these antibodies could reduce the therapeutic effect of the drug by either accelerating the drug blood clearance (ABC phenomenon) or cause complement activation‐related pseudoallergy (CARPA). There was no signal of either in the trial data. 27 , 28 Clinical symptoms after intravenous administration of liposome‐containing drugs such as PEG asparaginase, PEGylated IFN alpha can mimic IgE‐mediated allergy. Liposomes have a non‐specific complement activating potential depending on their surface structures and charge. 28 , 29 Moreover, vaccine mRNAs can directly interact with and activate mast cells. Addition of the RNAs to mast cells in vitro induced expression of type I interferons, TNF‐alpha and anti‐viral proteins but not degranulation. However, mast cell degranulation did not occur after intracellular RNA recognition in in vitro studies. 15 The frequency of suspected allergic reactions to the vaccine and placebo (0.9% NaCl) in the Pfizer and Moderna trials was similar which suggests that mast cell activation via mRNA was not relevant. However, as anaphylaxis events are rare it is possible, they may have not been picked up in the Phase III studies due to insufficient participant numbers.
7. COVID‐19 VACCINES: OTHER EXCIPIENTS
The Moderna vaccine, in addition to PEG, contains trometamol (tromethamine) known to cause not only contact dermatitis but also immediate hypersensitivity reactions. It is present in enteral and parenteral drugs: cotrimoxazole iv, Humalog insulin, Menitorix (Haemophilus type b and meningococcal group C conjugate) vaccine, midazolam, oxaliplatin as well as MRI and CT radiocontrast media. 30 The allergenic potential of other excipients: 1,2‐distearoyl‐sn‐glycero‐3‐phosphocholine (DSPC) and EDTA needs to be considered. 31 However, testing faces the same challenges as for PEG, due to the lack of standardized, commercially available test solutions.
8. MAIN QUESTIONS
8.1. How to manage patients who experienced a hypersensitivity reaction to a COVID‐19 vaccine?
Hypersensitivity reactions should be managed depending on their severity and according to the relevant guidelines. 32 Acute and baseline serum tryptase levels should be measured in case of all immediate reactions. The relevant differential diagnoses of anaphylaxis must be considered (syncope; anxiety attack; asthma; cardiac arrhythmia; exacerbation of preexistent chronic urticaria and angioedema etc.). All patients who experienced suspected anaphylaxis should be referred to an allergist/immunologist experienced in the management of drug/vaccine allergy. A detailed history should be taken, encompassing previous reactions to vaccines/drugs. Investigation with relevant skin tests with relevant excipients (PEG, PS80, tromethamine) should be considered although lack of availability of clinical‐grade reagents for test may make this difficult and safety considerations are important. If available, skin tests with the relevant vaccine should be undertaken. Undiluted Pfizer vaccine is nonirritant; data for other vaccines are needed. 33 If all investigations to the index vaccine and relevant excipients are negative, patients may then receive the second dose of the vaccine under the supervision of an allergist. A split‐dose administration can be considered. If skin tests are positive to the vaccine and/or the relevant excipient, an alternative vaccine should be administered. In selected cases, desensitization might be possible. The impact on the immune response of not administering the vaccine as a single dose may be a concern (Figure 2).
8.2. Who should not receive the currently available COVID‐19 vaccines?
The current edition of the UK Immunisation Green Book recommends that The vaccine should not be given to those who have had a previous systemic allergic reaction (including immediate‐onset anaphylaxis) to a previous dose of the same COVID‐19 vaccine and/or any component (excipient) of the COVID‐19 vaccine, for example polyethylene glycol. Anyone with any other allergies (such as a food allergy) can now have the vaccine. 3
This is a welcome revision to the initial MHRA guidance. These general recommendations will help avert the majority of the most severe allergic reactions to the COVID‐19 vaccines. As the worldwide vaccination campaign gains pace and detailed allergy workup data of cases of vaccine‐related anaphylaxis emerges, it will allow for the publication of a streamlined evidence‐based algorithm for the management of these reactions. For now, many questions remain: for how many patients are PEG or PS80 relevant as allergens in these vaccines? Will all PEG allergic patients (sensitized to different molecular weight PEGs) react to the vaccines that contain PEG2000 in a very small quantity? How many PEG allergic patients will react to PS80 due to cross‐reactivity? Do patients with unexplained anaphylaxis really need to avoid the Pfizer vaccine? ‘Unexplained anaphylaxis’ might suggest idiopathic anaphylaxis, that is anaphylaxis where no trigger (including no drugs which might contain PEG) has been identified after a thorough allergy workup. If there is no trigger for anaphylaxis (ie true idiopathic anaphylaxis), a PEG‐containing vaccine should be tolerated. In view of many unanswered questions, the European Academy of Allergy and Clinical Immunology encourages the allergy/immunology community to consider not only clinical and scientific aspects but also an ‘omics’ approach, for example proteomics, single‐cell RNA‐seq transcriptomics as well as ‘big data’ and legal, ethical and regulatory aspects of anaphylaxis to the COVID vaccine. 34
Global vaccination campaign gains pace and as of 25 March 2021, a total of 462 824 374 doses have been administered. 1 Adverse reactions will definitely continue to emerge. Systematic monitoring, detailed documentation and characterization of all potential anaphylactic events (immunological and non‐immunological) will be critical in delineating underlying mechanisms and informing future recommendations for safe vaccine administration.
CONFLICT OF INTEREST
All authors declare no relevant conflicts of interest.
AUTHOR CONTRIBUTION
All authors contributed equally to this work.
[Correction added on 7 May 2021, after first online publication: Key Messages were omitted and have been reinstated in this version.]
REFERENCES
- 1. https://covid19.who.int. Accessed 27/03/2021.
- 2. Beig Parikhani A, Bazaz M, Bamehr H, et al. The Inclusive Review on SARS‐CoV‐2 Biology, Epidemiology, Diagnosis, and Potential Management Options. Curr Microbiol. 2021. 10.1007/s00284-021-02396-x. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. UK Green Book: Immunisation against infectious disease . COVID‐19. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/961287/Greenbook_chapter_14a_v7_12Feb2021.pdf. Accessed 27/03/2021.
- 4. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA covid‐19 vaccine. N Engl J Med. 2020;383:2603‐2615. 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baden LR, El Sahly HM, Essink B, et al. Efficacy and Safety of the mRNA‐1273 SARS‐CoV‐2 Vaccine. N Engl J Med. 2020; NEJMoa2035389. doi: 10.1056/NEJMoa2035389. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ramasamy MN, Minassian AM, Ewer KJ, et al. Safety and immunogenicity of ChAdOx1 nCoV‐19 vaccine administered in a prime‐boost regimen in young and old adults (COV002): a single‐blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396:1979‐1993. 10.1016/S0140-6736(20)32466-1. Epub 2020 Nov 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sellaturay P, Nasser S, Islam S, Gurugama P, Ewan P. Polyethylene glycol (PEG) is a cause of anaphylaxis to the Pfizer/BioNTech mRNA COVID‐19 vaccine. Clin Exp Allergy. 2021; 10.1111/cea.13874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wenande W, Garvey LH. Immediate type hypersensitivity to polyethylene glycols: a review. CEA. 2016;46:907‐922. [DOI] [PubMed] [Google Scholar]
- 9. Lu IN, Rutkowski K, Kennard L, et al. Polyethylene glycol may be the major allergen in depot medroxyprogesterone acetate. J Allergy Clin Immunol Pract. 2020;8:3194‐3197. [DOI] [PubMed] [Google Scholar]
- 10. Kennard L, Rutkowski K, Mirakian R, Wagner A. Polyethylene glycol: not just a harmless excipient. J Allergy Clin Immunol Pract. 2018;6:2173. [DOI] [PubMed] [Google Scholar]
- 11. Stone CA, Yiwei L, Relling MV, et al. Immediate hypersensitivity to polyethylene glycols and polysorbates: more common than we have recognized. JACI Pract. 2019;7:1533‐1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li PH, Wagner A, Thomas I, et al. Steroid allergy: clinical features and the importance of excipient testing in a diagnostic algorithm. J Allergy Clin Immunol Pract. 2018;6:1655‐2166. [DOI] [PubMed] [Google Scholar]
- 13. Rutkowski K, Wagner A, Rutkowski R. Immediate hypersensitivity reactions to steroids and steroid containing medications. Curr Opin Allergy Clin Immunol. 2020;20:362‐366. [DOI] [PubMed] [Google Scholar]
- 14. Krantz MS, Liu Y, Phillips EJ, Stone CA. COVID‐19 vaccine anaphylaxis: PEG or not? Allergy. 2021; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cabanillas B, Akdis C, Novak N. COVID‐19 vaccine anaphylaxis: IgE, complement or what else? A reply to: “COVID‐19 2 vaccine anaphylaxis: PEG or not? Allergy. 2021; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. MMWR 1 Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer‐BioNTech COVID‐19 vaccine ‐ United States, December 14–23, 2020. MMWR Morb Mortal Wkly Rep. 2021;70:46‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shimabukuro T, Nair N. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer‐BioNTech COVID‐19 vaccine. JAMA. 2021;325(8):780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. MMWR2 allergic reactions including anaphylaxis after receipt of the first dose of Moderna COVID‐19 Vaccine ‐ United States. December 21, 2020‐January 10, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(4):125‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McNeil MM, Weintraub ES, Duffy J, et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137:868‐878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Su JR, Moro PL, Ng CS, et al. Anaphylaxis after vaccination reported to the VAERS 1990–2016. J Allergy Clin Immunol. 2019;143:1465‐1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li P, Wagner A, Rutkowski R, Rutkowski K. Vaccine allergy: a decade of experience from 2 large UK allergy centers. Ann Allergy Asthma Immunol. 2017;118:729‐731. [DOI] [PubMed] [Google Scholar]
- 22. Kelso JM. Misdiagnosis of systemic allergic reactions to mRNA COVID‐19 vaccines. Ann Allergy Asthma Immunol. 2021. doi: 10.1016/j.anai.2021.03.024. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Caminati M, Guarnieri G, Senna G. Who is really at risk for anaphylaxis due to COVID‐19 vaccine? Vaccines. 2021;9:38. 10.3390/vaccines9010038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jover Cerdá V, Rodríguez Pacheco R, Doménech Witek J, et al. Immediate hypersensitivity to polyethylene glycols in unrelated products: when standardization in the nomenclature of the components of drugs, cosmetics, and food becomes necessary. Allergy Asthma. Clin Immunol. 2019;19(15):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhou ZH, Stone CA, Jakubovic B, et al. Anti‐PEG IgE in anaphylaxis associated with polyethylene glycol. J Allergy Clin Immunol Pract. 2020;S2213‐2198(20)31231‐9. 10.1016/j.jaip.2020.11.011. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pitlick MM, Sitek AN, Kinate SA, Joshi AY, Park MA. Polyethylene glycol and polysorbate skin testing in the evaluation of COVID‐19 vaccine reactions: early report. Ann Allergy Asthma Immunol. 2021; 10.1016/j.anai.2021.03.012. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Klimek L, Novak N, Cabanillas B, et al. Allergenic components of the mRNA‐1273 vaccine for COVID‐19: possible involvement of polyethylene glycol and IgG‐mediated complement activation. Allergy. 2021; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mohamed M, Abu Lila AS, Shimizu T, et al. PEGylated liposomes: immunological responses. Sci Technol Adv Mater. 2019;20:710‐724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Inglut CT, Sorrin AJ, Kuruppu T, et al. Immunological and toxicological considerations for the design of liposomes. Nanomaterials. 2020;10(2):190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lukawska J, Mandaliya D, Chan AWE, et al. Anaphylaxis to trometamol excipient in gadolinium‐based contrast agents for clinical imaging. J Allergy Clin Immunol Pract. 2019;7:1086‐1087. [DOI] [PubMed] [Google Scholar]
- 31. Russo PAJ, Banovic T, Wiese MD, Whyte AF, Smith WB. Systemic allergy to EDTA in local anesthetic and radiocontrast media. J Allergy Clin Immunol Pract. 2014;2:225‐229. [DOI] [PubMed] [Google Scholar]
- 32. Resuscitation Council UK . Anaphylaxis guidance for vaccination settings 2021. https://www.resus.org.uk/about‐us/news‐and‐events/rcuk‐publishes‐anaphylaxis‐guidance‐vaccination‐settings
- 33. Marcelino J, Farinha S, Silva R, et al. Non‐irritant concentrations for skin testing with SARS‐CoV‐2 mRNA vaccine. J Allergy Clin Immunol Pract. 2021; 10.1016/j.jaip.2021.03.022. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sokolowska M, Eiwegger T, Ollert M, et al. EAACI statement on the diagnosis, management and prevention of severe allergic reactions to COVID‐19 vaccines. Allergy. 2021; 10.1111/all.14739 [DOI] [PMC free article] [PubMed] [Google Scholar]


