Abstract
Background and purpose
Mounting evidence supports an association between Guillain−Barré syndrome spectrum (GBSs) and severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection. However, GBSs in the setting of coronavirus disease 2019 (COVID‐19) remains poorly characterized, whilst GBSs prevalence amongst COVID‐19 patients has not been previously systematically evaluated using a meta‐analytical approach.
Methods
A systematic review and meta‐analysis of observational cohort and case series studies reporting on the occurrence, clinical characteristics and outcomes of patients with COVID‐19‐associated GBSs was performed. A random‐effects model was used to calculate pooled estimates and odds ratios (ORs) with corresponding 95% confidence intervals (CIs), compared to non‐COVID‐19, contemporary or historical GBSs patients.
Results
Eighteen eligible studies (11 cohorts, seven case series) were identified including a total of 136,746 COVID‐19 patients. Amongst COVID‐19 patients, including hospitalized and non‐hospitalized cases, the pooled GBSs prevalence was 0.15‰ (95% CI 0%–0.49‰; I 2 = 96%). Compared with non‐infected contemporary or historical controls, patients with SARS‐CoV‐2 infection had increased odds for demyelinating GBSs subtypes (OR 3.27, 95% CI 1.32%–8.09%; I 2 = 0%). In SARS‐CoV‐2‐infected patients, olfactory or concomitant cranial nerve involvement was noted in 41.4% (95% CI 3.5%–60.4%; I 2 = 46%) and 42.8% (95% CI 32.8%–53%; I 2 = 0%) of the patients, respectively. Clinical outcomes including in‐hospital mortality were comparable between COVID‐19 GBSs patients and non‐infected contemporary or historical GBSs controls.
Conclusion
GBSs prevalence was estimated at 15 cases per 100,000 SARS‐CoV‐2 infections. COVID‐19 appears to be associated with an increased likelihood of GBSs and with demyelinating GBSs variants in particular.
Keywords: COVID‐19, Guillain−Barré syndrome, acute inflammatory demyelinating polyneuropathy, prevalence, mortality
INTRODUCTION
Guillain−Barré syndrome spectrum (GBSs) has been increasingly recognized as a neurological manifestation of coronavirus disease 2019 (COVID‐19) in patients with severe acute respiratory coronavirus 2 (SARS‐CoV‐2) infection [1]. Since the first reported GBSs case in January 2020 in a COVID‐19 patient from Wuhan [2], the number of published cases, case series and observational studies postulating a positive correlation and a possible pathogenetic link between SARS‐CoV‐2 and GBSs has grown exponentially [1, 3, 4]. In contrast, recent epidemiological data have emerged contradicting this hypothesis and demonstrating a reduced GBSs incidence during the COVID‐19 pandemic compared to the pre‐COVID era amongst the UK population [5].
Guillain−Barré syndrome spectrum comprises a spectrum of acute, immune‐mediated polyneuropathies that can be distinguished into different subtypes according to clinical features and electrophysiological findings, such as acute inflammatory demyelinating polyneuropathy (AIDP), acute motor axonal neuropathy (AMAN) [6] and acute motor sensory axonal neuropathy (AMSAN), and encompasses the clinical GBS variants Miller−Fisher syndrome (MFS) and Bickerstaff's brainstem encephalitis [7, 8]. According to epidemiological studies, the overall incidence of GBSs is estimated between 0.8 and 1.9/100,000/year in the general population [9].
Although the epidemiological evidence on post‐infectious GBSs is equivocal [9], approximately two‐thirds of total GBSs cases are considered to be related to antecedent infections [10]. Amongst the infectious agents associated with GBSs are bacteria, including Campylobacter jejuni, Mycoplasma pneumoniae and Haemophilus influenzae, and viruses, including cytomegalovirus (CMV), influenza, enteroviruses, Epstein−Barr virus, herpes simplex virus, hepatitis, human immunodeficiency virus and Zika virus [10, 11, 12]. Only for few pathogens, however, including C. jejuni and CMV, has a causal link via ‘molecular mimicry’ or cross‐reactive antibodies against ganglioside epitopes been established, particularly for AMAN, AMSAN and MFS [13]. To date, the pathophysiological mechanisms underlying the neurological manifestations of COVID‐19 remain only partially elucidated [11]. Moreover, as the COVID‐19 pandemic still progresses, epidemiological associations between GBSs and SARS‐CoV‐2 remain tentative.
The aim of the present systematic review and meta‐analysis was to critically appraise the published literature related to GBSs and COVID‐19, and to evaluate the prevalence, clinical features and outcomes of GBSs in patients with COVID‐19 compared to non‐COVID‐19 contemporary or historical controls.
METHODS
Study design, search strategy and selection criteria
A systematic literature search was performed to identify all published research referring to GBSs and COVID‐19 with publication date between 30 December 2019 (the day of declaration of the first COVID‐19 case) and 18 December 2020, by three independent researchers (LP, MIS, AHK). Records were retrieved from MEDLINE and Scopus, without prior application of language or other restrictions for the database search. The complete search algorithm used in the MEDLINE search is provided in the Supplementary material. Reference lists of included articles were also screened to identify potential studies missed by the initial literature search. Any disagreements between the three researchers performing the literature search were resolved after discussion with the corresponding author (GT).
Amongst studies identified by the systematic literature search, eligibility for inclusion in the meta‐analysis was assessed based on pre‐specified criteria by three independent researchers (LP, MIS, MP). The inclusion criteria for the cohort and case series studies included (i) COVID‐19 diagnosis confirmed by positive polymerase chain reaction (PCR) for viral RNA or positive serological test for anti‐SARS‐CoV‐2 immunoglobulin M or immunoglobulin G; (ii) clinically probable COVID‐19 diagnosis based on the European Centre for Disease Prevention and Control case definitions [14]; (iii) GBSs diagnosis with typical clinical presentation and/or confirmatory diagnostic findings, including electrophysiological or cerebrospinal fluid (CSF) studies. Excluded from further analysis were (i) case reports; (ii) commentaries, narrative and systematic reviews; (iii) studies with overlapping data; and (iv) studies thematically unrelated to the study objective.
An aggregate data meta‐analysis was performed including observational cohort studies and case series reporting on the occurrence and clinical features of patients presenting with GBSs in association with COVID‐19. The meta‐analysis is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA) guidelines [15] and was written according to the Meta‐analysis of Observational Studies in Epidemiology (MOOSE) proposal [16]. The pre‐specified study protocol has been registered in the International Prospective Register of Ongoing Systematic Reviews PROSPERO (CRD42020227361).
Quality control and bias assessment
Eligible observational cohort studies and case series were subjected to quality control and bias assessment employing the Newcastle−Ottawa Scale and the checklist developed by the US National Heart Lung and Blood Institute for case series studies, respectively [17, 18]. The quality control and bias assessment was conducted independently by three reviewers (LP, MIS, CM), and disagreements were resolved via consensus and discussion with the corresponding author (GT).
Outcomes
Our predefined primary outcome measures were twofold: (i) the pooled prevalence rate of GBSs amongst COVID‐19 patients; and (ii) GBSs prevalence amongst COVID‐19 patients compared to either contemporary or historical non‐COVID‐19 GBSs controls.
As secondary outcomes of interest the rates of (i) clinical improvement, (ii) intensive care unit admission, (iii) mechanical ventilation and (iv) all‐cause mortality amongst COVID‐19 GBSs patients were further assessed; they were compared to their non‐COVID‐19 contemporary or historical GBSs counterparts. Finally, potential differences in demographics and clinical GBSs features between groups of patients stratified by COVID‐19 status were evaluated.
As an additional exploratory analysis, the number of reported to the number of expected GBSs cases were compared between COVID‐19 patients, assuming a similar GBSs incidence between SARS‐CoV‐2 positive patients and the general population. To calculate the total number of reported COVID‐19‐associated GBSs cases, cohort and case series studies were considered, along with case reports that were identified by the literature search but were excluded from the meta‐analysis. After exclusion of possible overlapping data, the reported cases were stratified by country of origin. To estimate the number of expected GBSs cases, the number of patients diagnosed with COVID‐19 in each country, as reported in the Coronavirus Resource Center of the Johns Hopkins University and Medicine until 18 December 2020 [19], was multiplied by the overall incidence of GBS in the general population (1.89/100,000) [9].
Statistical analysis
The prevalence of GBSs amongst COVID‐19 patients was calculated by dividing the number of patients with COVID‐19 and GBSs (including all GBS subtypes and variants) by the total number of COVID‐19 individuals, after implementation of the variance‐stabilizing double arcsine transformation. The random‐effects model of meta‐analysis (DerSimonian and Laird) [20] was used to calculate the pooled estimates. Pairwise comparisons were conducted between COVID‐19 patients with GBSs and controls (GBSs cases without COVID‐19) and were reported using odds ratios (ORs) and corresponding 95% confidence intervals (95% CIs). Additionally, all previous analyses were performed after subgroup stratification according to study design (cohort or case series).
Heterogeneity between included studies was assessed with the Cochran Q and I 2 statistics. For the qualitative interpretation of heterogeneity, I 2 values of at least 50% were considered to represent substantial heterogeneity, whilst values of at least 75% indicated considerable heterogeneity [21]. The significance level for the Q statistic was set at 0.1, and the equivalent z test for each pooled OR with a two‐tailed p value <0.05 was considered as statistically significant. Publication bias across individual studies was assessed when more than four studies were included in each analysis, using both funnel plot inspection and Egger's linear regression test [22].
All statistical analyses were conducted using the OpenMetaAnalyst [23] and Stata Statistical Software Release 13 for Windows (StataCorp LP).
RESULTS
Literature search and included studies
The systematic database search yielded a total of 280 and 264 records from the MEDLINE and Scopus databases, respectively (Figure 1). After excluding duplicates and out‐of‐scope articles, the full text of 104 records that were considered potentially eligible for inclusion were retrieved. After reading the full‐text articles, 82 were excluded (Table S1). Finally, 18 observational studies (11 cohorts and seven case series) were identified including a total of 136,746 COVID‐19 patients that were in line with our predefined inclusion/exclusion criteria (Table 1).
FIGURE 1.
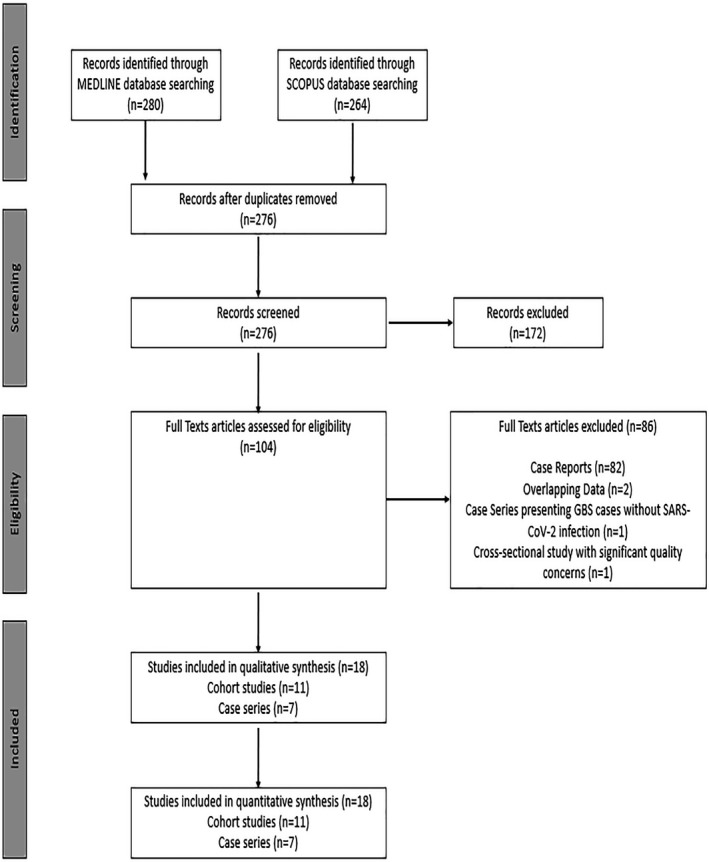
Flow chart presenting the selection of eligible studies
TABLE 1.
Overview of included studies
| Study name | Study location | Study period | Population | No. of COVID‐19 patients | No. of GBSs COVID‐19 patients | Controls (yes/no) |
|---|---|---|---|---|---|---|
| Cohorts | ||||||
| Espíndola et al. [45] | Brazil | April–June 2020 | Neurological admissions | 58 | 6 | No |
| Filosto et al. [4] | Italy | March–April 2020 | COVID‐19 patients | NR | 30 | Yes |
| General hospital admissions | 12711 | |||||
| Foresti et al. [39] | Bergamo, Italy | 23 February–21 May 2020 | General hospital admissions | 1832 | 17 | No |
| Fragiel et al. [24] | Spain | 1 March–30 April 2020 | COVID‐19 patients | 71904 | 11 | Yes |
| Guilmot et al. [46] | Brussels, Belgium | 23 March–24 April 2020 | General hospital admissions | 349 | 3 | No |
| Neurological admissions | 15 | |||||
| Keddie et al. [5] | London, UK | 1 March–27 April 2020 | COVID‐19 patients | NR | 25 | Yes |
| Koh et al. [47] | Singapore | 19 March–19 July 2020 | COVID‐19 patients | 47572 | 1 | No |
| Neurological admissions | 39 | |||||
| Kushwaha et al. [48] | India | April–July 2020 | Neurological admissions | 14 | 1 | No |
| Meppiel et al. [49] | France | 16 March–27 April 2020 | Neurological admissions | 222 | 15 | No |
| Paterson et al. [50] | London, UK | 9 April–15 May 2020 | Neurological admissions | 43 | 7 | No |
| Romero‐Sánchez et al. [51] | Albacete, Spain | 1 March–1 April 2020 | General hospital admissions | 841 | 1 | No |
| Case series | ||||||
| Abolmaali et al. [52] | Iran | April 2020 | NR | NR | 3 | No |
| Garnero et al. [53] | Italy | 15 February–3 May 2020 | NR | NR | 6 | Yes |
| Gigli et al. [3] | Italy | 1 March–15 April 2020 | NR | NR | 1 | Yes |
| Lascano et al. [54] | Switzerland | March–April 2020 | NR | NR | 3 | No |
| Manganotti et al. [55] | Italy | March–April 2020 | NR | NR | 5 | No |
| Nanda et al. [56] | India | NR | NR | NR | 4 | No |
| Toscano et al. [57] | Italy | Study period | General hospital admissions | 1200 | 5 | No |
Abbreviations: COVID‐19, coronavirus disease 2019; GBSs, Guillain−Barré syndrome spectrum; NR, not reported.
Quality control of included studies
The risk of bias in included cohort studies was assessed by the Newcastle−Ottawa scale and is presented in Table S2 [18]. The overall score was 71 of 99 (72%), which is considered to be indicative of moderate quality. The risk of bias in included case series studies was assessed according to the National Heart, Lung and Blood Institute Study Quality Assessment Tools and the results are presented in Table S3 [17]. The overall score was 52 of 63 (83%), which is considered to be indicative of satisfying quality.
Overall and subgroup analyses
The pooled prevalence rates of all GBSs cases stratified amongst different included COVID‐19 populations are presented in Figure 2. Amongst COVID‐19 patients in the community (including hospitalized and non‐hospitalized cases), the pooled GBSs rate was 0.15‰ (95% CI 0%–0.49‰; three studies; I 2 = 96%; p for Cochran Q < 0.001; Figure 2a), corresponding to 15 GBSs cases per 100,000 SARS‐CoV‐2 infections. The pooled prevalence rates of GBSs amongst COVID‐19 hospital and neurological admissions was 0.4% (95% CI 0.2%–0.8%; five studies; I 2 = 78%; p for Cochran Q < 0.001; Figure 2b) and 8.7% (95% CI 5%–13.4%; six studies; I 2 = 38%; p for Cochran Q 0.151; Figure 2c), respectively. COVID‐19 status was not associated with increased odds for GBSs (OR 5.90, 95% CI 0.98–35.47; two studies; I 2 = 91%; p for Cochran Q 0.001; Figure S1), when COVID‐19 GBSs patients were compared with non‐infected contemporary or historical GBSs controls.
FIGURE 2.
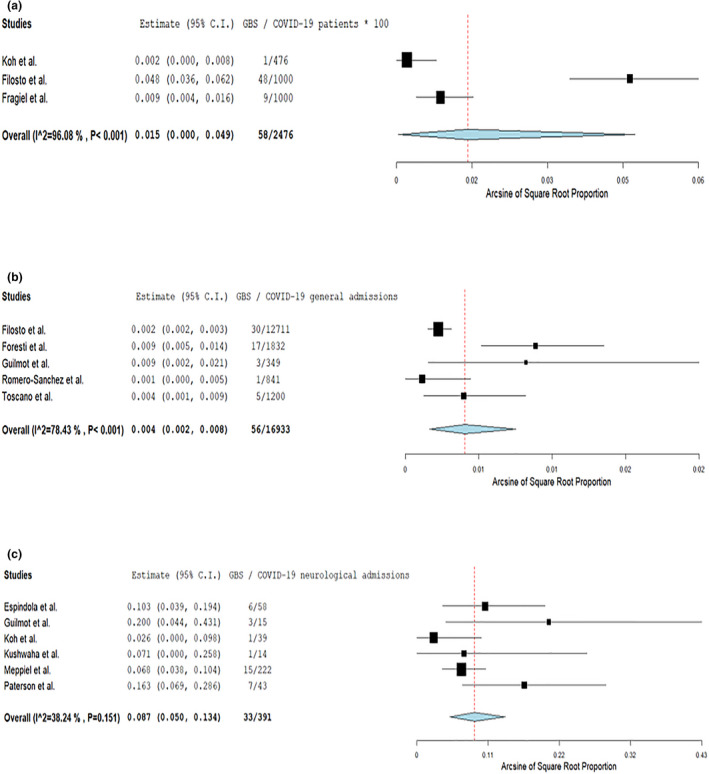
Pooled analysis on the prevalence of Guillain−Barré syndrome spectrum cases amongst COVID‐19 patients (a), COVID‐19‐associated general hospital admissions (b) and COVID‐19‐associated neurological admissions (c) [Colour figure can be viewed at wileyonlinelibrary.com]
The results of all analyses regarding the secondary outcomes of interest (demographics, GBS subtypes, diagnostics, treatment and outcomes) are presented in Table 2. The mean age at GBSs diagnosis was 58.9 ± 1.7 years amongst COVID‐19 patients (Figure S2a). No mean age differences between COVID‐19 patients and non‐COVID‐19 controls (Figure S2b) were identified. Additionally, male sex was more prevalent amongst COVID‐19 GBSs cases (pooled rate 72.6%, 95% CI 64.5%–80.0%; 16 studies; I 2 = 97%; p for Cochran Q 0.362; Figure S3a), but it was not related to COVID‐19 status amongst GBSs cases (OR 1.58, 95% CI 0.79–3.19; five studies; I 2 = 0%; p for Cochran Q 0.521; Figure S3b).
TABLE 2.
Overview of analyses on demographics, diagnostics, treatments and outcomes
| Variable | Prevalence | Associations with COVID‐19 status | ||||
|---|---|---|---|---|---|---|
| No. of studies | Pooled estimates (95% CI) | I 2, p for Cochran Q | No. of studies | Estimates (95% CI) | I 2, p for Cochran Q | |
| Demographics | ||||||
| Age (mean, years) | 13 | 59 (57–61) | 0%, 0.705 | 4 | MD = 3.7 (−1.8 to 9.1) | 0%, 0.619 |
| Male sex | 16 | 72.6% (64.5–80%) | 8%, 0.362 | 5 | OR = 1.58 (0.79–3.19) | 0%, 0.521 |
| GBSs subtypes | ||||||
| AIDP | 15 | 73.3% (60–84.7%) | 47%, 0.023 | 4 | OR = 3.27 (1.32–8.09) | 0%, 0.494 |
| Axonal GBSs | 15 | 21.3% (9.9–35.7%) | 59%, 0.002 | 4 | OR = 0.52 (0.08–3.46) | 56%, 0.078 |
| Miller−Fisher | 14 | 7.2% (3.2–12.7%) | 0%, 0.715 | 4 | OR = 1.58 (0.31–8.07) | 0%, 0.976 |
| Specific symptoms | ||||||
| Anosmia/ageusia | 7 | 41.4% (23.5–60.4%) | 46%, 0.088 | 2 | OR = 15.7 (1.89–130.50) | 0%, 0.615 |
| Other cranial nerve involvement | 12 | 42.8% (32.8–53%) | 0%, 0.591 | NA | ||
| Diagnostics | ||||||
| Cytoalbuminologic dissociation | 15 | 56.6% (42.3–70.4%) | 55%, 0.006 | 5 | OR = 1.22 (0.54–2.77) | 16%, 0.311 |
| Ganglioside antibodies | 7 | 18% (2.7–42.6%) | 64%, 0.01 | 3 | OR = 0.19 (0.03–1.39) | 0%, 0.517 |
| Treatment | ||||||
| Intravenous immunoglobulin | 14 | 83.4% (76.2–89.5%) | 5%, 0.397 | 3 | OR = 0.23 (0.05–0.98) | 0%, 0.715 |
| Plasmapheresis | 13 | 13.3% (5.5–23.8%) | 45%, 0.039 | 3 | OR = 0.49 (0.1–2.36) | 0%, 0.77 |
| Outcomes | ||||||
| ICU admission | 10 | 41.4% (30.3–52.8%) | 20%, 0.257 | 3 | OR = 2.41 (0.58–10.04) | 66%, 0.052 |
| Mechanical ventilation | 12 | 34.9% (22.7–48.2%) | 40%, 0.077 | 2 | OR = 3.31 (0.28–39.51) | 56%, 0.132 |
| Clinical improvement | 10 | 71.7% (60.3–81.8%) | 0%, 0.844 | 2 | OR = 1.77 (0.08–38.89) | 73%, 0.054 |
| In‐hospital mortality | 14 | 6% (2.7–10.6%) | 0%, 0.484 | 4 | OR = 1.67 (0.31–9.10) | 0%, 0.640 |
Abbreviations: AIDP, acute inflammatory demyelinating polyneuropathy; CI, confidence interval; COVID‐19, coronavirus disease 2019; GBSs, Guillain−Barré syndrome spectrum; ICU, intensive care unit; MD, mean difference; OR, odds ratio.
Regarding the clinical characteristics of GBSs, amongst COVID‐19 patients 41.4% (95% CI 23.5%–60.4%; seven studies; I 2 = 46%; p for Cochran Q 0.088; Figure S4a) were reported to suffer from anosmia or ageusia, and SARS‐CoV‐2 infection was associated with increased odds for olfactory or gustatory dysfunction (OR 15.67, 95% CI 1.88–130.51; two studies; I 2 = 0%; p for Cochran Q 0.615; Figure S4b) amongst GBSs cases. Additional cranial nerve involvement was also frequent amongst COVID‐19 GBSs patients (pooled rate 42.8%, 95% CI 32.8%–53%; 12 studies; I 2 = 0%; p for Cochran Q 0.591; Figure S5). Finally, no association was observed between MFS and COVID‐19 status (OR 1.58, 95% CI 0.31–8.07; four studies; I 2 = 0%; p for Cochran Q 0.976; Figure S6).
The predominant electrophysiological GBSs subtype corresponded to AIDP in 73.3% of COVID‐19 GBSs cases (95% CI 60%–84.7%; 15 studies; I 2 = 47%; p for Cochran Q 0.023; Figure 3a), whilst SARS‐CoV‐2 infection appeared associated with increased odds for AIDP (OR 3.27, 95% CI 1.32–8.09; four studies; I 2 = 0%; p for Cochran Q 0.494; Figure 3b) amongst GBSs cases. Conversely, SARS‐CoV‐2 infection was not related to axonal GBSs amongst GBSs cases (Figure S7).
FIGURE 3.
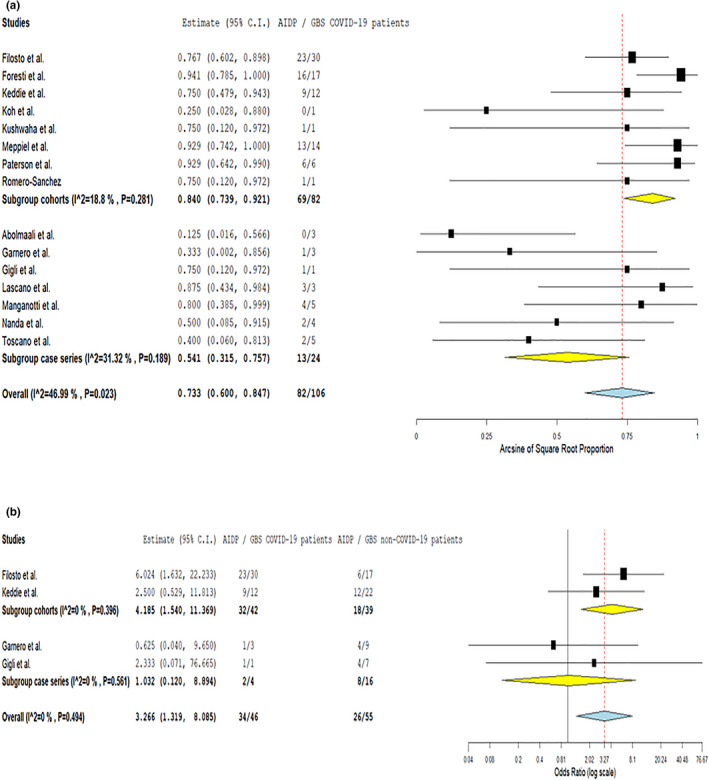
Pooled prevalence of acute inflammatory demyelinating polyneuropathy subtype amongst COVID‐19‐associated Guillain−Barré syndrome spectrum cases, stratified by study design (a) and probability of acute inflammatory demyelinating polyneuropathy subtype in COVID‐19‐associated Guillain−Barré syndrome spectrum cases compared to contemporary or historical controls (b) [Colour figure can be viewed at wileyonlinelibrary.com]
With respect to laboratory findings amongst COVID‐19 GBSs patients, the corresponding rates of cytoalbuminologic dissociation in CSF and ganglioside antibodies in serum were 56.6% (95% CI 42.3%–70.4%; 15 studies; I 2 = 55%; p for Cochran Q 0.006; Figure S8) and 18% (95% CI 2.7%–42.6%; seven studies; I 2 = 64%; p for Cochran Q 0.01; Figure S9), respectively. Yet, COVID‐19 status was not associated with the odds of detecting cytoalbuminologic dissociation and ganglioside antibodies amongst GBSs cases. Reverse transcription PCR revealed evidence of SARS‐CoV‐2 RNA in the CSF of only one patient with COVID‐19‐associated GBSs [24]; thus, pooled analysis could not be performed.
Treatment with either intravenous immunoglobulin or plasmapheresis was not different amongst GBSs patients with SARS‐CoV‐2 infection status compared to contemporary or historical controls (Figures S10 and S11).
Finally, with respect to clinical outcomes, COVID‐19 GBSs patients were found to have comparable odds for clinical improvement (OR 1.77, 95% CI 0.08–38.89; two studies; I 2 = 73%; p for Cochran Q 0.054, Figure S12), intensive care unit admission (OR 2.41, 95% CI 0.58–10.04; three studies; I 2 = 66%; p for Cochran Q 0.052, Figure S13), mechanical ventilation (OR 3.31, 95% CI 0.28–39.51; two studies; I 2 = 56%; p for Cochran Q 0.132, Figure S14) and in‐hospital mortality (OR 1.67, 95% CI 0.31–9.1; four studies; I 2 = 0%; p for Cochran Q 0.640, Figure 4) compared to their non‐COVID‐19 contemporary or historical counterparts.
FIGURE 4.
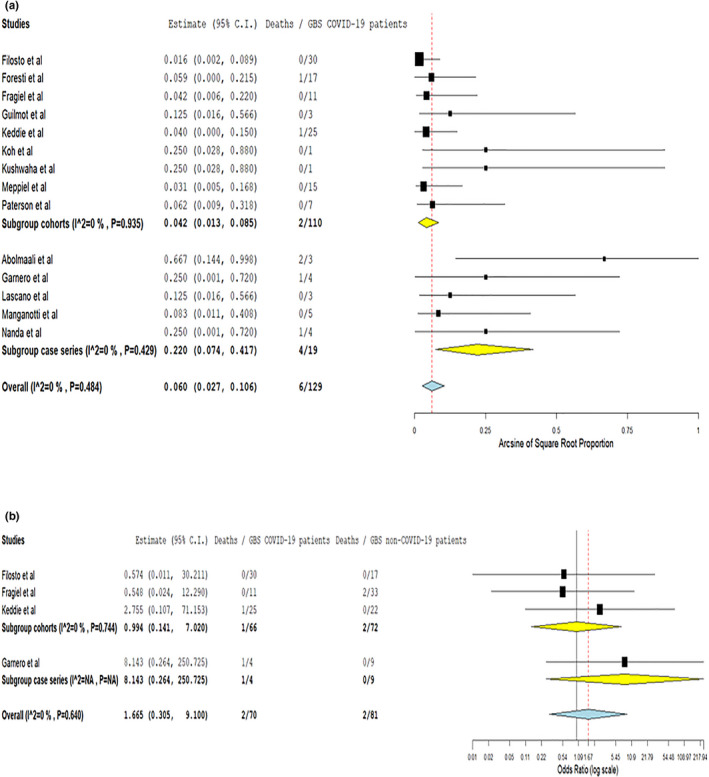
Pooled in‐hospital mortality rate amongst COVID‐19‐associated Guillain−Barré syndrome spectrum cases, stratified by study design (a) and probability of in‐hospital mortality amongst COVID‐19‐associated Guillain−Barré syndrome spectrum cases compared to contemporary or historical controls (b) [Colour figure can be viewed at wileyonlinelibrary.com]
Publication bias was evaluated using funnel plots; asymmetry and evidence of small study effects (i.e., publication bias) were uncovered in the reported mechanical ventilation rates of COVID‐19‐associated GBSs cases (p for Egger's test 0.006; Figure S15). No publication bias was uncovered in the rest of the outcomes investigated (Figures S16−S20).
After adding the number of GBSs cases derived from all case reports to those reported in case series and cohort studies for each country separately, it was demonstrated that in Italy the reported COVID‐19‐associated GBSs cases (n = 80) far exceeded those expected according to the number of COVID‐19 patients (n = 36; Table S4). The corresponding comparison regarding the other included countries did not yield similar results.
DISCUSSION
In the present systematic review and meta‐analysis, an overall GBSs prevalence of 0.15‰ in the COVID‐19 population (including hospitalized and non‐hospitalized patients) is reported, corresponding to 15 GBSs cases per 100,000 SARS‐CoV‐2 infections. The estimated GBSs prevalence amongst total COVID‐19‐associated hospital and neurological admissions was 0.4% and 7.6%, respectively. In addition, an approximately threefold increase in the likelihood of AIDP amongst patients infected with SARS‐CoV‐2 was documented compared to non‐infected contemporary or historical controls. Similarly, COVID‐19 increased the odds of olfactory or gustatory dysfunction substantially amongst GBSs patients. Finally, GBSs appeared not to be associated with adverse clinical outcomes including intensive care unit admission, mechanical ventilation and in‐hospital mortality.
The observed GBSs prevalence in SARS‐CoV‐2‐infected patients exceeds the corresponding average rate of GBSs in the general population (≈2/100,000) [9]. In a large‐scale epidemiological study during the COVID‐19 pandemic in the UK, Keddie et al. reported an estimated GBSs incidence of between 0.016 and 0.82 cases per 1000 COVID‐19 infections [5]. At population level, the authors also noted that the absence of a significant increase in total GBSs cases during the pandemic might indicate a lack of ‘causal’ association between SARS‐CoV‐2 and GBSs, or might be attributed to the reduced transmission of common infective GBSs triggers following the implementation of lockdown and public hygiene measures [5]. Nonetheless, this epidemiological analysis included all GBSs cases irrespective of their SARS‐COV‐2 infection status and thus the reported incidence rates are not comparable to our results.
Accordingly, in our analyses, the number of published GBSs cases did not exceed the estimates of expected GBS cases amongst the COVID‐19 patients in the majority of countries, with the exception of an important GBSs cluster associated with COVID‐19 in Italy. Nonetheless, publication bias may exist and could account for discrepancies between actual and reported GBSs cases across countries. With respect to the ‘Italian paradox’, previous research has indicated that environmental and genetic factors may underlie the increased susceptibility and severity of COVID‐19 noted in Italy during the pandemic [25, 26]. Also, since GBSs incidence has been shown to be significantly higher in Italy compared to the rest of Europe both during the pre‐COVID‐19 and in the COVID‐19 era [27], it would be plausible to assume that genetic factors could confer a higher susceptibility of the Italian population to GBSs also in the setting of SARS‐CoV‐2 infection.
Compared with occurrence rates of GBSs following infection with pathogens that have been ‘causally’ linked to GBSs, such as C. jejuni and CMV with corresponding occurrence rates between 0.25 to 0.65 and 0.6 to 2.2 GBSs per 1000 infections, respectively [28], the results of the present meta‐analysis suggest a weaker association between GBSs and SARS‐CoV‐2 infection. Although causality (or lack thereof) cannot be inferred by the available epidemiological evidence, there are no scientific accounts of a ‘molecular mimicry’ link between SARS‐CoV‐2 and GBSs to date [5]. In particular, SARS‐CoV‐2 appears to share no homology with human nerve axonal or myelin proteins and glycoproteins, at either the protein or the nucleic acid level. However, taking into consideration the high variability in genetic and biosynthetic pathways of human gangliosides, and the fact that new SARS‐CoV‐2 variants continue to emerge [29], the extent to which ‘molecular mimicry’ might underlie GBSs manifestation in COVID‐19 patients remains to be verified.
In our meta‐analysis, demyelinating GBSs was more frequently encountered amongst SARS‐CoV‐2‐infected patients compared to their non‐infected contemporary or historical GBSs counterparts. This observation is in line with the low prevalence of ganglioside antibodies amongst SARS‐CoV‐2 positive GBSs patients, and is also consistent with previous research that supports a stronger correlation of ganglioside antibodies with axonal rather than demyelinating GBSs variants [30]. Our findings also show that blood−brain barrier dysfunction was prevalent amongst GBSs COVID‐19 cases (as indicated by the evidence of cytoalbuminologic dissociation); yet, no association was noted between SARS‐CoV‐2 infection status and blood−brain barrier dysfunction. In addition, no evidence of SARS‐CoV‐2 RNA was found in the CSF of SARS‐CoV‐2 positive GBSs patients, although scarce cases with positive PCR CSF findings have been reported previously [24]. Our findings of the predominance of the demyelinating variants of GBSs in COVID‐19 patients, the satisfactory response to standard treatments (intravenous immunoglobulin or plasmapheresis) and the mainly good outcome upon hospital discharge have also been confirmed by another systematic review and individual participant data meta‐analysis that has recently been published and included 61 patients derived by 45 case reports and case series studies [31].
Taken together, these results lend support to the hypothesis of a post‐infectious GBSs aetiology, which suggests an immune‐mediated rather than a direct SARS‐CoV‐2‐mediated cause of GBSs in COVID‐19 patients [11]. Blood−brain barrier dysfunction and demyelinating disorders have been previously reported in conjunction with COVID‐19 [32] and are also currently considered to reflect an aberrant, SARS‐CoV‐2‐induced upsurge of pro‐inflammatory cytokines in the setting of a COVID‐19 cytokine storm [33]. Amongst the cytokines/chemokines implicated in the COVID‐19 cytokine storm, tumour necrosis factor α, interleukin‐1β (IL‐1β), IL‐6, IL‐17 and interferon‐γ have been shown to hold a pivotal role also in GBSs propagation [33]. Modulation of cytokine function has thus been proposed as a therapeutic target for the management of both COVID‐19 and GBSs.
In accordance with previously published studies reporting the presence of olfactory or gustatory dysfunction in up to 75% of COVID‐19 patients [34, 35, 36], our meta‐analysis revealed a highly significant association between SARS‐CoV‐2 infection status and olfactory or gustatory dysfunction in patients with GBSs. Currently, the pathophysiological mechanisms implicated in the development of olfactory symptoms in COVID‐19 patients remain only partially understood, with recent histological studies pointing to microvascular injury and inflammatory changes in the olfactory bulb [37, 38]. To date, it remains unclear to what extent the olfactory nerve impairment in COVID‐19 might reflect SARS‐CoV‐2 neurovirulence or, conversely, a cranial neuropathy of autoimmune origin. Intriguingly, further autoimmune neuropathies have been associated with COVID‐19, with mounting evidence suggesting that an autoimmune vagal neuropathy may be the underlying cause of cardiorespiratory failure in COVID‐19 [38]. In line with the autoimmune‐mediated hypothesis, our results show that cranial neuropathies were frequent amongst SARS‐CoV‐2 positive GBSs patients, although SARS‐CoV‐2 RNA was undetectable in the CSF in the vast majority of cases.
This is the first study that systematically evaluates the prevalence of GBSs cases in association with COVID‐19 using a meta‐analytical approach, based on cohort studies and case series. The prevalence, the clinical characteristics and the outcomes of COVID‐19‐associated GBSs cases have also been compared against contemporary or historical GBSs cases without history of SARS‐CoV‐2 infection. However, some limitations should be acknowledged in the current systematic review and meta‐analysis. First, there was a limited number of published cohorts and case series reporting on COVID‐19‐associated GBSs, with corresponding small sample size. Thus, the generalizability of the present results warrants corroboration in larger population‐based studies.
Second, there were few studies providing estimates of the latency period between COVID‐19 and GBSs, but significant discrepancies in the definition of latency period (e.g., based on first clinical symptoms, first diagnosis or clinical nadir) and the associated reported descriptive statistics were noted across studies [4, 5, 39]. Thus, these results could not be included in the aggregate meta‐analysis. Previous systematic reviews of GBSs cases in the context of COVID‐19, however, have shown that COVID‐19 manifestations consistently precede GBSs symptoms (median interval 14 days, interquartile range 7–20) [1] and support further the hypothesis of a post‐infectious GBSs aetiology [11]. Notably, the 2‐week interval between SARS‐CoV‐2 infection and GBSs also coincides with the second phase of COVID‐19, when the cytokine storm, the respiratory failure and the multiorgan dysfunction typically peak [40]. During this phase, in critically ill COVID‐19 patients, GBSs may masquerade as critical illness polyneuropathy, and should always be considered when difficulty in weaning from mechanical ventilation is noted. In these cases, ancillary electrophysiological and CSF testing is typically required for an accurate differential diagnosis [41]. However, unless these cases come to the attention of a neurologist, GBSs may remain underdiagnosed in critically ill COVID‐19 patients, possibly limiting the estimation of the prevalence and mortality rates mainly to non‐severe COVID‐19 cases. Furthermore, clinicians should also be aware that at least a third of SARS‐CoV‐2 patients may be asymptomatic [42], further obscuring the diagnosis of post‐COVID‐19 GBSs and highlighting the need for SARS‐CoV‐2 screening in all GBSs cases during the pandemic.
Third, due to the substantial heterogeneity in reported outcomes and patient populations of the included studies, our meta‐analysis cannot yield evidence comparable to results of large‐scale epidemiological studies. Nonetheless, as epidemiological data on COVID‐19‐associated GBSs are not currently available, the findings of the present meta‐analysis are considered to be of high relevance, as they provide preliminary estimates of GBSs prevalence amongst the COVID‐19 population. In the upcoming months, as COVID‐19 vaccines become widely available, whilst COVID‐19 continues to spread, estimates of GBSs prevalence for GBSs surveillance will be urgently needed [43]. To this end, experience gained from the 2009 H1N1 pandemic and the H1N1 vaccine safety surveillance programmes underlines the role of early monitoring of GBSs both in the vaccinated and unvaccinated populations [44].
In conclusion, the present systematic review and meta‐analysis provides evidence of an overall GBSs prevalence of 0.15‰ amongst the COVID‐19 population. Although an increased risk for GBSs was found in SARS‐CoV‐2‐infected patients compared with contemporary or historical controls, our results suggest a weaker association of GBSs with SARS‐CoV‐2 compared to infective pathogens ‘causally’ related to GBSs. The findings of (i) predominantly demyelinating GBSs subtypes amongst COVID‐19 patients, (ii) satisfactory GBSs response to immunomodulatory treatment and (iii) negative serological and CSF testing for ganglioside antibodies and SARS‐CoV‐2 RNA, respectively, indicate that GBSs most probably comprises another facet of autoimmunity in the setting of COVID‐19. Further, international cohort studies are required to independently confirm these preliminary observations and provide additional insight on the potential cause−effect relationship between COVID‐19 and GBSs.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
Lina Palaiodimou: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); visualization (equal); writing original draft (equal). Maria Ioanna Stefanou: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); visualization (equal); writing original draft (equal). Aristeidis H Katsanos: Formal analysis (equal); investigation (equal); methodology (equal); visualization (equal); writing original draft (equal). Paraskevi C. Fragkou: Investigation (equal); methodology (equal); writing review and editing (equal). Marianna Papadopoulou: Investigation (equal); methodology (equal); writing review and editing (equal). Christos Moschovos: Investigation (equal); methodology (equal); writing review and editing (equal). Ioannis Michopoulos: Data curation (equal); writing review and editing (equal). Panagiotis Kokotis: Data curation (equal); writing review and editing (equal). Christos Bakirtzis: Data curation (equal); Writing review and editing (equal). Androniki Naska: Data curation (equal); writing review and editing (equal). Theodoros I. Vassilakopoulos: Data curation (equal); writing review and editing (equal). Elisabeth Chroni: Data curation (equal); writing review and editing (equal). Sotirios Tsiodras: Supervision (equal); writing review and editing (equal). Georgios Tsivgoulis: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); methodology (equal); supervision (equal); writing original draft (equal).
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
None.
Lina Palaiodimou and Maria‐Ioanna Stefanou contributed equally to this work.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Abu‐Rumeileh S, Abdelhak A, Foschi M, Tumani H, Otto M. Guillain−Barré syndrome spectrum associated with COVID‐19: an up‐to‐date systematic review of 73 cases. J Neurol. 2020;268(4):1133–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhao H, Shen D, Zhou H, Liu J, Chen S. Guillain−Barré syndrome associated with SARS‐CoV‐2 infection: causality or coincidence? Lancet Neurol. 2020;19(5):383‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gigli GL, Bax F, Marini A, et al. Guillain−Barré syndrome in the COVID‐19 era: just an occasional cluster? J Neurol. 2021;268:1195–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Filosto M, Cotti Piccinelli S, Gazzina S, et al. Guillain−Barré syndrome and COVID‐19: an observational multicentre study from two Italian hotspot regions. J Neurol Neurosurg Psychiatry. 2020. 10.1136/jnnp-2020-324837. [DOI] [PubMed] [Google Scholar]
- 5. Keddie S, Pakpoor J, Mousele C, et al. Epidemiological and cohort study finds no association between COVID‐19 and Guillain−Barré syndrome. Brain. 2021;144(2):682–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McGrogan A, Madle GC, Seaman HE, de Vries CS. The epidemiology of Guillain−Barré syndrome worldwide. A systematic literature review. Neuroepidemiology. 2009;32(2):150‐163. [DOI] [PubMed] [Google Scholar]
- 7. Willison HJ, Jacobs BC, van Doorn PA. Guillain−Barré syndrome. Lancet. 2016;388(10045):717‐727. [DOI] [PubMed] [Google Scholar]
- 8. Wakerley BR, Soon D, Chan YC, Yuki N. Atypical Bickerstaff brainstem encephalitis: ataxic hypersomnolence without ophthalmoplegia. J Neurol Neurosurg Psychiatry. 2013;84(11):1206‐1207. [DOI] [PubMed] [Google Scholar]
- 9. Sejvar JJ, Baughman AL, Wise M, Morgan OW. Population incidence of Guillain−Barré syndrome: a systematic review and meta‐analysis. Neuroepidemiology. 2011;36(2):123‐133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wakerley BR, Yuki N. Infectious and noninfectious triggers in Guillain−Barré syndrome. Expert Rev Clin Immunol. 2013;9(7):627‐639. [DOI] [PubMed] [Google Scholar]
- 11. Dalakas MC. Guillain−Barré syndrome: the first documented COVID‐19‐triggered autoimmune neurologic disease: more to come with myositis in the offing. Neurol Neuroimmunol Neuroinflamm. 2020;7(5):e781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Parra B, Lizarazo J, Jiménez‐Arango JA, et al. Guillain−Barré syndrome associated with Zika virus infection in Colombia. N Engl J Med. 2016;375(16):1513‐1523. [DOI] [PubMed] [Google Scholar]
- 13. Yuki N. Infectious origins of, and molecular mimicry in, Guillain−Barré and Fisher syndromes. Lancet Infect Dis. 2001;1(1):29‐37. [DOI] [PubMed] [Google Scholar]
- 14. European Centre for Disease Prevention and Control . Case definition for coronavirus disease 2019 (COVID‐19), as of 3 December 2020. 2020. [Available from: https://www.ecdc.europa.eu/en/covid‐19/surveillance/case‐definition]. on Dec 18, 2020.
- 15. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1‐e34. [DOI] [PubMed] [Google Scholar]
- 16. Stroup DF, Berlin JA, Morton SC, et al. Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008‐2012. [DOI] [PubMed] [Google Scholar]
- 17. National Heart, Lung and Blood Institute . Study quality assessment tools. [Available from: https://www.nhlbi.nih.gov/health‐topics/study‐quality‐assessment‐tools. on 20 December 2020.
- 18. Wells G, Shea B, O'Connell D, et al. The Newcastle−Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta‐analyses. [Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. on December 18, 2020.
- 19. Coronavirus Resource Center of the Johns Hopkins University and Medicine . COVID‐19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). [Available from: https://coronavirus.jhu.edu/map.html. on December 18 2020.
- 20. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7(3):177‐188. [DOI] [PubMed] [Google Scholar]
- 21. Deeks JJ, Higgins JP, Altman DG, Group CSM . Analysing data and undertaking meta‐analyses. In: Higgins JP, Thomas J, Chandler M, Cumpston T, Page MJ, Welch VA, eds. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2019:241‐284. [Google Scholar]
- 22. Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wallace BC, Dahabreh IJ, Trikalinos TA, Lau J, Trow P, Schmid CH. Closing the gap between methodologists and end‐users: R as a computational back‐end. J Stat Softw. 2012;49(5):1‐15. [Google Scholar]
- 24. Fragiel M, Miro O, Llorens P, et al. Incidence, clinical, risk factors and outcomes of Guillain−Barré in COVID‐19. Ann Neurol. 2021;89(3):598–603. [DOI] [PubMed] [Google Scholar]
- 25. Liotta G, Marazzi MC, Orlando S, Palombi L. Is social connectedness a risk factor for the spreading of COVID‐19 among older adults? The Italian paradox. PLoS One. 2020;15(5):e0233329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Benetti E, Tita R, Spiga O, et al. ACE2 gene variants may underlie interindividual variability and susceptibility to COVID‐19 in the Italian population. Eur J Hum Genet. 2020;28(11):1602‐1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Benedetti L, Briani C, Beronio A, et al. Increased incidence of axonal Guillain−Barré syndrome in La Spezia area of Italy: a 13‐year follow‐up study. J Peripher Nerv Syst. 2019;24(1):80‐86. [DOI] [PubMed] [Google Scholar]
- 28. Yuki N, Hartung HP. Guillain−Barré syndrome. N Engl J Med. 2012;366(24):2294‐2304. [DOI] [PubMed] [Google Scholar]
- 29. Baric RS. Emergence of a highly fit SARS‐CoV‐2 variant. N Engl J Med. 2020;383(27):2684‐2686. [DOI] [PubMed] [Google Scholar]
- 30. Sekiguchi Y, Uncini A, Yuki N, et al. Antiganglioside antibodies are associated with axonal Guillain−Barré syndrome: a Japanese−Italian collaborative study. J Neurol Neurosurg Psychiatry. 2012;83(1):23‐28. [DOI] [PubMed] [Google Scholar]
- 31. Hasan I, Saif‐Ur‐Rahman KM, Hayat S, et al. Guillain−Barré syndrome associated with SARS‐CoV‐2 infection: a systematic review and individual participant data meta‐analysis. J Peripher Nerv Syst. 2020;25(4):335‐343. [DOI] [PubMed] [Google Scholar]
- 32. Tsivgoulis G, Palaiodimou L, Katsanos AH, et al. Neurological manifestations and implications of COVID‐19 pandemic. Ther Adv Neurol Disord. 2020;13:1756286420932036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hussain FS, Eldeeb MA, Blackmore D, Siddiqi ZA. Guillain−Barré syndrome and COVID‐19: possible role of the cytokine storm. Autoimmun Rev. 2020;19(12):102681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tsivgoulis G, Fragkou PC, Delides A, et al. Quantitative evaluation of olfactory dysfunction in hospitalized patients with coronavirus [2] (COVID‐19). J Neurol. 2020;267(8):2193‐2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tsivgoulis G, Fragkou PC, Lachanis S, et al. Olfactory bulb and mucosa abnormalities in persistent COVID‐19‐induced anosmia: a magnetic resonance imaging study. Eur J Neurol. 2021;28(1):e6‐e8. [DOI] [PubMed] [Google Scholar]
- 36. Tong JY, Wong A, Zhu D, Fastenberg JH, Tham T. The prevalence of olfactory and gustatory dysfunction in COVID‐19 patients: a systematic review and meta‐analysis. Otolaryngol Head Neck Surg. 2020;163(1):3‐11. [DOI] [PubMed] [Google Scholar]
- 37. Lee MH, Perl DP, Nair G, et al. Microvascular injury in the brains of patients with COVID‐19. N Engl J Med. 2021;384(5):481‐483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Matschke J, Lutgehetmann M, Hagel C, et al. Neuropathology of patients with COVID‐19 in Germany: a post‐mortem case series. Lancet Neurol. 2020;19(11):919‐929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Foresti C, Servalli MC, Frigeni B, et al. COVID‐19 provoking Guillain−Barré syndrome: the Bergamo case series. Eur J Neurol. 2020. 10.1111/ene.14549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pfortmueller CA, Spinetti T, Urman RD, Luedi MM, Schefold JC. COVID‐19‐associated acute respiratory distress syndrome (CARDS): current knowledge on pathophysiology and ICU treatment—a narrative review. Best Pract Res Clin Anaesthesiol. 2020. 10.1016/j.bpa.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. de Letter MA, Visser LH, van der Meché FG, Ang W, Savelkoul HF. Distinctions between critical illness polyneuropathy and axonal Guillain−Barré syndrome. J Neurol Neurosurg Psychiatry. 2000;68(3):397‐398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Oran DP, Topol EJ. The proportion of SARS‐CoV‐2 infections that are asymptomatic : a systematic review. Ann Intern Med. 2021. 10.7326/M20-6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lunn MP, Cornblath DR, Jacobs BC, et al. COVID‐19 vaccine and Guillain−Barré syndrome: let's not leap to associations. Brain. 2021;144(2):357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vellozzi C, Iqbal S, Broder K. Guillain−Barré syndrome, influenza, and influenza vaccination: the epidemiologic evidence. Clin Infect Dis. 2014;58(8):1149‐1155. [DOI] [PubMed] [Google Scholar]
- 45. Espíndola OM, Brandão CO, Gomes YCP, et al. Cerebrospinal fluid findings in neurological diseases associated with COVID‐19 and insights into mechanisms of disease development. Int J Infect Dis. 2020;102:155‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Guilmot A, Maldonado Slootjes S, Sellimi A, et al. Immune‐mediated neurological syndromes in SARS‐CoV‐2‐infected patients. J Neurol. 2021;268(3):751‐757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Koh JS, De Silva DA, Quek AML, et al. Neurology of COVID‐19 in Singapore. J Neurol Sci. 2020;418:117118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kushwaha S, Seth V, Bapat P, et al. Neurological associations of COVID‐19—do we know enough: a tertiary care hospital based study. Front Neurol. 2020;11:588879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Meppiel E, Peiffer‐Smadja N, Maury A, et al. Neurologic manifestations associated with COVID‐19: a multicentre registry. Clin Microbiol Infect. 2021;27(3):458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Paterson RW, Brown RL, Benjamin L, et al. The emerging spectrum of COVID‐19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143(10):3104‐3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Romero‐Sánchez CM, Díaz‐Maroto I, Fernández‐Díaz E, et al. Neurologic manifestations in hospitalized patients with COVID‐19: the ALBACOVID registry. Neurology. 2020;95(8):e1060‐e1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Abolmaali M, Heidari M, Zeinali M, et al. Guillain−Barré syndrome as a parainfectious manifestation of SARS‐CoV‐2 infection: a case series. J Clin Neurosci. 2021;83:119‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Garnero M, Del Sette M, Assini A, et al. COVID‐19‐related and not related Guillain−Barré syndromes share the same management pitfalls during lock down: the experience of Liguria region in Italy. J Neurol Sci. 2020;418:117114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lascano AM, Epiney JB, Coen M, et al. SARS‐CoV‐2 and Guillain−Barré syndrome: AIDP variant with a favourable outcome. Eur J Neurol. 2020;27(9):1751‐1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Manganotti P, Bellavita G, D'Acunto L, et al. Clinical neurophysiology and cerebrospinal liquor analysis to detect Guillain−Barré syndrome and polyneuritis cranialis in COVID‐19 patients: a case series. J Med Virol. 2021;93(2):766‐774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nanda S, Handa R, Prasad A, et al. COVID‐19 associated Guillain−Barré syndrome: contrasting tale of four patients from a tertiary care centre in India. Am J Emerg Med. 2021;39:125‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Toscano G, Palmerini F, Ravaglia S, et al. Guillain−Barré syndrome associated with SARS‐CoV‐2. N Engl J Med. 2020;382(26):2574‐2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


