Abstract
Background
Lung ultrasound (LUS) and lung ultrasound score (LUSS) have been successfully used to diagnose neonatal pneumonia, assess the lesion distribution, and quantify the aeration loss. The present study design determines the diagnostic value of LUSS in the semi‐quantitative assessment of pneumonia in coronavirus disease 2019 (COVID‐19) neonates.
Methods
Eleven COVID‐19 neonates born to mothers with COVID‐19 infection and 11 age‐ and gender‐matched controls were retrospectively studied. LUSS was acquired by assessing the lesions and aeration loss in 12 lung regions per subject.
Results
Most of the COVID‐19 newborns presented with mild and atypical symptoms, mainly involving respiratory and digestive systems. In the COVID‐19 group, a total of 132 regions of the lung were examined, 83 regions (62.8%) of which were detected abnormalities by LUS. Compared with controls, COVID‐19 neonates showed sparse or confluent B‐lines (83 regions), disappearing A‐lines (83 regions), abnormal pleural lines (29 regions), and subpleural consolidations (2 regions). The LUSS was significantly higher in the COVID‐19 group. In total, 49 regions (37%) were normal, 73 regions (55%) scored 1, and 10 regions (8%) scored 2 by LUSS. All the lesions were bilateral, with multiple regions involved. The majority of the lesions were located in the bilateral inferior and posterior regions. LUS detected abnormalities in three COVID‐19 neonates with normal radiological performance. The intra‐observer and inter‐observer reproducibility of LUSS was excellent.
Conclusions
LUS is a noninvasive, convenient, and sensitive method to assess neonatal COVID‐19 pneumonia, and can be used as an alternative to the use of diagnostic radiography. LUSS provides valuable semi‐quantitative information on the lesion distribution and severity.
Keywords: COVID‐19, lung ultrasound, neonate, pneumonia
1. INTRODUCTION
The outbreak of coronavirus disease 2019 (COVID‐19) has become a pandemic and is severely affecting public health worldwide. The epidemic is geographically focused in the city of Wuhan, Hubei, mainland China from January to March 2020. As increasing of new infections in Wuhan, the number of pregnant women with COVID‐19 had been on the rise. The neonates born to mothers with COVID‐19 were quarantined immediately after birth, some of whom were severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) positive and developed COVID‐19 pneumonia. 1 Chest computed tomography (CT) scan is an important diagnostic tool for COVID‐19, with specific imaging findings in the pediatric population. 2 However, radiation safety concern for the neonates is essential.
Lung ultrasound (LUS) examination is convenient, nonionizing, and widely used in the assessment or monitoring of patients with infective pneumonia and dyspnea. COVID‐19 lung lesions in children begin from and are mainly located in the peripheral thoracic area, and most pediatric patients are in the early stage of the disease. Therefore, LUS has the advantage of detecting COVID‐19 pneumonia lesions, and especially becomes essential for COVID‐19 neonates. A lung ultrasound score (LUSS) 3 is helpful to semi‐quantitatively evaluate regional lung aeration, and has been successfully used in various pulmonary disease assessments.
The morbidity of COVID‐19 in the pediatric population was low. Our hospital was the exclusive children's hospital in Wuhan for treating COVID‐19. Data regarding the lung lesion distribution and aeration loss of COVID‐19 neonates using LUS are rare. Therefore, the present study aimed to investigate the ultrasonic features, lung lesion distribution, the severity of aeration loss of neonatal COVID‐19 pneumonia using semi‐quantitative LUSS method.
2. METHODS
2.1. Study population and clinical information
We searched the medical database and retrospectively surveyed all neonates born to mothers with COVID‐19 (n = 60) in Wuhan Children's Hospital from January 31 to March 31, 2020. Eleven neonates (three males, 3.8 ± 5.2 days) with confirmed SARS‐CoV‐2 infection (COVID‐19 group), as well as 11 age‐ and gender‐matched controls (control group) at the same time, were recruited in this single‐center retrospective study. The systematic LUS images of the subjects in two groups were properly achieved.
This study was approved by the Ethics Committee of Wuhan Children's Hospital (Approval No. 2020R111‐E01). The guardians of the neonates agreed to participate in this study. The diagnosis and management of a neonate with or at risk of COVID‐19 were in accordance with guidelines provided by the National Health Commission and the Chinese Perinatal‐Neonatal SARS‐CoV‐2 Committee. 4
Demographic, epidemiologic, and clinical features were obtained from the Electronic Health Records Sharing System. In addition, SARS‐CoV‐2 real‐time reverse transcription‐polymerase chain reaction (RT‐PCR) tests (Novel Coronavirus PCR Fluorescence Diagnostic Kit; BGI) were conducted using nasopharyngeal swab samples. SARS‐CoV‐2 serological antibodies (immunoglobulin M [IgM] and IgG) test was detected by magnetic particle chemiluminescence (Yahuilong). All the neonates underwent influenza virus tests. Chest X‐ray or CT scan was performed at admission.
The inclusion criteria were: neonates born to mothers with COVID‐19; neonates testing positive for SARS‐CoV‐2 real‐time RT‐PCR, or for virus‐specific IgM. The exclusion criteria were neonates with transient tachypnea of the newborn, with bacterial pneumonia, with positive influenza virus test, and with concomitant congenital anomalies.
2.2. LUS and LUSS
LUS images were acquired by one doctor with over 5‐year experience within 3 days after admission. Pacifier could be used in some uncooperative neonates. LUS was performed in the supine, prone, and decubitus position, using a portable ultrasound system (Mindray M9) and a linear array probe with a frequency of 4–12 MHz (L12‐4s).
Lung aeration loss can be estimated using a validated score called the Lung Ultrasound Score. As previously recommended, 3 the chest wall was divided into 12 regions (6 regions per hemithorax, Figure 1). We delineated the anterior and posterior axillary lines as practical landmarks that defined the anterior, lateral, and posterior areas of both lungs, and delineated the nipple line defining the upper and lower halves. One video clip was obtained for each region. Each region of interest was extensively examined.
Figure 1.
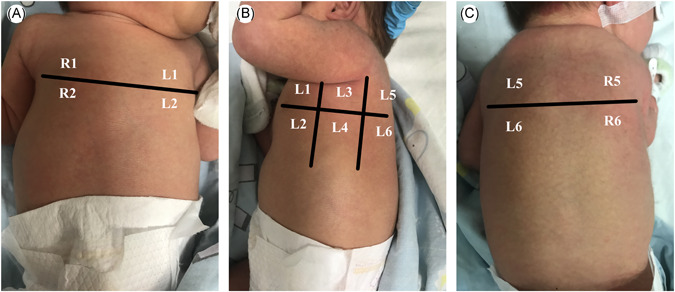
Neonates were placed in supine position (A), lateral position (B), and prone position (C), and the bilateral lungs were divided into 12 lung regions using the anterior axillary line, posterior axillary line, and nipple line [Color figure can be viewed at wileyonlinelibrary.com]
A semi‐quantitative LUSS was proposed for identifying four progressive stages of lung aeration loss, assigning a score from 0 to 3. See Table 1 and Figure 2 for a detailed description of LUSS.
Table 1.
Lung Ultrasound Scoring System
| Score | Ultrasonographic findings |
|---|---|
| Score 0—normal aeration | A‐lines presence—max 2 B‐lines |
| Score 1—moderate loss of aeration | ≥ 3 well‐spaced B‐lines |
| Score 2—severe loss of aeration | Confluent B‐lines |
| Score 3—complete loss of aeration | Tissue‐like pattern |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Figure 2.
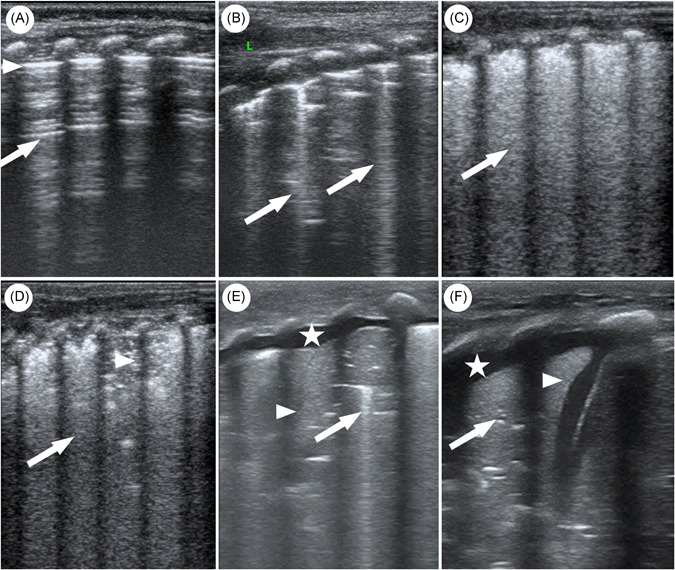
Lung ultrasound scoring system (A–D figures displayed ultrasound images of neonates with COVID‐19 pneumonia. Because there were no COVID‐19 neonates scored 3, so E and F figures displayed ultrasound images of other neonatal pneumonia for demonstration). (A) Score 0: normal aeration, normal pleural line (triangle), and A‐lines (arrow). (B) Score 1: moderate loss of aeration and ≥3 well‐spaced B‐lines (arrow). (C) Score 2: severe loss of aeration and confluent B‐lines (arrow). (D) Score 2: severe loss of aeration, confluent B‐lines (arrow), and small subpleural consolidation (triangle) with interrupted pleural line. (E) Score 3: lung consolidation (arrow) with air‐bronchograms (triangle) and pleural effusion (star). (F) Score 3: lung consolidation (arrow) with fluid‐bronchograms (triangle) and pleural effusion (star). COVID‐19, coronavirus disease 2019 [Color figure can be viewed at wileyonlinelibrary.com]
LUSS was independently assessed by two experienced reviewers for inter‐observer reproducibility. Intra‐observer analysis was performed using the recorded loops 4 weeks after the initial reading was conducted.
2.3. Statistical analysis
Statistical analysis was performed using SPSS Version 23.0 (IBM Corp.) and MedCalc Version 16.2.1 (MedCalc Software). Continuous variables were expressed as mean ± standard deviations. Variances were compared through F test. Continuous variables were compared via the independent sample t test. Categorical variables were compared through the χ 2 test.
The inter‐ and intra‐observer reproducibility of LUSS was assessed using Bland–Altman plot, coefficient of variation, Pearson's correlation, and intra‐class correlation coefficient (ICC). The coefficient of variation was defined as the standard deviation of differences between two readings in the percentage of the mean. p < .05 were considered statistically significant.
3. RESULTS
3.1. Clinical information
Demographic characteristics and clinical features of the COVID‐19 group and control group were described in Table 2. There were no differences in demographic characteristics between the two groups. In the COVID‐19 group, two babies born preterm and nine babies born at term. Most of the neonates initially experienced atypical and mild symptoms, that is, shortness of breath, vomiting, bucking, and lethargy. Two babies showed low‐grade fever, with the others normal. There were two asymptomatic newborns. Radiographic findings commonly showed peripheral ground‐glass opacities (GGO) and patch shadows, with nonspecific findings in three babies. Most of the babies showed elevated creatine kinase myocardial band (CK‐MB) (83.5 ± 39.5 U/L, reference range 24 U/L), with normal echocardiographic results. There were five (45%) neonates with SARS‐CoV‐2 by nasopharyngeal swabs testing positive, and six neonates with SARS‐CoV‐2 IgM positive. The serum IgM and IgG levels in the COVID‐19 group were 29.5 ± 27.7 AU/ml and 73.9 ± 31.7 AU/ml, respectively.
Table 2.
Demographic characteristics and clinical features
| Variables | COVID‐19 group (n = 11) | Control group (n = 11) | p |
|---|---|---|---|
| Males (%) | 3 (27.3%) | 3 (27.2%) | .647 |
| Age (days) | 3.8 ± 5.2 | 3.5 ± 4.7 | .848 |
| Wt (g) | 3050 ± 609 | 3240 ± 477 | .761 |
| Ht (cm) | 50.0 ± 2.0 | 49.1 ± 2.3 | .979 |
| HR (bpma) | 136.4 ± 8.0 | 136.7 ± 11.0 | .356 |
| R (bpmb) | 41.2 ± 7.4 | 40.3 ± 4.0 | .133 |
| T (°C) | 36.5 ± 0.4 | 36.5 ± 0.5 | .687 |
| RBC (109/L) | 3.8 ± 0.6 | 4.7 ± 1.0* | .008 |
| WBC (1012/L) | 9.6 ± 3.7 | 10.2 ± 2.6 | .177 |
| LYM (109/L) | 3.3 ± 1.6 | 4.5 ± 1.4 | .632 |
| NEU (109/L) | 3.6 ± 0.6 | 5.5 ± 2.5 | .849 |
| hsCPR (mg/L) | 1.1 ± 1.0 | 1.5 ± 0.8 | .605 |
Note: Variables are expressed as means ± SD.
Abbreviations: HR, heart rate; hsCPR, hypersensitive C‐reactive protein; Ht, height; LYM, lymphocyte count; NEU, neutrophil count; R, frequency of respiratory; RBC, red blood cell count; T, temperature; WBC, white blood cell count; Wt, weight.
Beats per minute.
Breaths per minute.
p < .05 is statistically significant.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
3.2. LUS and LUSS
In the COVID‐19 group, there were a total of 132 regions reviewed and 83 regions (62.8%) with detected abnormities. Each patient showed lesions in bilateral lungs and multiple regions. There were maximal 10 regions, average 7.5 regions involved among 12 regions per subject. The majority of detectable ultrasonic findings were sparse or confluent B‐lines in the nonconsolidated regions (83 regions, 62.8%), including sparse (73 regions, 55.3%) B‐lines and confluent B‐lines (10 regions, 7.6%); disappearing A‐lines (83 regions, 62.8%); irregular and interrupted pleural lines (29 regions, 21.9%), small subpleural consolidations (2 regions, 1.5%), and no pleural effusion in patients. Forty‐nine regions (37%) were normal and scored 0, 73 regions (55%) scored 1, 10 regions (8%) scored 2, and no regions scored 3. The global LUSS was higher in the COVID‐19 group than that in the control group. The regions with higher scores were primarily located in bilateral inferior and posterior regions (regions L4, L5, L6, R3, R4, R5, and R6) (Table 3 and Figure 3). LUS detected abnormalities in 3 COVID‐19 neonates with normal radiographic imaging.
Table 3.
Comparison of regional lung ultrasound score between the COVID‐19 group and the control group
| Lung region | COVID‐19 group (n = 11) | Control group (n = 11) | P |
|---|---|---|---|
| L1 | 0.18 ± 0.40 | 0.09 ± 0.30 | .557 |
| L2 | 0.45 ± 0.50 | 0.18 ± 0.41 | .187 |
| L3 | 0.09 ± 0.30 | 0 | .341 |
| L4 | 1.00 ± 0.00 | 0.18 ± 0.41* | .000 |
| L5 | 1.18 ± 0.41 | 0.27 ± 0.47* | .000 |
| L6 | 1.00 ± 0.63 | 0.36 ± 0.51* | .017 |
| R1 | 0.09 ± 0.30 | 0 | .341 |
| R2 | 0.36 ± 0.51 | 0.18 ± 0.41 | .362 |
| R3 | 0.64 ± 0.51 | 0.09 ± 0.30* | .007 |
| R4 | 0.91 ± 0.30 | 0.09 ± 0.30* | .000 |
| R5 | 1.18 ± 0.60 | 0.27 ± 0.47* | .000 |
| R6 | 1.27 ± 0.47 | 0.55 ± 0.52* | .003 |
| All regions | 8.40 ± 1.70 | 2.30 ± 1.40* | 1.9 × 10−8 |
Note: Variables are expressed as means ± SD.
p < .05 is statistically significant.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Figure 3.
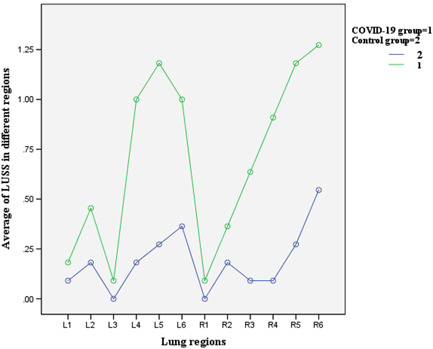
Comparison of LUSS in different lung regions between the COVID‐19 group and the control group. COVID‐19, coronavirus disease 2019; LUSS, lung ultrasound score [Color figure can be viewed at wileyonlinelibrary.com]
3.3. Intra‐ and inter‐observer reproducibility of LUSS
Bland–Alterman plot revealed that the LUSS derived by two readings from intra‐ and inter‐observers, were highly consistent (Figure 4). Both the intra‐ and inter‐observer reproducibility were excellent, with ICCs of 0.930 and 0.854, respectively. Intra‐observer reproducibility of LUSS slightly better than that of inter‐observer (Table 4).
Figure 4.
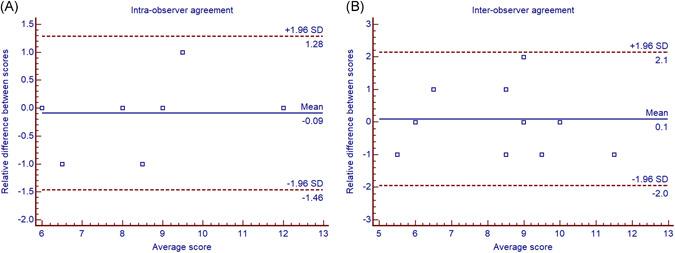
Bland–Altman plot showing the intra‐observer and inter‐observer agreement on LUSS. LUSS, lung ultrasound score [Color figure can be viewed at wileyonlinelibrary.com]
Table 4.
Intra‐ and inter‐observer reproducibility of LUSS
| Intra‐observer | Inter‐observer | |||||
|---|---|---|---|---|---|---|
| CV (%) | R (Pearson's) | ICC (95% CI) | CV (%) | R (Pearson's) | ICC (95% CI) | |
| LUSS | 8.5% | 0.931* | 0.930 (0.765–0.981) | 13.0% | 0.844* | 0.854 (0.543–0.959) |
Abbreviation: LUSS, lung ultrasound score.
p < .05 is statistically significant.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
4. DISCUSSION
The SARS‐CoV‐2 particles enter the airways and lungs, and could reach the bronchioles and alveoli. Therefore, the lesions are mostly located in the terminal alveoli and subpleural. 5 The pathologic findings 6 of COVID‐19 showed diffuse lesions of the alveoli with mucus exudation, which caused consolidation of the lung. Infants and children are also considered to be susceptible to SARS‐CoV‐2 infection, and mainly infected from family cluster outbreaks in China. 7 Compared with infected adults, infected children experience differently, and most of them appear to bear milder clinical course. Our colleague has reported 1 that three neonates were identified the COVID‐19 pneumonia in 33 neonates born to mothers with COVID‐19. The clinical data in the present study demonstrated a similar scene, and most of the COVID‐19 neonates showed mild symptoms, mainly involving respiratory and digestive systems. Meanwhile, transient mild myocardial injury may exist in the majority of patients, due to rapid CK‐MB recovery and normal cardiac function. However, asymptomatic patients are not uncommon in the pediatric population, some of whom show radiologic findings of pneumonia. 8 Therefore, it is important for us to be able to recognize the pediatric COVID‐19 patients, using medical imaging modalities. 9
CT should be a primary diagnostic tool for COVID‐19, with different features in the pediatric population when compared to adults. Most children recover in the early stage, and very few of them progress into the advanced and critical stages. Hence, subpleural lesions with localized inflammatory infiltration, along with no signs of pleural effusion or lymphadenopathy, are easily detected in most pediatric patients on chest CT. 2 , 9 Meanwhile, compared to adults, the lesions distribution is less extensive and some atypical GGO appear in children. In the current investigation, chest CT or X‐ray demonstrated similar nonspecific or mild changes in neonates. The peripheral distribution of lesions makes ultrasound detection relatively easier. 10
LUS cannot create direct imaging of the pulmonary parenchyma, but can be used for the diagnosis through utilizing artifacts produced by different pathological changes. The state of aeration of lung parenchyma is a measure of its “air/fluid ratio.” This ratio determines the characteristics of the image produced by LUS. All LUS findings described in adults are alike in neonates and children, in both normal and pathological conditions. 11 Given the small size of the neonates' chest, a linear probe allows the best visualization of the lungs in most cases, irrespective of the depth of the main target of the examination. A number of studies have described the benefits of LUS in the diagnosis of transient tachypnea of the newborn, respiratory distress syndrome, bronchiolitis in neonates. Compared with chest X‐ray, LUS is valuable in detecting pediatric pneumonia with excellent sensitivity and specificity, especially companied with lung consolidation. 12 However, it can reduce 38.8% chest X‐ray usage 13 in the pediatric population.
Considering children's higher radio‐sensitivity and free radiation of this technique, neonates may benefit from LUS. Launching a LUS in the neonatal intensive care unit (NICU) roughly halved the number of chest radiograms and significantly decreased the mean radiation dose. 14 In fact, LUS exam during the COVID‐19 outbreak should be as focused as necessary to obtain diagnostic views, 10 but should also be adequate to avoid return to the isolation ward. Each exam should be tailored to the indication and planned in advance.
In the current study, LUS presented most lung regions involved, with the lesions being identified as bilateral and diffuse. Lesions distribution suggested that bilateral inferior and posterior regions were mostly involved, which were similar to previous CT findings 5 , 9 and the lesion distribution on radiographic images in the present study. Abnormalities in B‐lines and A‐lines were the most common signs, covering all of the infected regions. There may be a few B‐lines in the lung fields of normal neonates at the age of 3–7 days. The abnormal visibility of B‐lines, usually accompanied with the disappearance of A‐lines, represents fluid accumulation at the alveolar level and lobular space, decreased air/liquid ratio, and pulmonary function impairment in varying degrees. Pulmonary edema, which is a typical sign in neonatal patients detected by LUS, has been previously reported as one of the major pathological findings in patients with COVID‐19 pneumonia. 6 When confluent B‐lines spread throughout the lung field, the ultrasound shows a compact B‐line pattern representing severe pulmonary edema, which was not observed in our study due to mildly symptomatic neonatal patients. 15 LUS shows a high sensitivity and specificity to detect lung edema throughout B‐line appearance, however, it is challenging to identify the etiology of edema, that is, cardiogenic, nephrogenic, or pneumonia. 16 We propose that it could be combined with other clinical and ultrasonic assessments to give a comprehensive judgment of the situation. Subpleural consolidation is another typical sign in neonatal COVID‐19. It indicates that the lung tissue becomes non‐aerated, resulting in tissue‐like echotexture. If residual gas or liquid in the bronchi is visible, air bronchogram or fluid bronchogram can be presented. In our study, we only observed two small subpleural consolidations, one of which was consistent with a small subpleural nodule in CT image, and the other was undetectable in X‐ray image. In the meantime, the abnormal LUS findings detected in patients with normal radiography made us believe that LUS is a sensitive diagnostic tool of neonatal COVID‐19 pneumonia. According to previous research regarding LUS application in the COVID‐19 pediatric population, when compared with the gold standard of chest CT, chest X‐ray displays false‐negative results for pulmonary involvement in 75%, whereas for LUS it is 16.7%. 10 Furthermore, LUS findings are more sensitive than chest X‐ray in pediatric patients with COVID‐19 infection, especially in the early stage of the disease and in mild cases. 17
Since LUS detects the artifacts generated by the accumulation of fluid, we could rank the artifacts according to the air/liquid ratio and create a score reflecting lung aeration. LUSS, a three‐stage classification system, could comprehensively and semi‐quantitatively reflect the lung aeration function and disease severity. LUSS is well correlated with indices of oxygenation in both term and preterm neonates, 18 guiding surfactant therapy and weaning ventilator support. 19 LUSS could assess global and regional lung aeration, and well correlated with CT quantitative analysis indices 20 in ARDS. In the present study, the global LUSS was obviously higher in the COVID‐19 group, in consistent with patients' symptoms. Patients with two lowest scores were asymptomatic. Two patients, in whom regions scored 2 were more than three regions, presented with obvious shortness of breath and digestive symptoms, along with positive chest radiographic findings. The regional score also reflected variant loss of lung aeration and severity of infectious lesions, especially in bilateral inferior and posterior regions. LUSS calculation shows a high intra‐ and inter‐observer agreement regardless of the observer's experience, 18 which has also been validated in our study.
One limitation of the study was the small sample size due to low morbidity in neonates and control of epidemics in Wuhan. The other limitation was the retrospective study, and few positive cases were not enrolled due to lack of timely LUS examination. However, the missing cases were fundamentally with mild clinical course, and the study was representative.
5. CONCLUSIONS
In the present study, we presented the typical ultrasonic features of neonatal COVID‐19 pneumonia using a bedside ultrasound machine. LUSS, with excellent intra‐observer and inter‐observer reproducibility, can semi‐quantitatively assess global and regional lung aeration and disease severity. LUS findings are in accordance with clinical symptoms and radiographic manifestations. In clinic settings, we should keep in mind that combining the imaging findings with clinic, epidemiological, and etiological evidence to diagnose COVID‐19. However, LUS is a free radiational, convenient, noninvasive, reproducible, and reliable imaging modality, and can be used as an alternative to the use of diagnostic radiography in neonatal COVID‐19 pneumonia diagnosis and monitoring.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Wei Li, Manli Fu, and Li Yuan contributed to the study design, analysis, interpretation of the results, and draft of the manuscript. Chao Qian and Yue Hong contributed to the ultrasound image reading and scoring. Lingkong Zeng, Huan Zhou, and Xin Liu contributed to the data collection, analysis, and interpretation of the results. Xuehua Peng contributed to the radiographic reading and interpretation of the results. All authors critically revised the manuscript and approved the final version.
ACKNOWLEDGMENTS
This study was supported in part by the Health Commission of Hubei Province (WJ2019Q002), Science and Technology Department of Hubei Province (2019CFC892), and Health Commission of Wuhan City (EX20E07).
Li W, Fu M, Qian C, et al., Quantitative assessment of COVID‐19 pneumonia in neonates using lung ultrasound score. Pediatric Pulmonology. 2021;56:1419–1426. 10.1002/ppul.25325
Wei Li and Manli Fu contributed equally to this study.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study titled “Quantitative assessment of COVID‐19 pneumonia in neonates using lung ultrasound score” are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Zeng L, Xia S, Yuan W, et al. Neonatal early‐onset infection with SARS‐CoV‐2 in 33 neonates born to mothers with COVID‐19 in Wuhan, China. JAMA Pediatr. 2020;174(7):722‐725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ma H, Hu J, Tian J, et al. A single‐center, retrospective study of COVID‐19 features in children: a descriptive investigation. BMC Med. 2020;18(1):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mongodi S, Bouhemad B, Orlando A, et al. Modified lung ultrasound score for assessing and monitoring pulmonary aeration. Ultraschall Med. 2017;38(5):530‐537. [DOI] [PubMed] [Google Scholar]
- 4. Wang L, Shi Y, Xiao T, et al. Chinese expert consensus on the perinatal and neonatal management for the prevention and control of the 2019 novel coronavirus infection (First edition). Ann Transl Med. 2020;8(3):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xu YH, Dong JH, An WM, et al. Clinical and computed tomographic imaging features of novel coronavirus pneumonia caused by SARS‐CoV‐2. J Infect. 2020;80(4):394‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jiehao C, Jin X, Daojiong L, et al. A Case Series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin Infect Dis. 2020;71(6):1547‐1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lu X, Zhang L, Du H, et al. SARS‐CoV‐2 infection in children. N Engl J Med. 2020;382(17):1663‐1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xia W, Shao J, Guo Y, Peng X, Li Z, Hu D. Clinical and CT features in pediatric patients with COVID‐19 infection: different points from adults. Pediatr Pulmonol. 2020;55(5):1169‐1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hizal M, Aykac K, Yayla BCC, et al. Diagnostic value of lung ultrasonography in children with COVID‐19. Pediatr Pulmonol. 2020:551‐560. [DOI] [PubMed] [Google Scholar]
- 11. Lichtenstein DA, Mauriat P. Lung ultrasound in the critically ill neonate. Curr Pediatr Rev. 2012;8(3):217‐223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shah VP, Tunik MG, Tsung JW. Prospective evaluation of point‐of‐care ultrasonography for the diagnosis of pneumonia in children and young adults. JAMA Pediatr. 2013;167(2):119‐125. [DOI] [PubMed] [Google Scholar]
- 13. Jones BP, Tay ET, Elikashvili I, et al. Feasibility and safety of substituting lung ultrasonography for chest radiography when diagnosing pneumonia in children: a randomized controlled trial. Chest. 2016;150(1):131‐138. [DOI] [PubMed] [Google Scholar]
- 14. Escourrou G, De Luca D. Lung ultrasound decreased radiation exposure in preterm infants in a neonatal intensive care unit. Acta Paediatr. 2016;105(5):e237‐e239. [DOI] [PubMed] [Google Scholar]
- 15. Raimondi F, Migliaro F, Sodano A, et al. Can neonatal lung ultrasound monitor fluid clearance and predict the need of respiratory support? Crit Care. 2012;16(6):R220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dietrich CF, Mathis G, Blaivas M, et al. Lung B‐line artefacts and their use. J Thorac Dis. 2016;8(6):1356‐1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Türe E, Korkmaz MF, Aksoy FD, et al. Point‐of‐care lung ultrasound findings in the pediatric emergency clinic during the COVID‐19 pandemic. J Clin Ultrasound. 2021;49(2):85‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brat R, Yousef N, Klifa R, Reynaud S, Shankar Aguilera S, De Luca D. Lung ultrasonography score to evaluate oxygenation and surfactant need in neonates treated with continuous positive airway pressure. JAMA Pediatr. 2015;169(8):e151797. [DOI] [PubMed] [Google Scholar]
- 19. El Amrousy D, Elgendy M, Eltomey M, Elmashad AE. Value of lung ultrasonography to predict weaning success in ventilated neonates. Pediatr Pulmonol. 2020;55(9):2452‐2456. [DOI] [PubMed] [Google Scholar]
- 20. Chiumello D, Mongodi S, Algieri I, et al. Assessment of lung aeration and recruitment by CT scan and ultrasound in acute respiratory distress syndrome patients. Crit Care Med. 2018;46(11):1761‐1768. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study titled “Quantitative assessment of COVID‐19 pneumonia in neonates using lung ultrasound score” are available from the corresponding author upon reasonable request.


