Abstract
Aim
The object of this work was to study how National Health Service hospitals in England and Wales aimed to maintain effective and safe colorectal cancer (CRC) services during the first peak of the COVID‐19 pandemic (April 2020).
Method
A national survey was performed among all 148 hospitals providing CRC services. Information was collected about changes in referrals, diagnostic, staging and therapeutic procedures, as well as whether there was access to a ‘cold site’ (a hospital facility free of COVID‐19). Clinicians in each hospital were also asked to give the ‘single most important lesson learned’ about keeping services safe and effective.
Results
Full responses were received from 123 (83%) hospitals, and information about ‘cold sites’ was available for 146 (99%). Eighty hospitals (54%) had access to a ‘cold site’ and this was increased in regions with higher COVID‐19 infection rates (p <0.001). Of the 123 responding hospitals, 105 (85%) indicated that referrals of patients with suspected CRC had dropped by at least 30%, and 69 (56%) indicated that treatment plans were altered in at least 50% of CRC patients. However, ‘cold site’ availability protected the capacity for diagnostic colonoscopy (p = 0.013) and CRC resection (p = 0.010). Many ‘lessons learned’ highlighted the importance of adequate structural service organization, often mentioning ‘cold sites’ and regional coordination as examples, good communication and triage of patients based on clinical urgency.
Conclusion
Access to ‘cold sites’, as well as regional coordination, clear communication and strong leadership, were found to be pivotal in maintaining capacity for diagnosis and treatment of CRC during the COVID‐19 surge.
Keywords: cold site, colorectal cancer, COVID‐19
What does this paper add to the literature?
NHS hospitals providing colorectal cancer services in England and Wales have demonstrated that access to ‘cold sites’ helped to protect capacity for diagnostic colonoscopy and colorectal cancer resection during COVID‐19. The ‘lessons learned’ highlight the importance of adequate structural organization, good communication and triage of patients based on urgency
INTRODUCTION
The first peak of the COVID‐19 pandemic had a large impact on colorectal cancer (CRC) services in many countries. Early reports suggest that patients undergoing cancer treatments were at increased risk of COVID‐19 infection, the requirement for critical care, and mortality [1, 2, 3]. Despite the roll‐out of vaccination programmes, the COVID‐19 pandemic continues to disrupt CRC services due to the direct risks of COVID‐19 to vulnerable CRC patients as well as the diversion of resources to other areas in the healthcare system, increased levels of staff sickness, and the need for social isolation among the clinical workforce [4, 5].
Diagnostic delays as a result of suspended screening programmes, reduced access, and availability for diagnostic services and changes in health‐seeking behaviours due to fear of exposure to COVID‐19 have sparked concerns that a rise in late cancer diagnoses may increase cancer mortality in the long term [6]. For example, in the UK, urgent primary care referrals for suspected cancer dropped by 60% in April 2020 compared with the previous year, and modelling exercises have estimated that diagnostic delays may lead to an increase of up to 16.6% in avoidable deaths from CRC [7, 8].
To maintain treatment capacity and to protect patients during the COVID‐19 pandemic, cancer services have increased the use of nonoperative treatments [9] and created ‘cold sites’ for elective treatments [10]. ‘Cold sites’ are hospital facilities set up to be COVID‐19 free, or almost so. This environment was achieved in the initial wave by screening patients for COVID‐19 prior to admission, strict segregation of elective and emergency surgical services, and geographical separation of the ‘cold’ facility from where patients with COVID‐19 were treated. More recently, regular staff testing for asymptomatic COVID‐19 has also been used [11, 12].
The National Bowel Cancer Audit (NBOCA) carried out a national study of CRC services during COVID‐19 to map the response of the National Health Service (NHS) in England and Wales. The primary aim of the study was to assess the capacity for elective CRC surgery and diagnostic colonoscopy in relation to the provision of ‘cold sites’ during the first peak of the pandemic. These results were compared at a regional level to assess whether the availability of ‘cold sites’ for CRC surgery varied according to local COVID‐19 infection rates. We also created a national snapshot of the impact of the pandemic on other aspects of CRC diagnostic and treatment pathways in England and Wales.
In addition, we asked the CRC providers to describe the ‘single most important lesson learned’ about how to make services for CRC patients as safe and effective as possible during the COVID‐19 pandemic. We present these lessons according to whether they relate to the diagnostic and staging aspect or the therapeutic aspect of the CRC treatment pathway, COVID‐19‐specific measures, the structural organization of CRC services, or leadership and management (human factors).
METHOD
Regional cumulative COVID‐19 rates
Total confirmed ‘cumulative’ COVID‐19 diagnoses from 11 January 2020 to 15 April 2020 were obtained for English local authorities and Wales [13]. For England, local authorities were mapped to the 21 regions that represent the NHS Cancer Alliances, the organizational structures set up to enable cancer care to be more effectively planned across local cancer care pathways [14]. Regional cumulative COVID‐19 rates to 15 April 2020 per 100 000 population were calculated using English and Welsh population estimates [15].
National survey
All 148 NHS English hospital trusts and Welsh hospital boards providing CRC care in England and Wales according to the NBOCA were requested in June and July 2020, via a national survey, to provide information about the CRC services they provided in mid‐April 2020 (see Appendix 1). The survey was distributed to leading NBOCA clinicians, and was therefore predominantly completed by consultant colorectal surgeons.
NHS hospital trusts and hospital boards are the legal entities responsible for providing publicly funded secondary care in England and Wales, respectively. A hospital trust or hospital board can consist of one or more hospital sites, but in this paper, ‘hospital’ is used to refer to a hospital trust or hospital board.
The requested information covered five COVID‐19‐related changes with respect to numbers of referrals for newly diagnosed patients, planned CRC treatments, delivery of CRC services, access to COVID‐19 surgical ‘cold sites’, and the ‘single most important lesson learned’ (Appendix 1). The ‘lessons learned’ were provided as free text.
Quantitative analysis
The regional cumulative COVID‐19 rates were grouped into high and low based on a threshold corresponding to the overall median value of cumulative COVID‐19 rates for the English Cancer Alliances and Wales. English hospitals were assigned to a group according to which Cancer Alliance they are in. The two hospitals with missing data for access to a ‘cold site’ were considered not to have such access for the purposes of further analysis.
The primary outcome for the study was the capacity to maintain elective CRC surgery and diagnostic colonoscopy dependent on the provision of ‘cold sites’.
Chi‐square tests were used to compare proportions. Spearman's rank correlation coefficient was used to test the association between cumulative COVID‐19 rates and access to surgical ‘cold sites’. The level of statistical significance was set at 0.05.
Free‐text analysis
The free‐text responses to the ‘single most important lesson learned’ were independently analysed by two reviewers (JB and JvdM) in three steps. First, responses were broken down into statements. If responses contained more than one statement these were analysed separately. Second, the statements were grouped into broader ‘lessons learned’. Each statement could link to multiple ‘lessons learned’ if applicable. Third, the ‘lessons learned’ were organized into five categories (diagnostic and staging pathway, treatment pathway, COVID‐specific measures, structural organization, leadership and management) and linked to five generally recognized domains of healthcare quality which were defined a priori: ‘effectiveness’ (delivering care to all patients who are expected to benefit); ‘safety’ (avoiding harm to patients from care that is intended to help them); ‘efficiency’ (avoiding waste of facilities, equipment and staff); ‘responsiveness’ (responding to patients’ specific conditions and situations); and ‘patient experience’ (providing care that is respectful and guided by patient preferences) [16]. The ‘lessons learned’ could link to more than one domain as appropriate. Disagreements were resolved through discussion.
RESULTS
Full responses were received from 123 (83%) hospitals. Information about ‘cold sites’ was available for 146 (99%).
Cumulative COVID‐19 rates and access to surgical ‘cold sites’
Overall, 80 of the 148 hospitals (54%) were found to have access to ‘cold sites’ for CRC surgery (Table 1, Figure 1). Over half of these (41 hospitals), reported access to a ‘cold site’ in the independent sector. There was considerable regional variation among the 21 English regions and Wales. In four of these 22 regions (18%), 25% or fewer of the hospitals had access to a ‘cold’ site, and in four regions (18%), all hospitals had access to such a site. There was strong evidence of an association between the regional cumulative COVID‐19 infection rate per 100 000 population and the proportion of providers reporting access to surgical ‘cold sites’ (p < 0.001) (Figure 2).
TABLE 1.
Response rate and access to ‘cold sites’ for colorectal cancer surgery by English region and Wales, ranked according to cumulative COVID‐19 rate per 100 000 population mid‐April 2020
| English regions and Wales | Cumulative COVID−19 rate per 100 000 population | No. of hospitals providing CRC services | No. with surgical ‘cold site’ access (%) | No. of complete responders (%) |
|---|---|---|---|---|
| England and Wales | 159.0 | 148 | 80 (54) | 123 (83) |
| Peninsula | 65.2 | 5 | 1 (20) | 4 (80) |
| Somerset, Wiltshire, Avon and Gloucestershire | 85.4 | 8 | 5 (63) | 8 (100) |
| Humber, Coast and Vale | 86.0 | 4 | 0 (0) | 3 (75) |
| East of England – North | 86.3 | 7 | 2 (29) | 6 (86) |
| West Yorkshire and Harrogate | 105.1 | 6 | 0 (0) | 6 (100) |
| East Midlands | 105.9 | 6 | 3 (50) | 4 (67) |
| Wessex | 107.9 | 8 | 3 (38) | 6 (75) |
| Surrey and Sussex | 130.8 | 9 | 3 (33) | 8 (89) |
| Kent and Medway | 144.6 | 4 | 2 (50) a | 1 (25) |
| Thames Valley | 144.7 | 4 | 2 (50) | 4 (100) |
| East of England – South | 149.3 | 8 | 4 (50) a | 5 (63) |
| West Midlands | 151.5 | 14 | 9 (64) | 12 (86) |
| Greater Manchester | 183.2 | 8 | 6 (75) | 8 (100) |
| Lancashire and South Cumbria | 190.8 | 4 | 1 (25) | 4 (100) |
| South Yorkshire and Bassetlaw | 193.1 | 5 | 3 (60) | 5 (100) |
| Northern | 195.8 | 8 | 5 (63) | 5 (63) |
| Cheshire and Merseyside | 201.3 | 9 | 7 (78) | 7 (78) |
| North Central London | 204.7 | 4 | 4 (100) | 2 (50) |
| North East London | 212.1 | 3 | 3 (100) | 3 (100) |
| Wales | 226.0 | 11 | 4 (36) | 10 (91) |
| RM Partners (West London) | 256.3 | 9 | 9 (100) | 8 (89) |
| South East London | 272.9 | 4 | 4 (100) | 4 (100) |
These regions each had a single nonresponder. The nonresponders were assumed not to have access to ‘cold sites’.
Bold values indicate the overarching results for the whole of England and Wales.
FIGURE 1.
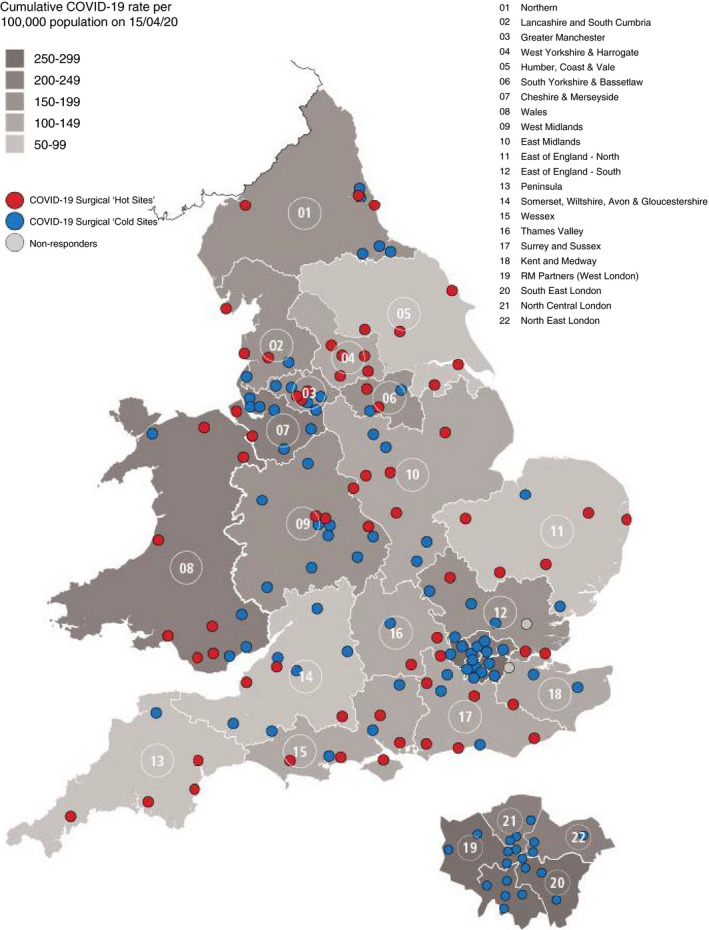
Map of reported ‘cold sites’ for colorectal cancer surgery by English region and Wales
FIGURE 2.
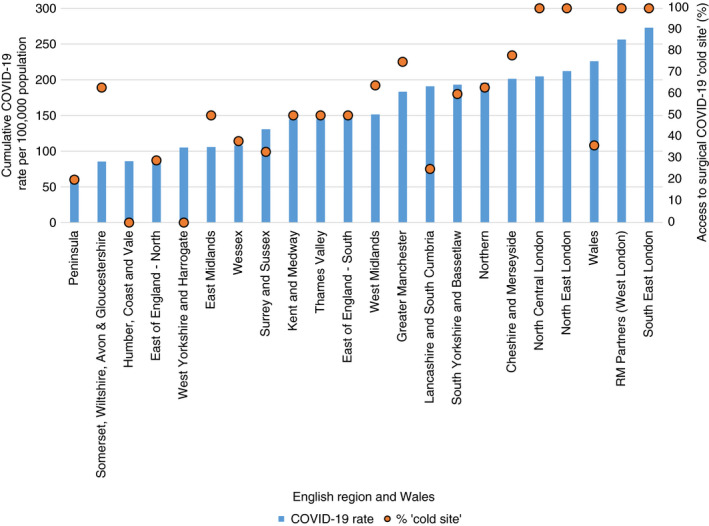
Cumulative COVID‐19 rate per 100 000 population and access to surgical COVID‐19 ‘cold site’ by English region and Wales
Referrals
The majority of hospitals reported a significant reduction in the number of urgent referrals: only 6 of the 123 hospitals (5%) that responded reported no reduction (more than 90% of the usual number of referrals), 12 hospitals (10%) reported a small reduction in numbers (71% to 90% of the usual number), 95 hospitals (77%) reported a large reduction in numbers (20% to 70% of the usual of number) and 10 hospitals (8%) reported very few referrals (0% to 19% of the usual number).
Changes to planned CRC treatments
Of the 123 responding hospitals, 69 (56%) indicated that, in response to COVID‐19 risks, treatment plans had been altered in more than 50% of their patients (Figure 3). Again, considering changes that affected more than 50% of their patients, 61 hospitals (50%) reported delays in treatments due to COVID‐19 infection risks, 61 (50%) delays in tissue diagnosis and radiological staging, and 57 (46%) delays in treatment due to diversion of resources. The COVID‐19 pandemic had a smaller impact on the length and type of chemotherapy treatment (47 hospitals (38%) reported changes in more than 50% of their patients), and on the use of temporizing treatments (23 hospitals (19%) reported changes in more than 50% of their patients) such as the stenting of obstructing cancers and radiotherapy for rectal cancer with a ‘long wait’.
FIGURE 3.
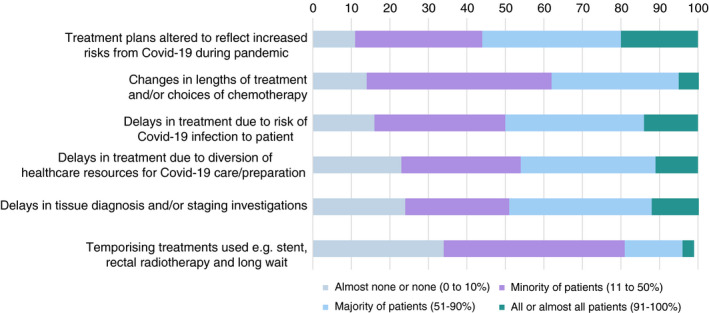
Impacts of the COVID‐19 pandemic on the management of colorectal cancer patients in England and Wales
Changes to provision of CRC services
The majority of providers reported that CRC services were running at less than 70% of their usual capacity for diagnostic and therapeutic activity (Table 2). Among the 123 responding hospitals, complete cessation of activity (0% to 10% of usual activity) was reported by 95 hospitals (77%) for diagnostic colonoscopy, 72 (59%) for lung resection, 67 (54%) for liver resection, 28 (23%) for CRC resection, 22 (18%) for adjuvant chemotherapy, and 21 (17%) for neo‐adjuvant chemoradiotherapy.
TABLE 2.
Provision of colorectal cancer services in mid‐April by cumulative COVID‐19 rate
| Response rate (%) (n = 123) | Cumulative COVID‐19 rate | p‐value | |||
|---|---|---|---|---|---|
| High (%) | Low (%) | ||||
| Diagnostic colonoscopy activity | |||||
| 0%–10% of usual | 95 (77) | 50 (74) | 45 (82) | ||
| 11%–70% of usual | 26 (21) | 17 (25) | 9 (16) | 0.505 | |
| 71%–100% of usual | 2 (2) | 1 (1) | 1 (2) | ||
| Colorectal resection activity | |||||
| 0%–10% of usual | 28 (23) | 17 (25) | 11 (20) | ||
| 11%–70% of usual | 53 (43) | 27 (40) | 26 (47) | 0.671 | |
| 71%–100% of usual | 42 (34) | 24 (35) | 18 (33) | ||
| Liver resection activity | |||||
| 0%–10% of usual | 67 (54) | 39 (57) | 28 (51) | ||
| 11%–70% of usual | 40 (33) | 20 (29) | 20 (36) | 0.708 | |
| 71%–100% of usual | 16 (13) | 9 (13) | 7 (13) | ||
| Lung resection activity | |||||
| 0%–10% of usual | 72 (59) | 43 (63) | 29 (53) | ||
| 11%–70% of usual | 36 (29) | 17 (25) | 19 (35) | 0.462 | |
| 71%–100% of usual | 15 (12) | 8 (12) | 7 (13) | ||
| Neo‐adjuvant chemoradiotherapy activity | |||||
| 0%–10% of usual | 21 (17) | 9 (13) | 12 (22) | ||
| 11%–70% of usual | 65 (53) | 39 (57) | 26 (47) | 0.383 | |
| 71%–100% of usual | 37 (30) | 20 (29) | 17 (31) | ||
| Adjuvant chemotherapy activity | |||||
| 0%–10% of usual | 22 (18) | 11 (16) | 11 (20) | ||
| 11%–70% of usual | 79 (64) | 47 (69) | 32 (58) | 0.433 | |
| 71%–100% of usual | 22 (18) | 10 (15) | 12 (22) | ||
There was no statistically significant association between high regional COVID‐19 rates and the provision of CRC services (Table 2). However, providers with access to ‘cold sites’ for CRC surgery were significantly more likely to retain higher levels of activity for diagnostic colonoscopy (p = 0.013), CRC resection (p = 0.010), lung resection (p = 0.026), neo‐adjuvant chemoradiotherapy (p = 0.010) and adjuvant chemotherapy (p = 0.050), with weaker statistical evidence for a similar difference for liver resection (p = 0.096) (Table 3).
TABLE 3.
Provision of colorectal cancer services in mid‐April by the availability of surgical ‘cold sites’
| Response rate (%) (n = 123) | Surgical ‘cold site’ access | P value | ||
|---|---|---|---|---|
| Yes (%) | No (%) | |||
| Diagnostic colonoscopy activity | ||||
| 0%–10% of usual | 95 (77) | 39 (66) | 56 (88) | |
| 11%–70% of usual | 26 (21) | 18 (31) | 8 (13) | 0.013 |
| 71%–100% of usual | 2 (2) | 2 (3) | 0 (0) | |
| Colorectal resection activity | ||||
| 0%–10% of usual | 28 (23) | 7 (12) | 21 (33) | |
| 11%–70% of usual | 53 (43) | 26 (44) | 27 (42) | 0.010 |
| 71%–100% of usual | 42 (34) | 26 (44) | 16 (25) | |
| Liver resection activity | ||||
| 0%–10% of usual | 67 (54) | 27 (46) | 40 (63) | |
| 11%–70% of usual | 40 (33) | 21 (36) | 19 (30) | 0.096 |
| 71%–100% of usual | 16 (13) | 11 (19) | 5 (8) | |
| Lung resection activity | ||||
| 0%–10% of usual | 72 (59) | 30 (51) | 42 (66) | |
| 11%–70% of usual | 36 (29) | 17 (29) | 19 (30) | 0.026 |
| 71%–100% of usual | 15 (12) | 12 (20) | 3 (5) | |
| Neo‐adjuvant chemoradiotherapy activity | ||||
| 0%–10% of usual | 21 (17) | 5 (8) | 16 (25) | |
| 11%–70% of usual | 65 (53) | 30 (51) | 35 (55) | 0.010 |
| 71%–100% of usual | 37 (30) | 24 (41) | 13 (20) | |
| Adjuvant chemotherapy activity | ||||
| 0%–10% of usual | 22 (18) | 6 (10) | 16 (25) | |
| 11%–70% of usual | 79 (64) | 14 (24) | 40 (63) | 0.050 |
| 71%–100% of usual | 22 (18) | 39 (66) | 8 (13) | |
Bold values indicate statistically significant P <0.05.
Single most important lesson learned
We received 108 responses, from which 154 statements were extracted. If responders had included multiple statements these were analysed separately (see Method section) (Figure 4). These statements were mapped to 31 ‘lessons learned’, with frequencies of statements classified as each lesson. Five statements could not be classified.
FIGURE 4.
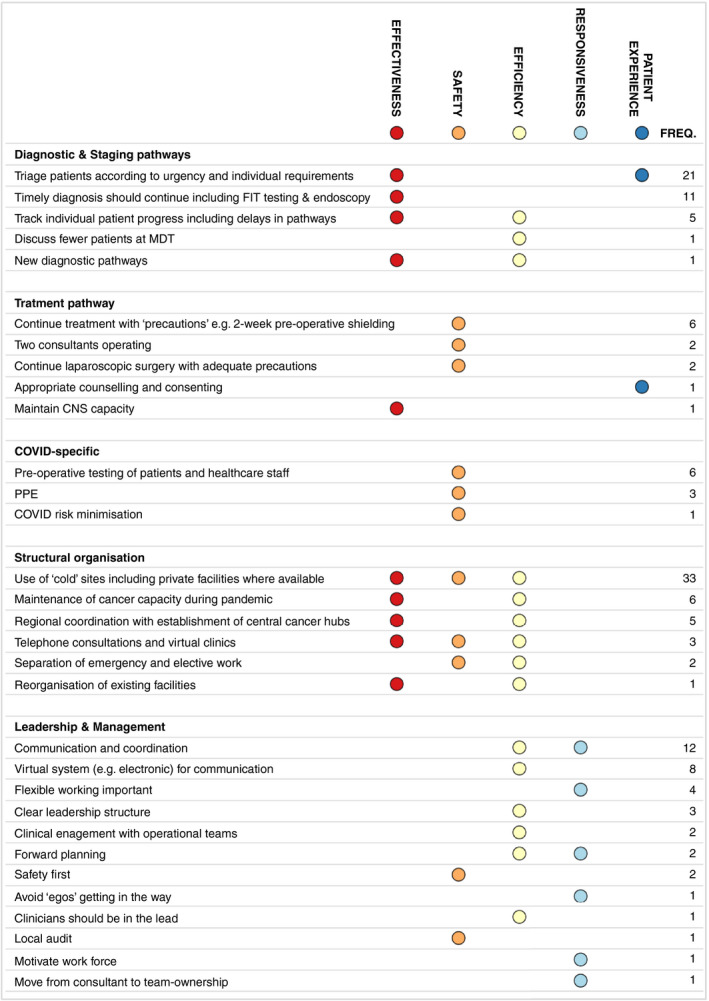
The single most important lesson about how to make CRC services as safe and effective as possible for patients during the COVID‐19 pandemic (CNS, clinical nurse specialist; FIT, faecal immunochemical testing; MDT, multidisciplinary team; PPE, personal protective equipment). (Note: FIT involves the detection of abnormal levels of blood within the stool. Patients with a negative FIT and normal haemoglobin with vague symptoms can be reassured that their risk of CRC is very low. In patients with low‐risk symptoms but a positive FIT test, an urgent referral should be completed. FIT is therefore useful as a triage tool to guide the prioritization of investigations if there is limited diagnostic capacity [37, 38])
Fifty statements related to the structural organization of the CRC services and 33 of these highlighted the importance of the use of ‘cold sites’. Thirty eight statements related to leadership and management with 12 mentioning aspects of communication and coordination and eight the role of virtual (e.g. electronic) methods of communication. Forty three statements related to aspects of the diagnostic and staging pathway with 21 focusing on the importance of triaging patients according to the urgency of their condition. Twelve statements related to aspects of the treatment pathway and 10 to COVID‐specific minimization measures.
Based on the five domains of healthcare quality (see Method section), the ‘lessons learned’ were most frequently linked to ‘effectiveness’ (87 statements), ‘efficiency’ (85 statements), and ‘safety’ (61 statements). Less frequently the ‘lessons learned’ were linked to ‘patient experience’ (22 statements) and ‘responsiveness’ (21 statements).
DISCUSSION
Main findings
During the first 2 months of the COVID‐19 pandemic there was considerable regional variation in the availability of ‘cold sites’ for CRC surgery in England and Wales, with just over half of NHS hospitals reporting access. ‘Cold sites’ were more likely to be available in regions with higher COVID‐19 rates, and their availability was significantly linked to higher retained levels of CRC surgery.
About three‐quarters of hospitals providing CRC services reported a major drop in referrals, and just over half indicated that treatment plans were altered in at least 50% of their patients, mainly because of delays in staging investigations and initiation of treatments. However, the availability of a ‘cold site’ also protected diagnostic colonoscopy activity, as well as other therapeutic activities including oncological treatments and the surgical management of advanced disease.
Many of the ‘lessons learned’ related to the structural organization of CRC services, with the setup of ‘cold sites’ often being mentioned as an example, and to human factors such as how the services were managed and led, highlighting the importance of communication and regional coordination. The ‘lessons learned’ that had a direct clinical perspective focused more frequently on the diagnostic and staging pathways than on the treatment pathway, reflecting major concerns about delays in the initiation of treatment for CRC.
Methodological considerations
The main limitation of this study is that the mapping of ‘cold sites’ is based on reports from clinicians at a time when there was no generally accepted definition of a ‘cold site’. Clinicians may have over‐reported the availability of ‘cold sites’ because this would have supported the local continuation of CRC services. Second, this study used responses from clinicians collected during the initial peak of the pandemic and therefore represents a time of greatest uncertainty with rapidly evolving and changing guidance. However, our results have enduring implications as they confirm the role of ‘cold sites’ in the maintenance of CRC surgery and diagnostic colonoscopy. Third, we were only able to establish COVID‐19 infection rates for Cancer Alliances rather than individual hospitals. We expect that if we could have captured infection rates at the hospital level our results would have shown stronger associations.
This study had an excellent response rate and therefore provides a representative picture of NHS hospitals delivering CRC services in England and Wales. An earlier national survey pertaining to UK CRC services during the pandemic included just 36 hospitals and primarily evaluated adherence to national guidelines [17].
Surgical ‘cold sites’
A key finding from this work, in line with other studies [18, 19, 20], is the pivotal role of ‘cold sites’ in maintaining CRC surgery. This is further supported by recent findings that patients undergoing elective cancer surgery at COVID‐19 ‘cold sites’ have lower rates of pulmonary complications and mortality [10]. As mentioned earlier, ‘cold sites’ were not precisely defined at the time we collected our data. Since, a broad definition has emerged that describes ‘cold sites’ as a COVID‐19‐free environment, either as a separate hospital or as separate facilities within a hospital, including an elective operating room, critical care and inpatient ward areas that are not shared with patients with COVID‐19 [10].
Additional measures employed to maintain these ‘cold sites’ have included pre‐ and postoperative self‐isolation for 14 days, preoperative COVID‐19 testing, including chest imaging, personal protective equipment (PPE) and workforce isolation with frequent and efficient point‐of‐care testing [11, 12].
Single‐centre studies have reported the use of nearby independent sector facilities, as found within our study [11, 12, 21]. However, the disadvantages of using geographically separate sites might include a lack of acute care facilities and the absence of a fixed workforce having a negative affect on the continuity of care. Careful risk assessment and selection for surgery, as well as clear communication regarding named clinician practice are vital.
Regions with higher rates of COVID‐19 were more likely to have access to ‘cold sites’. This may be explained by urban areas, with initially higher rates of COVID‐19, being more likely to have access to surgical ‘cold sites’ due to a higher density of NHS hospitals and greater availability of hospitals in the independent sector, such as the multicentre hub‐and‐spoke model for urgent cancer surgery in London [12].
Our results have also shown that ‘cold sites’ have a pivotal role in maintaining diagnostic activity, which is especially important given the 90% drop in endoscopy numbers in the initial phase of the pandemic [22, 23]. The risk of contracting COVID‐19 during colonoscopy has subsequently been deemed low [24].
Changes to CRC care pathways
Widespread disruptions to activity within CRC care pathways were reported, especially with respect to treatment delays and adjustments to standard pathways of patient care. Our results have shown that treatment plans tended to be delayed rather than alternative non‐conventional treatments used. This is an important finding, as delays in treatment of just 4 weeks have been shown to have negative impacts on survival [25].
It is important to note that changes to planned CRC treatments and activity levels were independent of regional COVID‐19 infection rates, which is in line with earlier findings [26]. This may be explained by the greater availability of ‘cold sites’ in regions with higher COVID‐19 rates. However, it could also be explained by the impact of rapidly changing national guidelines during the first wave of the pandemic [27, 28, 29, 30]. For example, initially only emergency and urgent CRC surgery were prioritized and laparoscopic surgery was discouraged [27, 31].
Ethical issues, especially those regarding the fairness of the allocation of resources that became scarce during the COVID‐19 pandemic, need to be explicitly addressed [32]. This is in line with our finding that many ‘lessons learned’ reported by participating clinicians related specifically to decisions about access to treatments. Strategies to minimize harm and prioritize patient care include using frailty and prognostic scoring in primary and secondary care in order to identify those patients for whom palliative care may be more appropriate [33].
Adjustments to treatment pathways could also include, for example, changes to oncological practice. Early guidance aimed to balance the risks of immunosuppression and the benefits of treatment against the risks of COVID‐19 infection, as well considering the effects on resource prioritization. In the context of CRC, these guidelines aimed to prioritize adjuvant chemotherapy instead of palliative chemotherapy [29]. A significant increase in the use of short‐course radiotherapy for rectal cancer was also observed during this period [34].
Human factors
Our results demonstrate the importance that clinicians placed on key human factors, including communication, coordination and leadership, during the first wave of the pandemic [35]. Human factor principles can help us understand the interaction between humans and other elements of a system in order to improve safety, which is particularly important for a healthcare system under stress [36]. For example, communication during the COVID‐19 pandemic may be negatively affected by the need to use PPE, workforce stress and fatigue, and unfamiliar working environments. We found that clinicians felt that a well‐led and responsive organization is better able to plan the most appropriate response to these challenging circumstances.
CONCLUSION
As many countries are experiencing subsequent waves of the COVID‐19 pandemic, it is vital that CRC services are protected and maintained in addition to tackling the existing shortfall in diagnostics and treatment from the first peak of the pandemic. The use of ‘cold sites’, particularly in regions with high rates of COVID‐19, appears to be pivotal for both surgical and diagnostic capacity, and highlights the need for regional coordination and cooperation as well as the importance of human factors such as clear communication and strong leadership.
CONFLICT OF INTERESTS
There are no conflicts of interest to disclose.
ETHICAL STATEMENT
Not required.
AUTHOR CONTRIBUTION
Jemma M. Boyle: Conceptualization, methodology, formal analysis, writing‐orginal draft, review and editing; Angela Kuryba: Formal analysis, writing‐review and editing; Helen A. Blake: Formal analysis, writing‐review and editing; Ajay Aggarwal: Methodology, writing‐review and editing; Jan van der Meulen: Conceptualization, methodology, formal analysis, writing‐review and editing, supervision; Kate Walker: Conceptualization, methodology, writing‐review and editing, supervision; Michael Braun: Coceptualization, writing‐review and editing, supervision; Nicola Fearnhead: Conceptualization, writing‐review and editing, supervision.
ACKNOWLEDGEMENTS
The National Bowel Cancer Audit is commissioned by the Healthcare Quality Improvement Partnership (HQIP) as part of the National Clinical Audit and Patient Outcomes Programme, and funded by NHS England and the Welsh Government (http://www.hqip.org.uk/national‐programmes). Neither HQIP nor the funders had any involvement in the study design, the collection, analysis and interpretation of data, the writing of the report or the decision to submit the article for publication.
APPENDIX 1.
Items included in the national survey of all NHS English hospital trusts and Welsh hospital boards providing colorectal cancer (CRC) services.
| 1. | In the middle of April, how many bowel cancer patients were referred to your MDT 1 via the 2‐week wait route compared to normal? | |
|
As many or almost as many as usual (91 to 100% of usual) Small reduction in numbers (71 to 90% of usual) Large reduction in numbers (20 to 70% of usual) Very few 2‐week wait referrals (0 to 19% of usual) |
||
| 2. | In the middle of April, how many patients at your MDT experienced each of the following changes to planned bowel cancer treatment because of the COVID−19 pande mic? |
All or almost all patients (91 to 100%) Majority of patients (51 to 90%) Minority of patients (11 to 50%) Almost none or none (0 to 10%) |
| i. Treatments delayed because the patient was diagnosed with COVID−19 | ||
| ii. Delays in tissue diagnosis and/or staging investigations | ||
| iii. Treatment plans altered to reflect increased risks from COVID−19 during epidemic | ||
| iv. Delays in treatment due to risk of COVID−19 infection to patient | ||
| v. Delays in treatment due to diversion of healthcare resources for COVID−19 care/preparation | ||
| vi. Temporising treatments used e.g. stent, rectal radiotherapy and long wait | ||
| vii. Emergency admissions while patients waited for diagnosis and/or treatment | ||
| viii. Changes in lengths of treatment and/or choices of chemotherapy | ||
| ix. Deaths due to complications of COVID−19 | ||
| 3. | In the middle of April, how were each of the following services (on‐ or off‐site) affected for elective bowel cancer patients at your MDT? | |
| i. Diagnostic colonoscopy |
Stopped entirely (0 to 10% of usual numbers) Substantially reduced (11 to 70% of usual numbers) Continued more or less the same as before the pandemic (71 to 100% of usual numbers) |
|
| ii. Colorectal resection | ||
| iii. Liver resection | ||
| iv. Lung resection | ||
| v. Neo‐adjuvant chemoradiotherapy | ||
| vi. Adjuvant chemotherapy | ||
| 4. | In the middle of April, did your MDT have access to a COVID−19 ‘cold site’ for elective colorectal surgery? | |
|
No Yes within every hospital in the trust Yes in certain hospitals in the trust |
||
| 5. | If you answered ‘Yes in certain hospitals in the trust’ or ‘Yes in another trust/private hospital’, please write the name(s) of these specific hospital sites in the box below. | |
| 6. | What would you describe as the single most important lesson your MDT has learned about how to make services for bowel cancer patients as safe and effective as possible during the pandemic? | |
Multidisciplinary team (MDT): group of CRC experts based within a hospital who discuss and plan the treatment of every CRC patient. The team contains surgeons, medical doctors, nurses, radiologists, and pathologists. Patients from smaller hospitals will be discussed in the closest specialist CRC MDT.
Michael Braun and Nicola Fearnhead are joint senior authors with an equal contribution.
Funding information
The National Bowel Cancer Audit is commissioned by the Healthcare Quality Improvement Partnership (HQIP) as part of the National Clinical Audit and Patient Outcomes Programme, and funded by NHS England and the Welsh Government (http://www.hqip.org.uk/national‐programmes). AA is supported by a National Institute for Health Research (NIHR) Advanced Fellowship (NIHR300599). This grant source had no involvement in the study design; collection, analysis and interpretation of data, the writing of the report or decision to submit the article for publication. The researchers were independent of the funding source and all authors had full access to the data and can take responsibility for the integrity of the data and accuracy of the data analysis.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the authors. Restrictions apply to the availability of these data, which were used under licence for this study. Data are available from the authors with the permission of HQIP.
REFERENCES
- 1. Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer patients in SARS‐CoV‐2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu Z, McGoogan JM. Characteristics of and important lessons from the Coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. J Am Med Assoc. 2020;323(13):1239–42. [DOI] [PubMed] [Google Scholar]
- 3. Onder G, Rezza G, Brusaferro S. Case‐fatality rate and characteristics of patients dying in relation to COVID‐19 in Italy. J Am Med Assoc. 2020;323(18):1775–6. [DOI] [PubMed] [Google Scholar]
- 4. Royal College of Surgeons of England . Guidance for surgeons working during the COVID‐19 pandemic, 2020. Available from: https://www.rcseng.ac.uk/coronavirus/joint‐guidance‐for‐surgeons‐v1. Accessed 4 January, 2021.
- 5. COVIDSurg Collaborative . Elective surgery cancellations due to the COVID‐19 pandemic: global predictive modelling to inform surgical recovery plans. Br J Surg. 2020;107(11):1440–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Macmillan Cancer Support . The forgotten ‘C’? The impact of Covid‐19 on cancer care; 2020. Available from: https://www.macmillan.org.uk/assets/forgotten‐c‐impact‐of‐covid‐19‐on‐cancer‐care.pdf. Accessed 4 January, 2021.
- 7. England NHS. Waiting times for suspected and diagnosed cancer patients for April 2020. 11 June 2020. Available from: www.gov.uk/government/statistics/waiting‐times‐for‐suspected‐and‐diagnosed‐cancer‐patients‐for‐april‐2020. Accessed 4 January, 2021.
- 8. Maringe C, Spicer J, Morris M, Purushotham A, Nolte E, Sullivan R, et al. The impact of the COVID‐19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population‐based, modelling study. Lancet Oncol. 2020;21(8):1023–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. COVIDSurg Collaborative . Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS‐CoV‐2 infection: an international cohort study. Lancet. 2020;396(10243):27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Glasbey JC, Bhangu A. Elective cancer surgery in COVID‐19‐free surgical pathways during the SARS‐CoV‐2 pandemic: an international, multicenter, comparative cohort study. J Clin Oncol. 2021;39(1):66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Iqbal MR, Dhahri AA, Darwish NMM, Vijay V. Single centre concept of ‘cold site’ elective surgery during the peak of COVID‐19 pandemic: a cohort study. Ann Med Surg. 2020;59:245–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kasivisvanathan V, Lindsay J, Rakhshani‐moghadam S, Elhamshary A, Kapriniotis K, Kazantzis G, et al. Evaluation of 30‐day mortality for 500 patients undergoing non‐emergency surgery in a COVID‐19 cold site within a multicentre regional surgical network during the COVID‐19 pandemic. Int J Surg. 2020;84:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Local Government Association . Local Government Inform Plus. Available from: https://home.esd.org.uk/reporting. Accessed 4 January, 2021.
- 14. NHS . Cancer alliances – improving care locally. Available from: https://www.england.nhs.uk/cancer/cancer‐alliances‐improving‐care‐locally/.Accessed 4 January, 2021.
- 15. Office for National Statistics . Estimates of the population for the UK, England and Wales, Scotland and Northern Ireland. Mid‐2019: April 2020 local authority district codes edition of this dataset. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/datasets/populationestimatesforukenglandandwalesscotlandandnorthernireland. Accessed 4 January, 2021.
- 16. Institute of Medicine Committee on Quality of Health Care in America . Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press; 2001. [Google Scholar]
- 17. Collaborative CCR . The impact of the COVID‐19 pandemic on colorectal cancer service provision. Br J Surg. 2020;107(11):e521–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Spinelli A, Pellino G. COVID‐19 pandemic: perspectives on an unfolding crisis. Br J Surg. 2020;107(7):785–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pellino G, Vaizey CJ, Maeda Y, tESoCG Committee. The COVID‐19 pandemic: considerations for resuming normal colorectal services. Colorectal Dis. 2020;22(9):1006–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Di Saverio S, Pata F, Gallo G, Carrano F, Scorza A, Sileri P, et al. Coronavirus pandemic and colorectal surgery: practical advice based on the Italian experience. Colorectal Dis. 2020;22(6):625–34. [DOI] [PubMed] [Google Scholar]
- 21. Huddy JR, Freeman Z, Crockett M, Hadjievangelou N, Barber N, Gerrard D, et al. Establishing a ‘cold’ elective unit for robotic colorectal and urological cancer surgery and regional vascular surgery following the initial COVID‐19 surge. Br J Surg. 2020;107(11):e466–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. British Society of Gastroenterology . Endoscopy activity and COVID‐19: BSG and JAG guidance; 2020.
- 23. Richards M, Anderson M, Carter P, Ebert BL, Mossialos E. The impact of the COVID‐19 pandemic on cancer care. Nat Cancer. 2020;1(6):565–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rees CJ, Rutter MD, Sharp L, Hayee B, East JE, Bhandari P, et al. COVID‐19 as a barrier to attending for gastrointestinal endoscopy: weighing up the risks. Lancet Gastroenterol Hepatol. 2020;5(11):960–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hanna TP, King WD, Thibodeau S, Jalink M, Paulin GA, Harvey‐Jones E, et al. Mortality due to cancer treatment delay: systematic review and meta‐analysis. BMJ. 2020;371:m4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brunner M, Krautz C, Kersting S, Weber GF, Stinner B, Benz SR, et al. Oncological colorectal surgery during the COVID‐19 pandemic‐a national survey. Int J Colorectal Dis. 2020;35(12):2219–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. ACPGBI . Urgent Intercollegiate General Surgery Guidance on COVID‐19; 2020. Available from: https://www.acpgbi.org.uk/content/uploads/2020/03/Updated‐Intercollegiate‐General‐Surgery‐Guidance‐on‐COVID‐19‐final‐with‐logos13.pdf. Accessed 4 January, 2021.
- 28. NICE Guideline . COVID‐19 rapid guideline: delivery of radiotherapy; 2020. Available from: https://www.nice.org.uk/guidance/ng162. Accessed 4 January, 2021.
- 29. NICE Guideline . COVID‐19 rapid guideline: delivery of systemic anticancer treatments; 2020. Available from: https://www.nice.org.uk/guidance/ng161/chapter/6‐Prioritising‐systemic‐anticancer‐treatments. Accessed 4 January, 2021. [PubMed]
- 30. NHS England . Clinical guide to surgical prioritisation during the coronavirus pandemic. Available from: https://fssa.org.uk/_userfiles/pages/files/covid19/prioritisation_master_240820.pdf. Accessed 4 January, 2021.
- 31. Academy of Medical Royal Colleges . Clinical guide for the management of essential cancer surgery for adults during the coronavirus pandemic. NHS England and NHS Improvement; 2020.
- 32. Emanuel EJ, Persad G, Upshur R, Thome B, Parker M, Glickman A, et al. Fair allocation of scarce medical resources in the time of Covid‐19. N Engl J Med. 2020;382(21):2049–55. [DOI] [PubMed] [Google Scholar]
- 33. ACPGBI Legacy Working Group . Legacy of COVID‐19 ‐ the opportunity to enhance surgical services for patients with colorectal disease. Colorectal Dis. 2020;22(10):1219– 28. [DOI] [PubMed] [Google Scholar]
- 34. Morris EJA, Goldacre R, Spata E, Mafham M, Finan PJ, Shelton J, et al. Impact of the COVID‐19 pandemic on the detection and management of colorectal cancer in England: a population‐based study. Lancet Gastroenterol Hepatol. 2021;6(3):199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Papautsky EL, Lustberg M, Krok‐Schoen JL, Lee C, Park KU, Attai DJ, et al. A human factors perspective to characterize treatment and surgery during the COVID‐19 pandemic. Proc Int Symp Hum Factors Ergon Health Care. 2020;9(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Alagha MA, Jaulin F, Yeung W, Celi LA, Cosgriff CV, Myers LC. Patient harm during COVID‐19 pandemic: using a human factors lens to promote patient and workforce safety. J Patient Saf. 2021;17:87–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. NICE . Quantitative faecal immunochemical tests to guide referral for colorectal cancer in primary care. Diagnostics guidance [DG30]. July 2017. Available from: https://www.nice.org.uk/guidance/dg30. Accessed 4 January, 2021.
- 38. Arasaradnam RP, Bhala N, Evans C, Greenaway J, Logan R, Penman I, et al. Faecal immunochemical testing in the COVID‐19 era: balancing risk and costs. Lancet Gastroenterol Hepatol. 2020;5(8):717–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the authors. Restrictions apply to the availability of these data, which were used under licence for this study. Data are available from the authors with the permission of HQIP.


