Abstract
Background
Descriptions of symptoms and medication use at end of life in COVID‐19 are limited to small cross‐sectional studies, with no Australian longitudinal data.
Aims
To describe end‐of‐life symptoms and care needs of people dying of COVID‐19.
Methods
This retrospective cohort study included consecutive admitted patients who died at a Victorian tertiary referral hospital from 1 January to 30 September directly due to COVID‐19. Clinical characteristics, symptoms and use of supportive therapies, including medications and non‐pharmacological interventions in the last 3 days of life were extracted.
Results
The cohort comprised 58 patients (median age 87 years, interquartile range (IQR) 81–90) predominantly admitted from home (n = 30), who died after a median of 11 days (IQR 6–28) in the acute medical (n = 31) or aged care (n = 27) wards of the hospital. The median Charlson Comorbidity Score was 7 (IQR 5–8). Breathlessness (n = 42), agitation (n = 36) and pain (n = 33) were the most frequent clinician‐reported symptoms in the final 3 days of life, with most requiring opioids (n = 52), midazolam (n = 40), with dose escalation commonly being required. While oxygen therapy was commonly used (n = 47), few (n = 13) required an anti‐secretory agent.
Conclusions
This study presents one of the first and largest Australian report of the end of life and symptom experience of people dying of COVID‐19. This information should help clinicians to anticipate palliative care needs of these patients, for example, recognising that higher starting doses of opioids and sedatives may help reduce prevalence and severity of breathlessness and agitation near death.
Keywords: COVID‐19, palliative care, death, signs and symptoms, infectious disease
Introduction
COVID‐19 emerged as a novel coronavirus infection in December 2019. As of 24 November 2020, it had caused more than 1.38 million cumulative deaths worldwide. 1 Australia has observed significantly fewer infections and consequently deaths compared to many other international settings, with just 907 deaths and 819 of these reported in the state of Victoria, mostly occurring from July 2020 during the second wave of infection. 1
End‐of‐life care for people dying of COVID‐19 infection has only been recently described in small cohorts, predominantly from settings with high prevalence of infection. 2 , 3 , 4 , 5 , 6 , 7 , 8 There are limited data describing COVID‐19 hospital deaths in an Australian setting, 9 and to our knowledge, none outside intensive care settings and few explicitly focussed on the symptom experience and supportive therapies administered in the final days of life.
This study aimed to describe the clinical characteristics, end‐of‐life care, symptomatology and use of supportive therapies in consecutive admitted patients who died from COVID‐19 in a large Victorian tertiary hospital to help familiarise clinicians with likely care needs.
Methods
Design
This was a retrospective cohort study of consecutive inpatient deaths due to COVID‐19 occurring in both the acute and subacute hospital campuses at The Royal Melbourne Hospital, based in metropolitan Melbourne. The study was approved by the Royal Melbourne Hospital Human Research Ethics Committee (QA2020141).
Participants
The study cohort included patients of all ages who died from 1 January to 30 September 2020 due to COVID‐19, with diagnosis determined by diagnosis‐related group coding identified from the patients' discharge summary performed post death. Deaths not directly caused by COVID‐19 as documented in the death certificates were excluded.
Data collection
De‐identified outcome data were extracted from the medical records of eligible cases by trained members of palliative care clinical staff using an electronic standardised case report form (REDCap, Vanderbilt University). A detailed data dictionary was available and data quality checks were completed by a senior consultant. Data collected included:
Demographic and clinical characteristics, including the Australian‐modified Karnofsky Performance Status (AKPS) 10 and the Charlson Comorbidity Index 11 ;
Final admission characteristics, including treating unit, length of stay, palliative care referral, goals of care documentation and place of death;
Symptom severity in the final 3 days of life as measured by the Palliative Care Outcome Scale Staff Questionnaire, 12 a validated, clinician‐rated assessment of common end‐of‐life symptom domains measured on a five‐point categorical scale (absent, slight, moderate, severe, and overwhelming);
Use of supportive pharmacological and non‐pharmacological therapies in the final 3 days of life as measured by background and breakthrough opioid use converted into oral morphine equivalent daily dose (oMEDD), 13 sedative use and anti‐secretory use; presence and mode of oxygen delivery; suction of respiratory secretions; and pressure area care. Medications audited were based on international guidelines on managing COVID‐19‐related end‐of‐life symptoms. 14
Analyses
Descriptive statistics were used to summarise each variable collected. An analysis plan was generated a priori to enable the standardised coding of descriptive data into relevant categorical and/or numerical variables for inclusion in results tables. Continuous variables were expressed as median with interquartile range (IQR) and categorical variables as number (percentage). Consistent with the descriptive aims of this cohort study, no sub‐group analyses were planned or conducted, and no missing data was imputed. All analyses were performed using stata version 15.1 (StataCorp, College Station, TX, USA).
Results
Description of the cohort
This study comprised 58 patients who died of COVID‐19 in hospital (Table 1). Thirty‐four were men, and the group had a median age of 87 years (IQR 81–90). Thirty (52%) lived at home prior to hospital admission, and the median baseline performance status was an AKPS of 50 (IQR 40–60) meaning they required considerable assistance and frequent medical care. Hypertension and dementia were the most common comorbidities.
Table 1.
Description of patient cohort (n = 58)
| Characteristic | n (%) |
|---|---|
| Age, median (IQR) (years) | 87 (81–90) |
| ≥50 to <60 | 4 (7) |
| ≥60 to <70 | 2 (3) |
| ≥70 to <80 | 6 (10) |
| ≥80 | 46 (79) |
| Usual place of residence | |
| Home | 30 (52) |
| Residential aged care facility | 21 (36) |
| Other supported accommodation (e.g. disability support) | 7 (12) |
| Baseline AKPS, median (IQR, range 0–100) | 50 (40–60) |
| Comorbidities | |
| Charlson Comorbidity Index Score (median, IQR) | 7 (5–8) |
| Hypertension | 40 (69) |
| Dementia | 28 (48) |
| Diabetes (with complications) | 18 (31) |
| Renal disease | 17 (29) |
| Myocardial infarction | 14 (24) |
| Congestive cardiac failure | 14 (24) |
| Chronic pulmonary disease | 12 (21) |
| Cerebrovascular disease | 8 (14) |
| Tumour (without metastasis) | 5 (9) |
| Hemiplegia | 4 (7) |
| Peripheral vascular disease | 3 (5) |
| Peptic ulcer disease | 3 (5) |
| Moderate – severe liver disease | 2 (3) |
| Diabetes (no complications) | 2 (3) |
| Leukaemia (acute or chronic) | 2 (3) |
| Metastatic solid tumour | 2 (3) |
| Connective tissue disease | 2 (3) |
| Goals of care | |
| Goals of care documented | 58 (100) |
| Timing of goals of care (days relative to death), median (IQR) | 7.5 (4–17) |
| Full resuscitation | 5 (9) |
| Limited resuscitation | 9 (16) |
| Symptom management only | 35 (60) |
| End‐of‐life care | 9 (16) |
AKPS, Australian‐modified Karnofsky Performance Status; IQR, interquartile range.
End‐of‐life admission characteristics
Thirty‐one people who died of COVID‐19 were initially cared for in acute medical wards, while 27 were initially on the aged care wards. At the time of death seven patients were in intensive care, while 23 died in the aged care ward and 28 were in the acute ward. The median length of hospital stay prior to death was 11 days (IQR 6–28). A minority (n = 8) were formally referred to palliative care for specific individual management and/or advice. Goals of care were documented in every patient, a median of 7.5 days (IQR 4–17) before death, with a small number for full resuscitation (n = 5; 9%) or limited resuscitation (n = 9; 16%).
Symptom severity in the final 3 days of life
The most commonly reported symptoms in the final 3 days of life were breathlessness (n = 42; 72%), agitation (n = 36; 62%) and pain (n = 33; 57%). These were also the most severe reported symptoms. Detailed symptom severity is presented in Figure 1. In total, 48 (83%) patients reported at least one of the symptoms of fever, cough or breathlessness in the last 3 days of life, with seven patients reporting all three symptoms.
Figure 1.
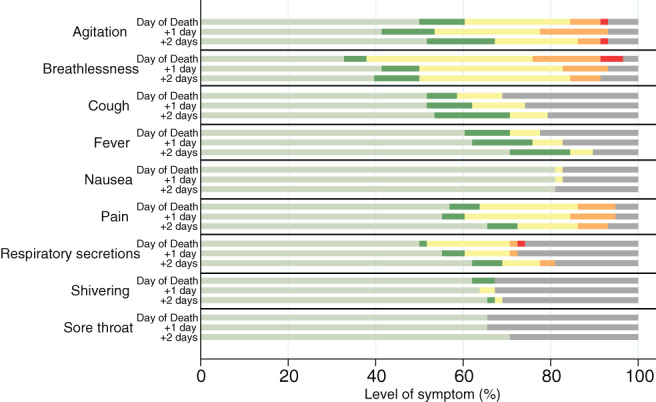
Symptom severity in final 3 days of life. ( ), Absent; (
), Absent; ( ), slight; (
), slight; ( ), moderate; (
), moderate; ( ), severe; (
), severe; ( ), overwhelming; (
), overwhelming; ( ), not documented.
), not documented.
Use of medications and supportive therapies in the final 3 days of life
End‐of‐life medications most frequently administered included opioids, sedatives and an anti‐secretory agent (Table 2).
Table 2.
Medication use in the final 3 days of life
| Medication | Number of participants | Median (IQR) (mg) |
|---|---|---|
| Morphine oral equivalent daily dose (oMEDD) | ||
| Day of death | 52 | 45 (22.5–95.8) |
| 1 Day before death | 39 | 30 (15–67.5) |
| 2 Days before death | 34 | 25 (9.4–75) |
| Midazolam | ||
| Day of death | 39 | 12.5 (5, 21) |
| 1 Day before death | 20 | 10.55 (6.3, 24) |
| 2 Days before death | 21 | 5 (2.5, 14.5) |
| Glycopyrrolate | ||
| Day of death | 13 | 0.2 (0.2, 0.6) |
| 1 Day before death | 5 | 0.2 (0.2, 1.2) |
| 2 Days before death | 4 | 0.4 (0.2, 0.6) |
On the day of death, 37 patients received a continuous opioid infusion, most frequently either morphine or fentanyl. Other opioids were buprenorphine, hydromorphone and oxycodone. Although median daily background dose remained stable in the final 3 days of life, cumulative opioid daily dose increased (oMEDD of 25 mg, 30 mg and 45 mg respectively), and number of patients requiring opioids also increased (34, 39 and 52 of patients respectively).
Thirty patients received a continuous sedative infusion on the day of death, most frequently midazolam (n = 23) while propofol was used for the seven patients who were intubated. Other sedatives (diazepam, clonidine, clonazepam, olanzapine, quetiapine) were administered to a small minority. A pattern of increasing sedative requirement was observed for midazolam in the last 3 days of life (5 mg, 10.6 mg and 12.5 mg, respectively, per 24 h, required by 21, 20 and 39 patients in the 3 days leading to death). Breakthrough pattern of these medications was similar on each day (mean breakthrough two to three per day).
A minority (n = 2) received glycopyrrolate (glycopyrronium bromide) infusion for respiratory secretions on day of death, while 12 received glycopyrrolate via breakthrough administration. Total number of patients requiring glycopyrrolate increased daily (4, 5 and 13 patients, respectively, in the 3 days prior to death).
Oxygen therapy was delivered to 43 patients on the day of death, 42 patients 1 day before death and 41 patients 3 days before death, while seven patients received mechanical ventilatory support. Suction of respiratory secretions was provided to nine patients and pressure care was documented to be performed on 51 patients over the last 3 days of life.
Discussion
This study is among the first to report on the symptoms and end‐of‐life care of a sample of consecutive Australian patients admitted to hospital who died from COVID‐19. Our cohort comprised an elderly population admitted from home with substantial comorbidities and a baseline performance status indicating significant pre‐existing care needs.
There are currently only few published studies internationally outlining symptom management in patients dying of COVID‐19 2 , 3 , 4 , 5 , 6 , 7 , 8 with only one that analysed trends in prevalence of symptoms over the 72 h prior to death. 4 Our findings are consistent with these reports, which suggest that breathlessness is the most prevalent symptom. However, while Alderman did not observe an increase in breathlessness prevalence during the final 72 h of life, 4 we did observe an increase in prevalence and breathlessness intensity, accompanied by a parallel escalation of opioid and midazolam use. The incidence of excess respiratory secretions in our cohort was lower than the 35% prevalence reported in a systematic review of the general population dying from all causes, despite COVID‐19 being largely a respiratory illness (and respiratory illnesses being a common cause of death in the population). 15 This suggests that the degree of respiratory secretions, though noted to be thick and of large quantity in the lower airways, still does not affect patients as much as within the general population. 16
Agitation was the second most common symptom, 2 , 4 , 8 the degree peaking between the final 48–72 h period of life before plateauing or improving on the day of death. This may reflect the comparatively higher doses of opioids and midazolam administered on the day of death, along with a reduced conscious state closer to death. The prevalence of agitation in our cohort (72.4%) is similar to that reported in a systematic review examining terminal delirium in the general population (59–88%). 17 Importantly, these data suggest that this symptom was controlled on the day of death.
We also observed an increasing number of patients experiencing moderate to severe pain in the last 24–48 h of life, although this was less common (56.8%), compared to a recent large population study (69.7%). 18 While cough is one of the commonest presenting symptoms of COVID‐19, 19 it was uncommon in this cohort of dying patients and the prevalence reduced with time. This may be related to the suppressive effect of opioids that were administered for dyspnoea and pain, and the loss of the cough reflex with deteriorating consciousness.
Our findings broadly confirm those limited data available internationally documenting medication use in this setting. Opioid medication is a common symptomatic treatment at end of life in COVID‐19 deaths, with a UK series reporting up to 67% of patients requiring opioids, 4 , 20 and in the United States the most commonly prescribed opioids being parenteral morphine and hydromorphone, used in 77% and 37% of patients respectively. 6 While in our study opioids were more frequently prescribed (90%) in the last 3 days of life, the doses used were similar to reported ranges of daily subcutaneous morphine equivalent doses of between 10 and 24 mg. 2 , 4 , 6 , 8
Of note, in our series while median background opioid dose was relatively static throughout the last 3 days, the median breakthrough dose doubled by the final day of death. The increase in median total oMEDD is likely reflected by increases in breakthrough dosing. This increase in breakthrough dose may have been done in response to both an increase in agitation 1 day before death, to good effect, and/or to treat breathlessness on the day of death. The number of patients using opioids also increased during this period, by just over 50%. It is possible that a higher starting dose and more liberal escalation may have prevented the observed spike in agitation and breathlessness noted.
In our series, midazolam was the most frequently used sedative, and similarly, the doses of midazolam we report are similar to reported dose ranges of 8.75–15 mg. 2 , 4 , 7 , 8 However, therapies used for agitation varied across the literature, with regular haloperidol commonly prescribed, 2 , 4 , 6 , 7 followed by levomepromazine. 4 , 7 In contrast, the use of such antipsychotics in our cohort was rare or absent. This may be due to the timing of our data collection – antipsychotics may have been used prior to the final days of life, and ceased once the goal of care was to optimise sedation. Midazolam use was proportionally increased more compared to opioid use, but with a relatively low peak median dose, and less patients requiring midazolam than morphine. Breakthrough frequency did not change. Similar to morphine, an earlier escalation or starting dose of midazolam may have prevented the escalation of symptoms near death.
While evidence to support the use of anti‐secretory agents to manage noise respiratory secretions remains scant, 21 they are listed in published international guidelines for management of this symptom. 22 Interestingly in our cohort, anti‐secretory medication use was low. Glycopyrrolate was the only anti‐secretory agent used. Although use of glycopyrrolate increased with time, only two decedents in the entire cohort required a glycopyrrolate infusion, and on the final day of life, only a fifth were administered glycopyrrolate, most of whom received it as a breakthrough, with mean breakthrough frequency of twice per day only, and median total daily dose of 0.2 mg.
Anticholinergic anti‐secretory agents work by reducing the production and accumulation of respiratory secretions, and are deemed to be more effective in patients with low amounts of secretions. 23 Critically ill COVID‐19 patients are known to have large amounts of thick sputum in the lower airways 16 ; as such, the use of anti‐secretory agents in this group of patients may not be as helpful.
Supportive therapies were used in addition to medication for management of symptoms in the final days of life for patients with COVID‐19 illness. The consensus from the European Respiratory Society International Task Force was for use of oxygen therapy for palliative treatment of breathlessness and hypoxaemia in COVID‐19 illness. 24 Similarly, the National Institute for Health and Care Excellence guidelines advise consideration of a trial of oxygen for breathlessness 14 despite a general lack of evidence of symptomatic benefit for patients who are not hypoxic. 25 Oxygen therapy was administered to more than two‐thirds of patients in this cohort in their final 3 days of life, lower than that reported in the United States where 97% of patients were found to have received supplemental oxygen prior to a palliative care unit admission. 6
Conclusions
This study presents one of the first and largest Australian reports of the end‐of‐life care and symptom experience in a sample of consecutive deaths from COVID‐19 at a major Australian tertiary hospital. Clinician‐assessed symptoms confirm the frequency of breathlessness, agitation and pain in the 3 days prior to death, which are similar to comparable international cohorts, although different in prevalence to the general dying population. Relatively low doses of opioids, sedatives and anti‐secretory agent were required. Opioids and midazolam showed the highest escalation on day of death, mostly for treating a peak in agitation and breathlessness. The use of a higher starting dose with earlier escalation of these medications may be a useful measure to prevent the anticipated worsening of these symptoms.
Acknowledgements
We acknowledge Amy Pascoe, Research Assistant, Parkville Integrated Palliative Care Service, Melbourne, Victoria, Australia for assistance with data management.
Funding: None.
Conflict of interest: None.
References
- 1. World Health Organization . WHO Coronavirus Disease (COVID‐19) Dashboard. Geneva, Switzerland: World Health Organization; 2020. [cited 2020 Nov 24]. Available from URL: https://covid19.who.int/ [Google Scholar]
- 2. Lovell N, Maddocks M, Etkind SN, Taylor K, Carey I, Vora V et al. Characteristics, symptom management and outcomes of 101 patients with COVID‐19 referred for hospital palliative care. J Pain Symptom Manage 2020; 60: e77–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jackson T, Hobson K, Clare H, Weegmann D, Moloughney C, McManus S. End‐of‐life care in COVID‐19: an audit of pharmacological management in hospital inpatients. Palliat Med 2020; 34: 1235–40. [DOI] [PubMed] [Google Scholar]
- 4. Alderman B, Webber K, Davies A. An audit of end‐of‐life symptom control in patients with corona virus disease 2019 (COVID‐19) dying in a hospital in the United Kingdom. Palliat Med 2020; 34: 1249–55. [DOI] [PubMed] [Google Scholar]
- 5. Turner J, Hodgson LE, Leckie T, Eade L, Ford‐Dunn S. A dual‐centre observational review of hospital based palliative care in patients dying with COVID‐19. J Pain Symptom Manage 2020; 60: e75–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sun H, Lee J, Meyer BJ, Myers EL, Nishikawa MS, Tischler JL et al. Characteristics and palliative care needs of COVID‐19 patients receiving comfort‐directed care. J Am Geriatr Soc 2020; 68: 1162–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Voo T, Senguttuvan M, Tam CC. Family presence for patients and separated relatives during COVID‐19: physical, virtual, and surrogate. J Bioeth Inq 2020; 17: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hetherington L, Johnston B, Kotronoulas G, Finlay F, Keeley P, McKeown A. COVID‐19 and hospital palliative care – a service evaluation exploring the symptoms and outcomes of 186 patients and the impact of the pandemic on specialist hospital palliative care. Palliat Med 2020; 34: 1256–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burrell AJ, Pellegrini B, Salimi F, Begum H, Broadley T, Campbell LT et al. Outcomes of COVID‐19 patients admitted to Australian intensive care units during the early phase of the pandemic. Med J Aust 2020; 1: 23–30. [DOI] [PubMed] [Google Scholar]
- 10. Abernethy AP, Shelby‐James T, Fazekas BS, Woods D, Currow DC. The Australia‐modified Karnofsky performance status (AKPS) scale: a revised scale for contemporary palliative care clinical practice. BMC Palliat Care 2005; 4: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Clin Epidemiol 1987; 40: 373–83. [DOI] [PubMed] [Google Scholar]
- 12. Masso M, Allingham SF, Johnson CE, Pidgeon T, Yates P, Currow D et al. Palliative care problem severity score: reliability and acceptability in a national study. Palliat Med 2016; 30: 479–85. [DOI] [PubMed] [Google Scholar]
- 13. Faculty of Pain Medicine, Australian and New Zealand College of Anaesthetists Opioid dose equivalence: calculation of oral morphine equivalent daily dose (oMEDD) [updated 2019 Mar; Cited 10 Mar 2021]. Available from URL: http://www.opioidcalculator.com.au/index.html
- 14. National Institute for Health and Care Excellence COVID‐19 rapid guideline: managing symptoms (including at the end‐of‐life) in the community. Oct 2020 [Cited 10 Mar 2021]. Available from URL: https://www.nice.org.uk/guidance/ng163 [PubMed]
- 15. Lokker M, van Zuylen L, van der Rijt CC, van der Heide A. Prevalence, impact, and treatment of death rattle: a systematic review. J Pain Symptom Manage 2014; 47: 105–22. [DOI] [PubMed] [Google Scholar]
- 16. Wang Y, Zhang M, Yu Y, Han T, Zhou J, Bi L. Sputum characteristics and airway clearance methods in patients with severe COVID‐19. Medicine 2020; 99: e23257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hosie ADP, Agar M, Sanderson CR, Phillips J. Delirium prevalence, incidence, and implications for screening in specialist palliative care inpatient settings: a systematic review. Palliat Med 2013; 27: 486–98. [DOI] [PubMed] [Google Scholar]
- 18. Hagarty AM, Bush SH, Talarico R, Lapenskie J, Tanuseputro P. Severe pain at the end of life: a population‐level observational study. BMC Palliat Care 2020; 19: 60. doi: 10.1186/s12904-020-00569-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Keeley P, Buchanan D, Carolan C, Pivodic L, Tavabie S, Noble S. Symptom burden and clinical profile of COVID‐19 deaths: a rapid systematic review and evidence summary. BMJ Support Palliat Care 2020; 10: 381–4. [DOI] [PubMed] [Google Scholar]
- 20. Heath L, Yates S, Carey M, Miller MP. Alliative care during COVID‐19: data and visits from loved ones. Am J Hosp Palliat Med 2020; 37: 988–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wee B, Hillier R. Interventions for noisy breathing in patients near to death. Cochrane Database Syst Rev 2008; CD005177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. National Institute for Health and Care Excellence . Care of Dying Adults in the Last Days of Life. London: NICE; 2015. [cited 2021 Mar 3]. Available from URL: https://www.nice.org.uk/guidance/ng31 [PubMed] [Google Scholar]
- 23. Mercadante S, Villari P, Ferrera P. Refractory death rattle: deep aspiration facilitates the effects of antisecretory agents. J Pain Symptom Manage 2011; 41: 637–9. [DOI] [PubMed] [Google Scholar]
- 24. Janssen D, Ekström M, Currow DC, Johnson MJ, Maddocks M, Simonds AK et al. COVID‐19: guidance on palliative care from a European Respiratory Society international task force. Eur Respir J 2020; 56: 2002583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Allsop M, Ziegler L, Fu Y, Rudd Sarah, Bennett Michael I Is oxygen an effective treatment option to alleviate the symptoms of breathlessness for patients dying with COVID‐19 and what are the potential harms? University of Oxford; 2020 [updated 2020 May 7; cited 2021 Mar 10]. Available from URL: https://www.cebm.net/covid-19/


