Abstract
Extracorporeal membrane oxygenation (ECMO) is a life‐saving therapy utilized for patients with severe life‐threatening cardiorespiratory failure. Patients treated with ECMO are among the most severely ill encountered in critical care and are at high‐risk of developing multiple organ dysfunction, including acute kidney injury (AKI) and fluid overload. Continuous renal replacement therapy (CRRT) is increasingly utilized inpatients on ECMO to manage AKI and treat fluid overload. The indications for renal replacement therapy for patients on ECMO are similar to those of other critically ill populations; however, there is wide practice variation in how renal supportive therapies are utilized during ECMO. For patients requiring both CRRT and ECMO, CRRT may be connected directly to the ECMO circuit, or CRRT and ECMO may be performed independently. This review will summarize current knowledge of the epidemiology of AKI, indications and timing of CRRT, delivery of CRRT, and the outcomes of patients requiring CRRT with ECMO.
1. INTRODUCTION
Extracorporeal membrane oxygenation (ECMO) is a life‐saving therapy utilized for patients of all ages with severe life‐threatening cardiopulmonary failure. 1 The indications for ECMO include reversible conditions with a high predicted mortality of ≥80%. Although ECMO was originally developed as a rescue therapy for neonates with respiratory failure, in recent years the number of adults placed on ECMO has surpassed the number of pediatric and neonatal patients combined. 1 , 2 , 3 Patients treated with ECMO represent the sickest patients encountered in critical care and are at high‐risk of developing multiple organ dysfunction and associated sequelae, including acute kidney injury (AKI) and fluid overload (FO). Continuous renal replacement therapy (CRRT) is being increasingly utilized in patients on ECMO to manage AKI, prevent and treat FO. This review will summarize our current understanding of the epidemiology and impact of AKI, indications and timing for CRRT, delivery of CRRT, and the outcomes associated with CRRT for patients on ECMO.
2. PATHOPHYSIOLOGY, EPIDEMIOLOGY, AND OUTCOMES ASSOCIATED WITH AKI
The pathophysiology and high incidence of AKI in those on ECMO is multifactorial in nature with significant contributions from the underlying disease and additional factors inherent to ECMO. Individuals being placed on ECMO are among the highest risk patients to develop AKI prior to cannulation related to their severity of illness as well as the etiology and treatment of their primary disease (respiratory failure, cardiac failure, hypotension requiring vasopressor support, cardiac arrest, ischemia, nephrotoxic exposures). 4 , 5
There are a multitude of pathophysiologic mechanisms inherent to ECMO that potentially contribute to the exacerbation of existing and/ or development of new AKI. The hemodynamic changes around the time of ECMO cannulation can impact renal blood flow resulting in ischemic/ reperfusion injury. 6 , 7 Additional variables associated with ECMO that can predispose to AKI include systemic inflammation, 8 , 9 hemolysis, 10 , 11 microcirculatory dysfunction, and platelet/ coagulation abnormalities. 12 , 13
Acute kidney injury has been shown to occur commonly in patients of all ages treated with ECMO (Table 1). AKI occurs commonly across all populations treated with ECMO with incidence ranging from 42% to 85%. 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 The existing data show clearly that a significant amount of AKI is present at the time of ECMO cannulation and the majority develops within 48 h of ECMO initiation. 15 , 16 , 18 , 19 , 22 The data evaluating outcomes associated with AKI consistently shows that the highest stage of AKI is associated with increased morbidity and mortality across populations. The small size, low power and single center nature of these studies likely explains the inconsistent association with low stages of AKI with outcomes. The only multicenter study to date has been performed by the KIDMO study group and clearly shows that AKI of any stage is associated with increased morbidity (increase length of ECMO in those with AKI, 149 vs 121 h) and mortality (adjusted odds ratio, 1.52; 1.04–2.21) in a cohort 832 pediatric and neonatal patients from six centers. 16 Further multicenter collaborative work is needed to understand the problem across medicine.
TABLE 1.
Incidence and outcomes associated with AKI on ECMO
| Author/year (N) | Study design/details | Incidence of AKI | Findings |
|---|---|---|---|
| Adults | |||
| Tsai, 2017 (n = 167) 21 |
|
Incidence of AKI within 48 h of ECMO initiation: 85% | Multivariable analysis: AKI, Glasgow Coma Scale on first ECMO day, Hg on first ECMO day associated with mortality |
| Antonucci, 2016 (n = 135) 14 |
|
70% | Multivariable analysis: ICU mortality was not associated with AKI or RRT |
| Haneya, 2015 (n = 262) 18 |
|
|
|
| Lee, 2015 (n =322) 19 |
|
Incidence of AKI within 24 h of ECMO initiation: 82% | Stage 3 AKI was associated with in‐hospital mortality, with a hazard ratio (HR) (95% CI) of 2.690 (1.472–4.915) |
| Chen, 2011 (n = 102) 15 |
|
|
AKI at 48 predicted in hospital mortality |
| Yan, 2010 (n = 67) 22 |
|
85% within 48 h of ECMO initiation | Stage 3 AKI associated with increased mortality |
| Pediatric and neonatal | |||
| Fleming, 2016 (n = 832) 16 |
|
74% |
|
| Zwiers, 2013 (n = 242) 23 |
|
64% | AKI stage F: Increased mortality (65%) |
| Smith et al, 2013 (n = 46) 20 |
|
71% | Increased mortality (aOR 4.7) |
| Gadepalli, 2009 (n = 68) 17 |
|
71% | AKI stage F associated with Increased mortality, length of stay, less ventilator free days |
2.1. Indications and Timing for CRRT
The indications for renal replacement therapy for patients on ECMO are similar to those of other critically ill populations and include: acidosis, electrolyte abnormalities, intoxications, FO, and uremia. 4 , 5 In 2010, the Kidney Interventions During Membrane Oxygenation (KIDMO) study group surveyed 65 participating Extracorporeal Life Support Organization (ELSO) centers and showed that the most common indications for initiating CRRT on ECMO were: FO (43%), FO prevention (16%), and AKI (35%). 24 In 2020, the KIDMO study group performed a similar survey focused on pediatric and neonatal centers and showed the treatment and/ or prevention of FO was the primary indication for CRRT on ECMO in 85% of centers (in press ASAIO). These data highlight the importance of FO in decision making surrounding the initiation of CRRT on ECMO.
For patients on ECMO the fluid status of the patient should be evaluated daily on rounds. The daily fluid balance and cumulative fluid balance should be utilized in medical decision making. The following equations can be utilized to describe the fluid status of patients on ECMO 25 , 26 :
Studies evaluating the impact of FO in patients on ECMO have commonly utilized these equations to describe the epidemiology of FO and impact of FO on outcomes. Changes in daily weight have also been reliably utilized to calculate fluid balance in pediatric patients and neonates on ECMO. 27 , 28 , 29 , 30
The deleterious impact of FO on outcomes for patients on ECMO was recognized early in the history of neonatal and pediatric ECMO. In 2000, Swaniker et al 29 reported that a failure to return to “dry weight” was associated with adverse outcomes in a cohort of 128 children treated with ECMO, which confirmed earlier reports in neonatal populations. 30 In recent years, there has been an expansion of our understanding of the epidemiology and impact of fluid accumulation and subsequent FO in patients on ECMO (Table 2). The KIDMO study group recently showed that FO occurs commonly (Peak FO: ≥10% in 84.8%, ≥20% in 67.2%, and ≥50% in 29%) and is independently associated with increase mortality (aOR per 10% rise in peak FO, 1.09; 95% CI, 1.04–1.15) and length of ECMO in a multicenter cohort of 656 pediatric and neonatal patients treated with ECMO. 27 In a planned secondary analysis of the KIDMO study, this group showed that the degree of FO at CRRT initiation consistently predicted adverse outcomes confirming earlier pediatric single center studies. 28 , 31 Fluid balance in the first 3–5 days on ECMO and cumulative fluid balance on ECMO have consistently been shown to be associated with outcomes in adult populations on ECMO. 25 , 32 , 33 , 34 The ELSO has provided the following recommendation surrounding volume management on ECMO, “return the extracellular fluid volume to normal (dry weight) and maintain it there.” 35 The current literature shows that fluid status and the development of FO adversely impacts outcomes across ECMO populations. Taken together this data suggests that there may be a role for early initiation of CRRT in volume management for patient on ECMO.
TABLE 2.
Studies evaluating the impact of fluid overload and the timing of the initiation of CRRT on Outcomes in ECMO
| Author, year (N) | Study population details | Study design | Method of FO measurement | Main findings |
|---|---|---|---|---|
| Adults | ||||
| Dado, 2020 (n = 48) 93 | Patients undergoing ECMO and CRRT | Retrospective single‐center | Fluid balance on ECMO over 3 days | |
| Fong, 2020 (n = 123) 32 | All patients treated with ECMO | Retrospective single‐center | Fluid balance while on ECMO |
|
| Besnier, 2020 (n = 101) 33 | All patients treated with VA ECMO | Retrospective single‐center | Fluid balance and weight changes over first 5 days on ECMO |
|
| McCanny, 2019 (n = 24) 34 | Patients treated with VV ECMO and CRRT, 2010–2015 | Retrospective single‐center | Fluid balance |
|
| Schmidt, 2014 (n = 172) 25 | All patients treated with ECMO | Retrospective single‐center | Fluid balance |
|
| Pediatric and neonatal | ||||
| Gorga, 2020 (n = 357) 31 | All patients <18 year old treated with ECMO and CRRT, 2007–2011 | Retrospective multicenter, 6 centers | Fluid balance while on ECMO |
|
| Selewski, 2017 (n = 756) 27 | All patients treated with ECMO, 2007–2011 | Retrospective multicenter, 6 centers | Fluid balance while on ECMO |
|
| Selewski, 2015 (n = 53) 28 | All patients treated with CRRT and ECMO, 2006–2010 | Retrospective, single‐center | Change in in daily weight |
|
| Blijdorp, 2009 (n = 61) 37 | Pre‐emptive CRRT during ECMO, <28 days, NICU | Retrospective case‐comparison study | Fluid balance while on ECMO | Pre‐emptive CRRT improves outcomes by decreasing time on ECMO because of improved fluid management |
| Hoover, 2008 (n = 52) 36 | All patients receiving ECMO, Age 1 month old–18 years, PICU, 1992–2006 | Retrospective case‐matched study (Patients receiving CRRT +ECMO vs. ECMO alone) | Fluid balance while on ECMO |
Use of CRRT with ECMO was associated with:
|
To date there has not been a systematic trial evaluating the timing of the initiation of CRRT on ECMO. Single center studies in pediatric and neonatal populations have suggested that the early initiation of CRRT in patients on ECMO is feasible and associated with improved outcomes (fluid balance, nutrition, improved chest X‐rays, and decreased length of ECMO). 36 , 37 , 38 , 39 , 40 A meta‐analysis performed in 21,642 adults on ECMO found an association between earlier initiation of CRRT in those that go on to require CRRT and improved survival. 41 Further study and clinical trials are greatly needed to better define the role and optimal timing of the initiation of CRRT in patients on ECMO. Until more definitive studies are available, the authors suggest that the need for CRRT be evaluated at initiation of ECMO and daily on rounds based on the patient's clinical course and fluid status. CRRT should be instituted when FO is anticipated or has developed and medical management (diuretics) are unlikely to be successful. 4 , 5 , 35
3. DELIVERY OF CRRT TO ECMO PATIENTS
There is wide practice variation in how renal supportive therapies are utilized during ECMO. 24 For patients requiring both CRRT and ECMO, the CRRT machine may be connected directly to the ECMO circuit, or CRRT and ECMO may be performed independently. There are advantages and disadvantages to both options, but it is important to note that connecting CRRT with ECMO is not currently a US Food and Drug Administration (FDA)‐approved strategy. CRRT can be performed by adding a hemofilter or CRRT device into the ECMO circuit (integrated system) or by placing a separate catheter for independent renal support. 4 , 49 Any CRRT modality—continuous veno‐venous hemofiltration (CVVH), continuous veno‐venous hemodialysis (CVVHD), continuous veno‐venous hemodiafiltration (CVVHDF) or slow continuous ultrafiltration (SCUF)—can be combined with ECMO. CRRT devices are designed to connect to venous pressures ranging from 0 to 20 mm Hg, while negative pressures are generated by the centrifugal pump (−20 to −100 mm Hg) in the drainage limb of the ECMO circuit, and positive pressures (100–500 mm Hg) are present in the limbs distal to the pump. 46 The CRRT machine may alarm due to a low inlet pressure and potentially stop dialysis. Occasionally, CRRT alarm ranges need to be modified to accommodate these pressure differences, although some newer CRRT devices can be programmed at start‐up to recognize the ECMO circuit. Overriding alarm limits may result in low negative pressures, hemolysis, flow turbulence, and air embolization.
The approach to initiating RRT with ECMO is the same for both VA‐ and VV‐circuits and can be classified as separate or integrated systems. Options for CRRT delivery with ECMO are further detailed below:
3.1. Independent CRRT and ECMO circuits
A separate vascular access is established, and the CRRT and ECMO circuits operate independently. A main advantage is the ability to adjust the settings and performance of each device without (or minimally) interfering with the function of the other. Disadvantages include the possibility of having limited sites for CRRT vascular access, particularly if remaining vascular sites are needed for ECMO support. 50 , 51 There may also be some increased bleeding risk during catheter insertion, or other procedures, due to anticoagulation use for the ECMO circuit.
3.1.1. Addition of an in‐line hemofilter into the ECMO circuit
An in‐line hemofilter or CRRT circuit may be integrated into the ECMO circuit. The inlet limb (access port) of a hemofilter can be connected after the blood pump, and the outlet limb (return port) is typically connected prior to the membrane oxygenator (Figure 1). This approach is less costly compared to CRRT, but disadvantages include a lack of pressure alarms and poor control of net ultrafiltration. A stopcock or similar instrument to restrict blood flow can be added but may increase the risk of thrombosis or hemolysis. SCUF is typically the most common modality used for RRT with a hemofilter, the blood flow through which is driven by the ECMO pump.
FIGURE 1.
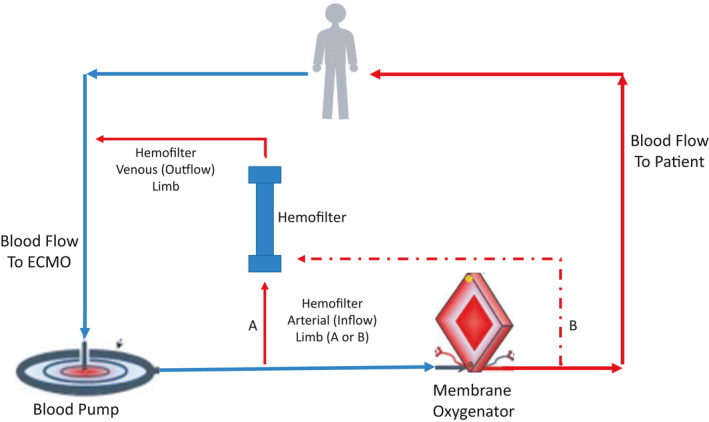
Inline Hemofilter combined with ECMO. The inflow to the hemofilter is typically distal to the blood pump, either between the pump and oxygenator or distal to the oxygenator. The outflow from the hemofilter typically returns prior to the blood pump, but can also return prior to the oxygenator
3.1.2. CRRT combined with ECMO
Combining CRRT with the ECMO circuit avoids additional catheter‐associated complications, including risks associated with catheter insertion, infection, and mechanical complications. However, combined CRRT and ECMO may result in abnormal pressures in the ECMO circuit (low‐pressure alarms when the CRRT drainage or return access is placed before the blood pump, and high‐pressure alarms when placed after the blood pump). 52 High pressures in the CRRT circuit may result in treatment interruptions or stop the circuit. As a result, alarm adjustments may be necessary on some CRRT devices. Newer generation CRRT devices can be programmed to account for pressure changes when connecting to the ECMO circuit or automatically recognize an ECMO connection. Whether connecting CRRT to the ECMO circuit ultimately reduces complications, as compared to providing each independently, is yet to be examined in a prospective manner.
Strategies for combining CRRT and ECMO have previously been described. 4 , 5 , 44 , 45 , 48 , 49 , 52 The CRRT and ECMO circuits can be joined, thereby allowing for circuit pressure monitoring and better net ultrafiltration control. Depending on the ECMO device utilized, the inflow to the CRRT device can be placed before or after the blood pump, or in some cases between the blood pump and oxygenator when these components are separated (Figures 2, 3, 4). Blood from the CRRT device is typically returned to the ECMO circuit before the membrane oxygenator to reduce the risk of systemic emboli. Extracorporeal carbon dioxide removal can also be achieved by inserting a membrane oxygenator, rather than full ECMO support, into the CRRT circuit. 53 , 54 This technique has been used to permit protective lung ventilation in severe acute respiratory distress syndrome (ARDS) and to improve acidosis in hypercapnic respiratory failure.
FIGURE 2.
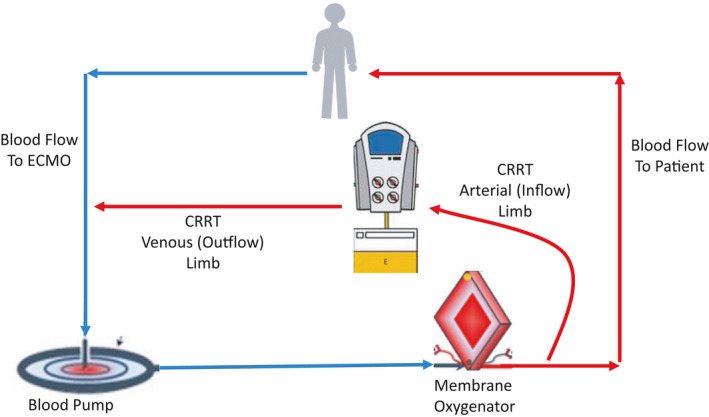
CRRT combined with ECMO. In this example, the inflow to the CRRT machine is distal to the oxygenator, and the outflow from the CRRT machine returns to the ECMO circuit proximal to the blood pump
FIGURE 3.
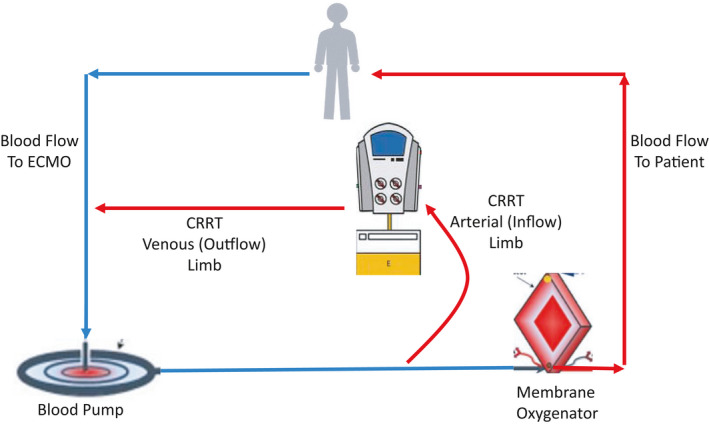
CRRT combined with ECMO. In this example, the inflow to the CRRT machine is between the blood pump and the oxygenator, and the outflow from the CRRT machine returns to the ECMO circuit proximal to the blood pump
FIGURE 4.
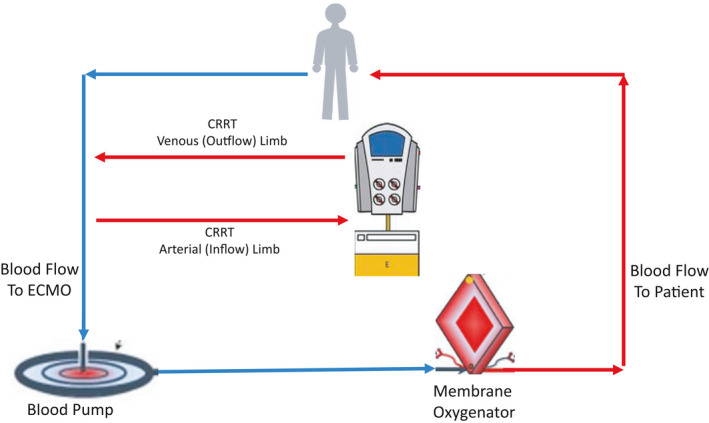
CRRT combined with ECMO. In this example, both the inflow to and outflow from the CRRT machine are connected proximal to the blood pump
There are several ways that a CRRT circuit can be combined with an ECMO circuit. The CRRT inflow line can be connected to the ECMO circuit before or after the centrifugal pump. The CRRT outflow line can be connected before the centrifugal pump or between the centrifugal pump and membrane oxygenator. There is an increased risk of clot or air embolism when the CRRT outflow line is connected prior to the centrifugal pump, where the pressure is negative. Beyond the centrifugal pump, ECMO circuit pressure changes from negative to positive. Some ECMO circuits have a blood flow limb separating the pump and the oxygenator, while other circuits may have an integrated pump and oxygenator (and thus no blood flow limb in‐between). Where the pump and oxygenator are separated, the CRRT inflow line may arise after the centrifugal pump, and the CRRT outflow may return to the ECMO circuit proximal to the oxygenator. The advantage of this configuration is that the oxygenator serves as a bubble trap, while the positive circuit pressure at this site should lower the risk of air entry. Third, the CRRT inlet line can be connected distal to the oxygenator, and the CRRT outlet line returns to a site prior to the centrifugal pump. This configuration offers optimal blood flow into the CRRT circuit and no resistance to outflow.
4. ANTICOAGULATION
Giani et al 55 performed a retrospective review of 48 adult patients who received CRRT while on VV‐ECMO support between 2009 and 2018. They examined the safety and efficacy of adding regional citrate anticoagulation (RCA + UFH group) for CRRT anticoagulation, compared with anticoagulation with systemic heparin alone (UFH group). The study's end points included filter life span, occurrence of CRRT circuit clotting, coagulation parameters, and any citrate‐related complications. The median duration of ECMO support was 16 (7–26) days, and 18 (37%) patients were on CRRT before ECMO. By their protocol, CVVHDF was used when UFH is the sole anticoagulant, while CVVHD was used when RCA was added and UFH remained unchanged. They found fewer CRRT clotting events in the RCA +UFH group (11% vs 38%, p < 0.001). Survival analysis showed a longer circuit lifetime for the RCA + UFH group. There were no significant complications related to the use of citrate anticoagulation. In‐hospital mortality for ECMO with CRRT patients was 38% and did not differ significantly from the other cohorts.
When using UFH anticoagulation alone for both the CRRT and ECMO circuits, UFH is administered systemically, and anticoagulation is targeted to an aPTT level of 60–80 s (1.5–2.0 × baseline) or anti‐factor Xa level of 0.3–0.7. 56 Thromboelastography reaction (R) times have also been used to monitor anticoagulation with ECMO. 57 , 58 , 59 , 60 , 61 , 62 , 63 However, the anticoagulation goal and frequency of monitoring may vary according to the desired intensity of anticoagulation and center‐specific protocols. 56 , 73 Antithrombin III (ATIII) levels should also be monitored occasionally, with levels maintained >50%. In situations where UFH cannot be used, anticoagulation for ECMO has been achieved using direct thrombin inhibitors. 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 There is no change in the management of systemic anticoagulation for ECMO whether RCA is used for the CRRT circuit or not. Use of RCA allows for continued anticoagulation of the CRRT circuit, should systemic anticoagulation need to be reduced or suspended. ECMO without use of therapeutic anticoagulation has been reported, but this has not yet included patients on combined CRRT with ECMO. 85 , 86 , 87 , 88
5. COMPLICATIONS
Complications related to CRRT include problems with vascular access, bleeding, electrolyte disturbances, nutrient losses, arrhythmias, and hypothermia. Vascular access complications may be varied and include vascular injury, arterial puncture or dilation, hematoma, fistula formation, or thrombosis. Additionally, placement of a cannula may (depending on the location) result in pneumothorax, hemothorax, cardiac perforation, pericardial tamponade, or retroperitoneal hemorrhage. The most common electrolyte disturbances include hypokalemia and hypophosphatemia, which may contribute to cardiac rhythm abnormalities, hemolysis, or rhabdomyolysis. When CRRT is combined with ECMO, precautions must be taken to minimize the risk of air embolization into the circuit, which may result during central venous line placement, connecting or disconnecting CRRT to or from the ECMO circuit, or connecting infusions to the lines or circuit. Often, ECMO blood flow rates may be lowered, thereby reducing the negative circuit access pressure and risk of air entry.
6. OUTCOMES
An important consideration surrounding CRRT and ECMO management is whether combining the two modalities is safe. Studies have consistently demonstrated the safety and feasibility of combining CRRT with ECMO. Chen et al 42 performed a systematic review to examine the combination of CRRT and ECMO in critically ill patients. Nineteen studies were identified that described the methods for performing CRRT with ECMO: independent CRRT access, placement of a hemofiltration filter into the ECMO circuit, and placement of a CRRT device into the ECMO circuit. For ECMO survivors receiving CRRT, overall fluid balance was less than that in non‐CRRT survivors. They identified a higher mortality (OR 5.89; 95% CI 4.38–7.92; p < 0.0001) and longer ECMO duration when CRRT was added to ECMO but concluded that the two modalities could be combined and performed together in a safe manner.
Studies examining factors predictive of outcome for patients receiving CRRT with ECMO are emerging in the literature. Lee et al 89 studied the impact of different CRRT modalities on survival in patients receiving ECMO using claims data from Taiwan's National Health Insurance Research Database. The authors identified 1077 patients, of which 849 received CVVH and 228 received CVVHD. An important study limitation included a lack of data on whether CRRT and ECMO were performed independently or combined, and while the ECMO indication was reported, ECMO modality (VA vs VV) was unknown. The most common ECMO indication was post‐cardiotomy shock (49.2%), followed by respiratory—non‐pneumonia (13.0%), CV—non‐ischemia (myocarditis) (11.5%), and respiratory—pneumonia (10.7%). The CVVH group had a lower risk of in‐hospital mortality (68.4% vs 76.9%; OR 0.65; 95% CI 0.50–0.85) compared with the CVVHD group. The CVVH group also had a shorter mean ICU stay as compared with the CVVHD group (mean difference −4.59 days, 95% CI −9.15 to −0.03 days). While the authors concluded that CVVH may be associated with a lower risk of in‐hospital mortality in ECMO patients with AKI, this finding has not yet been validated in larger, prospective studies.
He et al 90 analyzed 32 patients that received CRRT with ECMO between 2007 and 2017. Patients received VA‐ECMO support, and CRRT was provided as CVVH or CVVHD. For all patients, CRRT was combined with the ECMO circuit, rather than performed independently. Multivariable analysis identified fluid balance at day 3 (median value for survivors vs non‐survivors, 210 ml vs 1090 ml; OR 5.27; 95% CI 1.38–20.09; p = 0.015) and lactate at CRRT initiation (mean value for survivors vs. non‐survivors, 4.66 vs 7.07; OR 2.12; 95% CI 1.10–4.06; p = 0.024) as independently associated with survival. The authors suggest that earlier CRRT initiation into the ECMO circuit may reduce the harmful effects of fluid accumulation and improve outcomes. The deleterious effect of early fluid accumulation on mortality has also been reported for children receiving ECMO. 91
Initial reports suggested a high mortality, up to 80%, associated with the combination of CRRT with ECMO. 92 More recently, Dado et al. evaluated 92 patients who underwent ECMO support between 2012 and 2018 at a single institution. 93 They identified 48 (53.3%) patients supported by CRRT (CVVH) with ECMO (a majority of which had combined circuits) and compared them with 42 patients supported by ECMO alone. Seventy‐three of 90 (81.1%) patients received VV ECMO. Of those patients receiving CRRT with ECMO, the mortality rate was 39.5%. The mortality rate for those receiving ECMO alone was 31.4% (p = 0.074). Of 29 survivors, 6 (20.7%) were dialysis‐dependent at hospital discharge. In a multivariable analysis, increasing age (OR 1.07; 95% CI 1.01–1.13; p = 0.01) and positive net fluid balance (OR 1.02; 95% CI 1.01–1.04; p = 0.0009) were independently associated with mortality.
Deatrick et al 94 reported outcomes for 187 patients who received VV ECMO from 2014 to 2018 at a specialty center. The most common indications for VV ECMO were bacterial pneumonia (22.5%), ARDS not postop (19.8%), viral pneumonia (18.2%), and aspiration pneumonia (17.0%). Overall survival to hospital discharge was 74.6%. Ninety‐four (50.3%) patients had CRRT (CVVH) while on VV ECMO, with 57 (61.0%) surviving to hospital discharge. By comparison, 82 (88%) patients in the VV ECMO‐alone group survived to discharge (p < 0.001). Patients requiring CRRT had a lower pH (7.16 vs 7.25), lower Respiratory ECMO Survival Prediction (RESP) score (2 vs 4), higher Sequential Organ Failure Assessment (SOFA) score (14 vs 10), more pre‐existing CKD (7 vs 0), and a higher median serum creatinine (2.2 mg/dl vs 0.98 mg/dl), (for all comparisons, p < 0.001). Notably, there was a 93% (n = 53) rate of renal recovery before hospital discharge, with only 4 (6.8%) patients needing IHD at discharge. Devasagayaraj et al 95 reported similar outcomes in 54 patients supported by VV ECMO for ARDS. ECMO survival in the presence of AKI was lower compared to the non‐AKI group (56% vs 87%, p = 0.014). The AKI group also had more ECMO‐related complications, including liver failure and bleeding, despite comparable ECMO durations (14 and 12 days, respectively, p = 0.31).
In one of the largest series to date, Kuo et al identified 2272 patients the Taiwan National Insurance Research Database who received first‐time CRRT with ECMO between 2007 and 2013. 96 Their aim was to assess outcomes and complications according to the duration of CRRT received: ≤3 days, 4–6 days, and ≥7 days. Survival did not significantly differ among the CRRT duration groups; however, patients receiving CRRT ≥7 days had a higher risk of ESRD (aHR 3.46; 95% CI 1.47–8.14), ventilator dependence (aHR 2.45; 95% CI 1.32–4.54), and readmission rate (aHR 1.67; 95% CI 1.13–2.47), compared to patients receiving ≤3 days of CRRT. These findings raise questions regarding how prolonged CRRT with ECMO may affect post‐discharge quality of life and risk for long‐term disability.
While prediction scores are available to estimate ECMO survival and presumably help select appropriate candidates for ECMO support, current models do not reliably factor the mortality contribution related to AKI or need for CRRT. The Predicting Death for Severe ARDS on VV‐ECMO (PRESERVE) index, published by Schmidt et al in 2013, utilized data from 140 ARDS patients at 3 French ICUs and identified factors independently associated with death by 6 months post‐ICU discharge: age, body mass index, immunocompromised status, prone positioning, days of mechanical ventilation, sepsis‐related organ failure assessment, plateau pressure, and positive end‐expiratory pressure. 97 Survival differed by PRESERVE score classification—97% (score 0–2), 79% (score 3–4), 54% (score 5–6), and 16% (score ≥7). While renal insufficiency was recorded, it was not associated with mortality in this cohort. The RESP score utilized ELSO registry data from 2000 to 2012 to develop a model for predicting hospital survival at the initiation of ECMO for respiratory failure. 98 The authors identified 12 pre‐ECMO variables (age, immunocompromised status, mechanical ventilation duration before ECMO initiation, acute respiratory failure diagnosis group, CNS dysfunction, acute non‐pulmonary‐associated infection, neuromuscular blocking agent use, nitric oxide use, bicarbonate infusion, cardiac arrest, PaCO2, and peak inspiratory pressure) associated with survival and developed a scoring system that predicted survival, with a step‐wise decline in survival by RESP score risk class (ranging from 92% at RESP class I to 18% at RESP class V). While renal dysfunction was initially identified for potential inclusion, it was not independently predictive of survival and thus not included in the final RESP score. The Survival after Veno‐arterial ECMO (SAVE) score, by contrast, utilizes pre‐ECMO factors associated with survival for patients with refractory cardiogenic shock requiring VA ECMO to create a predictive survival model. 99 SAVE incorporates both acute renal dysfunction (Cr >1.5 mg/dl, with or without RRT) and chronic renal failure (kidney damage or glomerular filtration rate <60 ml/min/1.73 m2 for ≥3 months) into the score, and in‐hospital survival decreases with increasing risk class (ranging from 75% at SAVE class I to 18% at SAVE class V). Additional predictive scoring models have been published for acute myocardial infarction with cardiogenic shock (ENCOURAGE), VA‐ECMO use and cardiopulmonary resuscitation patients (PREDICT‐VA ECMO), and after coronary artery bypass grafting (REMEMBER), with measures of renal function variably incorporated in the models. 100 , 101 , 102
To date, there is limited data on the impact of SARS‐CoV‐2 (COVID‐19) on ECMO‐related AKI outcomes. Barbaro et al utilized data from the ELSO Registry to describe the epidemiology, course, and outcomes of patients 16 years of age and older with confirmed COVID‐19 who underwent ECMO support at 213 hospitals in 36 countries. In patients with COVID‐19 receiving VV‐ECMO and characterized as having ARDS), the estimated cumulative incidence of in‐hospital mortality at 90 days after ECMO initiation was 38.0% (95% CI: 34.6–41.5). While only 2% of patients had pre‐existing renal insufficiency, AKI was present in 247 of 779 (32%), and renal replacement therapy was ultimately used during ECMO support in 444 of 1006 (44%) patients (with data missing for 29 patients). Multivariable Cox modeling identified AKI (along with increasing age, immunocompromise, chronic respiratory disease, pre‐ECMO cardiac arrest, and initial mode VA) as associated with in‐hospital mortality (for AKI, HR 1.38; 95% CI: 1.08–1.76). 103
7. RENAL RECOVERY
Renal recovery data in patients who have received ECMO with CRRT are limited. Baek et al 104 performed a retrospective observational study of 124 patients that received CRRT for AKI at their institution between January and December 2014. Their aim was to identify clinical factors that may predict CRRT duration for AKI survivors. There was a total of 21 patients that received ECMO support, 18 of which required long‐duration (>6 days) CRRT. ECMO duration ranged from 1 to 9 days and included patients supported by VA and VV modalities. While a minority of patients received ECMO, a multivariable analysis identified oliguria (<0.5 ml/kg/h) (OR 3.45; 95% CI 1.34–8.86; p = 0.01), mechanical ventilation use (OR 7.89; 95% CI 2.40–25.92; p = 0.001), and ECMO use (OR 6.52; 95% CI 1.57–27.16; p = 0.01) as predictors of long‐duration CRRT. In their study of 48 patients requiring CRRT with VV‐ECMO, Giani et al 55 found that renal recovery occurred in 23 of 30 survivors, whereas seven patients were discharged from the ICU with an ongoing requirement for renal replacement therapy. Other studies of neonate and pediatric as well as adult survivors observed higher rates (>90%) of renal recovery. 92 , 105 , 106 In their study, Thajudeen et al 92 found that all patients that had renal recovery received VA ECMO, and they proposed that VA ECMO facilitated this recovery by increasing oxygen delivery to the renal vessels.
8. FUTURE DIRECTIONS
With continued growth in the use of ECMO for critically ill patients, several opportunities arise that may help improve the safety and efficacy of this technology, particularly when CRRT is also required. An optimal anticoagulation strategy for ECMO patients requiring CRRT is yet to be defined. Similarly, the intensity of anticoagulation—standard vs low‐dose—and best strategies for monitoring efficacy and complications have not been widely studied. There is also limited data on pharmacokinetics and medication dosing, particularly with combined circuits. Given the effects of respiratory failure, hemodynamic compromise, mechanical ventilation, and ECMO initiation on the incidence of AKI, studies that aim to determine best management methods are warranted. Developing and validating better AKI predictive models for ECMO patients, and identifying factors predictive of survival and renal recovery, are needed for this population. How ECMO affects the reliability and utility of AKI biomarkers is unclear and not yet widely studied in this population. 107 While the deleterious impact of AKI and FO on outcomes for ECMO patients is clear, critical questions warranting further study remain regarding the role of CRRT in patient management, including device, modality, and optimal timing of initiation. Finally, management strategies that ultimately prevent the occurrence of AKI in the setting of ECMO and lower AKI‐associated morbidity and mortality are desired.
ACKNOWLEDGEMENT
The authors wish to acknowledge Mr. Leo Black and Ms. Kela Beans for their assistance with the critical review of this manuscript.
Selewski DT, Wille KM. Continuous renal replacement therapy in patients treated with extracorporeal membrane oxygenation. Semin Dial. 2021;34(6):537–549. 10.1111/sdi.12965
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1. Nasr VG, Raman L, Barbaro RP, et al. Highlights from the extracorporeal life support organization registry: 2006–2017. ASAIO J. 2019;65(6):537‐544. [DOI] [PubMed] [Google Scholar]
- 2. Barbaro RP, Paden ML, Guner YS, et al. Pediatric extracorporeal life support organization registry international report 2016. ASAIO J. 2017;63(4):456‐463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thiagarajan RR, Barbaro RP, Rycus PT, et al. Extracorporeal life support organization registry international report 2016. ASAIO J. 2017;63(1):60‐67. [DOI] [PubMed] [Google Scholar]
- 4. Askenazi DJ, Selewski DT, Paden ML, et al. Renal replacement therapy in critically ill patients receiving extracorporeal membrane oxygenation. Clin J Am Soc Nephrol. 2012;7(8):1328‐1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ostermann M, Connor M Jr, Kashani K. Continuous renal replacement therapy during extracorporeal membrane oxygenation: why, when and how? Curr Opin Crit Care. 2018;24(6):493‐503. [DOI] [PubMed] [Google Scholar]
- 6. Keckler SJ, Laituri CA, Ostlie DJ, et al. A review of venovenous and venoarterial extracorporeal membrane oxygenation in neonates and children. Eur J Pediatr Surg. 2010;20(1):1‐4. [DOI] [PubMed] [Google Scholar]
- 7. Kilburn DJ, Shekar K, Fraser JF. The complex relationship of extracorporeal membrane oxygenation and acute kidney injury: Causation or association? Biomed Res Int. 2016;2016:1094296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mildner RJ, Taub N, Vyas JR, et al. Cytokine imbalance in infants receiving extracorporeal membrane oxygenation for respiratory failure. Biol Neonate. 2005;88(4):321‐327. [DOI] [PubMed] [Google Scholar]
- 9. Al‐Fares A, Pettenuzzo T, Del Sorbo L. Extracorporeal life support and systemic inflammation. Intensive Care Med Exp. 2019;7(Suppl 1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gbadegesin R, Zhao S, Charpie J, et al. Significance of hemolysis on extracorporeal life support after cardiac surgery in children. Pediatr Nephrol. 2009;24(3):589‐595. [DOI] [PubMed] [Google Scholar]
- 11. Borasino S, et al. Impact of hemolysis on acute kidney injury and mortality in children supported with cardiac extracorporeal membrane oxygenation. J Extra Corpor Technol. 2018;50(4):217‐224. [PMC free article] [PubMed] [Google Scholar]
- 12. Cashen K, Meert K, Dalton HJ. Platelet count and function during pediatric extracorporeal membrane oxygenation. Semin Thromb Hemost. 2020;46(3):357‐365. [DOI] [PubMed] [Google Scholar]
- 13. Doyle AJ, Hunt BJ. Current understanding of how extracorporeal membrane oxygenators activate haemostasis and other blood components. Front Med (Lausanne). 2018;5:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Antonucci E, Lamanna I, Fagnoul D, et al. The impact of renal failure and renal replacement therapy on outcome during extracorporeal membrane oxygenation therapy. Artif Organs. 2016;40(8):746‐754. [DOI] [PubMed] [Google Scholar]
- 15. Chen Y‐C, Tsai F‐C, Chang C‐H, et al. Prognosis of patients on extracorporeal membrane oxygenation: the impact of acute kidney injury on mortality. Ann Thorac Surg. 2011;91(1):137‐142. [DOI] [PubMed] [Google Scholar]
- 16. Fleming GM, Sahay R, Zappitelli M, et al. The incidence of acute kidney injury and its effect on neonatal and pediatric extracorporeal membrane oxygenation outcomes: a multicenter report from the kidney intervention during extracorporeal membrane oxygenation study group. Pediatr Crit Care Med. 2016;17(12):1157‐1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gadepalli SK, Selewski DT, Drongowski RA, et al. Acute kidney injury in congenital diaphragmatic hernia requiring extracorporeal life support: an insidious problem. J Pediatr Surg. 2011;46(4):630‐635. [DOI] [PubMed] [Google Scholar]
- 18. Haneya A, Diez C, Philipp A, et al. Impact of acute kidney injury on outcome in patients with severe acute respiratory failure receiving extracorporeal membrane oxygenation. Crit Care Med. 2015;43(9):1898‐1906. [DOI] [PubMed] [Google Scholar]
- 19. Lee SW, Yu M‐Y, Lee H, et al. Risk factors for acute kidney injury and in‐hospital mortality in patients receiving extracorporeal membrane oxygenation. PLoS One. 2015;10(10):e0140674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smith AH, Hardison DC, Worden CR, et al. Acute renal failure during extracorporeal support in the pediatric cardiac patient. ASAIO J. 2009;55(4):412‐416. [DOI] [PubMed] [Google Scholar]
- 21. Tsai T‐Y, Chien H, Tsai F‐C, et al. Comparison of RIFLE, AKIN, and KDIGO classifications for assessing prognosis of patients on extracorporeal membrane oxygenation. J Formos Med Assoc. 2017;116(11):844‐851. [DOI] [PubMed] [Google Scholar]
- 22. Yan X, Jia S, Meng X, et al. Acute kidney injury in adult postcardiotomy patients with extracorporeal membrane oxygenation: evaluation of the RIFLE classification and the Acute Kidney Injury Network criteria. Eur J Cardiothorac Surg. 2010;37(2):334‐338. [DOI] [PubMed] [Google Scholar]
- 23. Zwiers AJM, de Wildt SN, Hop WCJ, et al. Acute kidney injury is a frequent complication in critically ill neonates receiving extracorporeal membrane oxygenation: a 14‐year cohort study. Crit Care. 2013;17(4):R151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fleming GM, Askenazi DJ, Bridges BC, et al. A multicenter international survey of renal supportive therapy during ECMO: the Kidney Intervention During Extracorporeal Membrane Oxygenation (KIDMO) group. ASAIO J. 2012;58(4):407‐414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schmidt M, Bailey M, Kelly J, et al. Impact of fluid balance on outcome of adult patients treated with extracorporeal membrane oxygenation. Intensive Care Med. 2014;40(9):1256‐1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goldstein SL, Currier H, Graf JM, et al. Outcome in children receiving continuous venovenous hemofiltration. Pediatrics. 2001;107(6):1309‐1312. [DOI] [PubMed] [Google Scholar]
- 27. Selewski DT, Askenazi DJ, Bridges BC, et al. The impact of fluid overload on outcomes in children treated with extracorporeal membrane oxygenation: a multicenter retrospective cohort study. Pediatr Crit Care Med. 2017;18(12):1126‐1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Selewski DT, Cornell TT, Blatt NB, et al. Fluid overload and fluid removal in pediatric patients on extracorporeal membrane oxygenation requiring continuous renal replacement therapy. Crit Care Med. 2012;40(9):2694‐2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Swaniker F, Kolla S, Moler F, et al. Extracorporeal life support outcome for 128 pediatric patients with respiratory failure. J Pediatr Surg. 2000;35(2):197‐202. [DOI] [PubMed] [Google Scholar]
- 30. Heiss KF, et al. Renal insufficiency and volume overload in neonatal ECMO managed by continuous ultrafiltration. ASAIO Trans. 1987;33(3):557‐560. [PubMed] [Google Scholar]
- 31. Gorga SM, Sahay RD, Askenazi DJ, et al. Fluid overload and fluid removal in pediatric patients on extracorporeal membrane oxygenation requiring continuous renal replacement therapy: a multicenter retrospective cohort study. Pediatr Nephrol. 2020;35(5):871‐882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fong KM, Au SY, Ng GWY, et al. Positive fluid balance and mortality in adult patients treated with extracorporeal membrane oxygenation: a retrospective study. J Intensive Care Soc. 2020;21(3):210‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Besnier E, Boubèche S, Clavier T, et al. Early positive fluid balance is associated with mortality in patients treated with veno‐arterial extra corporeal membrane oxygenation for cardiogenic shock: a retrospective cohort study. Shock. 2020;53(4):426‐433. [DOI] [PubMed] [Google Scholar]
- 34. McCanny P, Smith MW, O’Brien SG, et al. Fluid balance and recovery of native lung function in adult patients supported by venovenous extracorporeal membrane oxygenation and continuous renal replacement therapy. ASAIO J. 2019;65(6):614‐619. [DOI] [PubMed] [Google Scholar]
- 35. ExtrcoporealLife Support Organization – General guidelines for all cases. https://www.elso.org/Portals/0/ELSO%20Guidelines%20General%20All%20ECLS%20Version%201_4.pdf. Accessed January 19, 2021.
- 36. Hoover NG, Heard M, Reid C, et al. Enhanced fluid management with continuous venovenous hemofiltration in pediatric respiratory failure patients receiving extracorporeal membrane oxygenation support. Intensive Care Med. 2008;34(12):2241‐2247. [DOI] [PubMed] [Google Scholar]
- 37. Blijdorp K, Cransberg K, Wildschut ED, et al. Haemofiltration in newborns treated with extracorporeal membrane oxygenation: a case‐comparison study. Crit Care. 2009;13(2):R48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Murphy HJ, Cahill JB, Twombley KE, et al. Implementing a practice change: early initiation of continuous renal replacement therapy during neonatal extracorporeal life support standardizes care and improves short‐term outcomes. J Artif Organs. 2018;21(1):76‐85. [DOI] [PubMed] [Google Scholar]
- 39. Murphy HJ, Cahill JB, Twombley KE, et al. Early continuous renal replacement therapy improves nutrition delivery in neonates during extracorporeal life support. J Ren Nutr. 2018;28(1):64‐70. [DOI] [PubMed] [Google Scholar]
- 40. Murphy HJ, Eklund MJ, Hill J, et al. Early continuous renal replacement therapy during infant extracorporeal life support is associated with decreased lung opacification. J Artif Organs. 2019;22(4):286‐293. [DOI] [PubMed] [Google Scholar]
- 41. Han S‐S, Kim HJ, Lee SJ, et al. Effects of renal replacement therapy in patients receiving extracorporeal membrane oxygenation: a meta‐analysis. Ann Thorac Surg. 2015;100(4):1485‐1495. [DOI] [PubMed] [Google Scholar]
- 42. Chen H, Yu R‐G, Yin N‐N, et al. Combination of extracorporeal membrane oxygenation and continuous renal replacement therapy in critically ill patients: a systematic review. Crit Care. 2014;18(6):675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kielstein JT, Heiden AM, Beutel G, et al. Renal function and survival in 200 patients undergoing ECMO therapy. Nephrol Dial Transplant. 2013;28(1):86‐90. [DOI] [PubMed] [Google Scholar]
- 44. Santiago MJ, Sánchez A, López‐Herce J, et al. The use of continuous renal replacement therapy in series with extracorporeal membrane oxygenation. Kidney Int. 2009;76(12):1289‐1292. [DOI] [PubMed] [Google Scholar]
- 45. Seczyńska B, Królikowski W, Nowak I, et al. Continuous renal replacement therapy during extracorporeal membrane oxygenation in patients treated in medical intensive care unit: technical considerations. Ther Apher Dial. 2014;18(6):523‐534. [DOI] [PubMed] [Google Scholar]
- 46. Sidebotham D, Allen SJ, McGeorge A, et al. Venovenous extracorporeal membrane oxygenation in adults: practical aspects of circuits, cannulae, and procedures. J Cardiothorac Vasc Anesth. 2012;26(5):893‐909. [DOI] [PubMed] [Google Scholar]
- 47. Simons AP, Weerwind PW. Re: How to perform a haemodialysis using the arterial and venous lines of an extracorporeal life support. Eur J Cardiothorac Surg. 2011;39(6):1084‐1085. [DOI] [PubMed] [Google Scholar]
- 48. Kashani K, Ostermann M. Optimizing renal replacement therapy for patients who need extracorporeal membrane oxygenation: cross‐talk between two organ support machines. BMC Nephrol. 2019;20(1):404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Villa G, Katz N, Ronco C. Extracorporeal Membrane Oxygenation and the Kidney. Cardiorenal Med. 2015;6(1):50‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Brasseur A, Scolletta S, Lorusso R, et al. Hybrid extracorporeal membrane oxygenation. J Thorac Dis. 2018;10(Suppl 5):S707‐S715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sorokin V, MacLaren G, Vidanapathirana PC, et al. Choosing the appropriate configuration and cannulation strategies for extracorporeal membrane oxygenation: the potential dynamic process of organ support and importance of hybrid modes. Eur J Heart Fail. 2017;19(Suppl 2):75‐83. [DOI] [PubMed] [Google Scholar]
- 52. de Tymowski C, Augustin P, Houissa H, et al. CRRT Connected to ECMO: managing High Pressures. ASAIO J. 2017;63(1):48‐52. [DOI] [PubMed] [Google Scholar]
- 53. Nentwich J, Wichmann D, Kluge S, et al. Low‐flow CO2 removal in combination with renal replacement therapy effectively reduces ventilation requirements in hypercapnic patients: a pilot study. Ann Intensive Care. 2019;9(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Terragni PP, et al. Extracorporeal CO2 removal. Contrib Nephrol. 2010;165:185‐196. [DOI] [PubMed] [Google Scholar]
- 55. Giani M, Scaravilli V, Stefanini F, et al. Continuous renal replacement therapy in venovenous extracorporeal membrane oxygenation: a retrospective study on regional citrate anticoagulation. ASAIO J. 2020;66(3):332‐338. [DOI] [PubMed] [Google Scholar]
- 56. Colman E, Yin EB, Laine G, et al. Evaluation of a heparin monitoring protocol for extracorporeal membrane oxygenation and review of the literature. J Thorac Dis. 2019;11(8):3325‐3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Alexander DC, Butt WW, Best JD, et al. Correlation of thromboelastography with standard tests of anticoagulation in paediatric patients receiving extracorporeal life support. Thromb Res. 2010;125(5):387‐392. [DOI] [PubMed] [Google Scholar]
- 58. Giani M, et al. Thromboelastometry, thromboelastography, and conventional tests to assess anticoagulation during extracorporeal support: a prospective observational study. ASAIO J. 2021;67(2):196‐200. [DOI] [PubMed] [Google Scholar]
- 59. Gilman EA, Koch CD, Santrach PJ, et al. Fresh and citrated whole‐blood specimens can produce different thromboelastography results in patients on extracorporeal membrane oxygenation. Am J Clin Pathol. 2013;140(2):165‐169. [DOI] [PubMed] [Google Scholar]
- 60. Henderson N, et al. Use of thromboelastography to predict thrombotic complications in pediatric and neonatal extracorporeal membranous oxygenation. J Extra Corpor Technol. 2018;50(3):149‐154. [PMC free article] [PubMed] [Google Scholar]
- 61. Panigada M, E. Iapichino G, Brioni M, et al. Thromboelastography‐based anticoagulation management during extracorporeal membrane oxygenation: a safety and feasibility pilot study. Ann Intensive Care. 2018;8(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Roberts TR, Jones JA, Choi J‐H, et al. Thromboelastography on‐the‐go: evaluation of the TEG 6s device during ground and high‐altitude Aeromedical Evacuation with extracorporeal life support. J Trauma Acute Care Surg. 2019;87(1S Suppl 1):S119‐S127. [DOI] [PubMed] [Google Scholar]
- 63. Sleeper LA, Mulone M, Diallo F, et al. Stratification of bleeding risk using thromboelastography in children on extracorporeal membrane oxygenation support. Pediatr Crit Care Med. 2021. [DOI] [PubMed] [Google Scholar]
- 64. Toomasian JM, et al. The use of bound heparin in prolonged extracorporeal membrane oxygenation. Trans Am Soc Artif Intern Organs. 1984;30:133‐136. [PubMed] [Google Scholar]
- 65. Liveris A, Bello RA, Friedmann P, et al. Anti‐factor Xa assay is a superior correlate of heparin dose than activated partial thromboplastin time or activated clotting time in pediatric extracorporeal membrane oxygenation*. Pediatr Crit Care Med. 2014;15(2):e72‐e79. [DOI] [PubMed] [Google Scholar]
- 66. Nguyen T, Musick M, Teruya J. Anticoagulation monitoring during extracorporeal membrane oxygenation: is anti‐factor Xa assay (heparin level) a better test?*. Pediatr Crit Care Med. 2014;15(2):178‐179. [DOI] [PubMed] [Google Scholar]
- 67. Yeo HJ, Kim DH, Jeon D, et al. Low‐dose heparin during extracorporeal membrane oxygenation treatment in adults. Intensive Care Med. 2015;41(11):2020‐2021. [DOI] [PubMed] [Google Scholar]
- 68. Fitousis K, Klasek R, Mason PE, et al. Evaluation of a pharmacy managed heparin protocol for extracorporeal membrane oxygenation patients. Perfusion. 2017;32(3):238‐244. [DOI] [PubMed] [Google Scholar]
- 69. Padhya DR, Prutsky GJ, Nemergut ME, et al. Routine laboratory measures of heparin anticoagulation for children on extracorporeal membrane oxygenation: systematic review and meta‐analysis. Thromb Res. 2019;179:132‐139. [DOI] [PubMed] [Google Scholar]
- 70. Arnouk S, Altshuler D, Lewis TC, et al. Evaluation of anti‐Xa and activated partial thromboplastin time monitoring of heparin in adult patients receiving extracorporeal membrane oxygenation support. ASAIO J. 2020;66(3):300‐306. [DOI] [PubMed] [Google Scholar]
- 71. Figueroa Villalba CA, Brogan TV, McMullan DM, et al. Conversion from activated clotting time to anti‐Xa heparin activity assay for heparin monitoring during extracorporeal membrane oxygenation. Crit Care Med. 2020;48(12):e1179‐e1184. [DOI] [PubMed] [Google Scholar]
- 72. Gordon SE, et al. Evaluation of heparin anti‐factor Xa levels following antithrombin supplementation in pediatric patients supported with extracorporeal membrane oxygenation. J Pediatr Pharmacol Ther. 2020;25(8):717‐722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. McMichael ABV, Hornik CP, Hupp SR, et al. Correlation among antifactor xa, activated partial thromboplastin time, and heparin dose and association with pediatric extracorporeal membrane oxygenation complications. ASAIO J. 2020;66(3):307‐313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhong H, et al. Management of bivalirudin anticoagulation therapy for extracorporeal membrane oxygenation in heparin‐induced thrombocytopenia: a case report and a systematic review. Front Pharmacol. 2020;11:565013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yasuda N, Goto K, Mizoguchi T, et al. A new anticoagulation strategy using recombinant human thrombomodulin in patients on veno‐venous extracorporeal membrane oxygenation: a retrospective study. Ann Palliat Med. 2021;10(2):1834‐1841. [DOI] [PubMed] [Google Scholar]
- 76. Seelhammer TG, Rowse P, Yalamuri S. Bivalirudin for maintenance anticoagulation during venovenous extracorporeal membrane oxygenation for COVID‐19. J Cardiothorac Vasc Anesth. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ryerson LM, Balutis KR, Granoski DA, et al. Prospective exploratory experience with bivalirudin anticoagulation in pediatric extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2020;21(11):975‐985. [DOI] [PubMed] [Google Scholar]
- 78. Massicotte MP, Bauman ME. Anticoagulation and antithrombin in veno‐venous extracorporeal membrane oxygenation. Anesthesiology. 2020;132(3):421‐423. [DOI] [PubMed] [Google Scholar]
- 79. Kaseer H, Soto‐Arenall M, Sanghavi D, et al. Heparin vs bivalirudin anticoagulation for extracorporeal membrane oxygenation. J Card Surg. 2020;35(4):779‐786. [DOI] [PubMed] [Google Scholar]
- 80. Hamzah M, Jarden AM, Ezetendu C, et al. Evaluation of bivalirudin as an alternative to heparin for systemic anticoagulation in pediatric extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2020;21(9):827‐834. [DOI] [PubMed] [Google Scholar]
- 81. Grecu L. Anticoagulation for extracorporeal membrane oxygenation: between the rock and the hard place. Crit Care Med. 2020;48(2):264‐266. [DOI] [PubMed] [Google Scholar]
- 82. Bertini P, Guarracino F. Anticoagulation in extracorporeal membrane oxygenation: still a challenge. Minerva Anestesiol. 2020;86(1):7‐8. [DOI] [PubMed] [Google Scholar]
- 83. Netley J, et al. Bivalirudin anticoagulation dosing protocol for extracorporeal membrane oxygenation: a retrospective review. J Extra Corpor Technol. 2018;50(3):161‐166. [PMC free article] [PubMed] [Google Scholar]
- 84. Sanfilippo F, Asmussen S, Maybauer DM, et al. Bivalirudin for alternative anticoagulation in extracorporeal membrane oxygenation: a systematic review. J Intensive Care Med. 2017;32(5):312‐319. [DOI] [PubMed] [Google Scholar]
- 85. Fina D, Matteucci M, Jiritano F, et al. Extracorporeal membrane oxygenation without therapeutic anticoagulation in adults: a systematic review of the current literature. Int J Artif Organs. 2020;43(9):570‐578. [DOI] [PubMed] [Google Scholar]
- 86. Kurihara C, Walter JM, Karim A, et al. Feasibility of venovenous extracorporeal membrane oxygenation without systemic anticoagulation. Ann Thorac Surg. 2020;110(4):1209‐1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Martinez‐Solano J, et al. Emergent bedside and anticoagulation‐free veno‐venal extracorporeal oxygenation membrane cannulation in a patient with massive hemoptysis and unresponsive shock. Arch Bronconeumol. 2021;57(1):71‐73. [DOI] [PubMed] [Google Scholar]
- 88. Olson SR, Murphree CR, Zonies D, et al. Thrombosis and bleeding in extracorporeal membrane oxygenation (ECMO) without anticoagulation: a systematic review. ASAIO J. 2021;67(3):290‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lee C‐C, Chen S‐W, Cheng Y‐L, et al. The impact of CRRT modality in patients with AKI receiving ECMO: a nationwide registry study in Taiwan. J Crit Care. 2020;57:102‐107. [DOI] [PubMed] [Google Scholar]
- 90. He P, Zhang S, Hu B, et al. Retrospective study on the effects of the prognosis of patients treated with extracorporeal membrane oxygenation combined with continuous renal replacement therapy. Ann Transl Med. 2018;6(23):455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Anton‐Martin P, Quigley R, Dhar A, et al. Early fluid accumulation and intensive care unit mortality in children receiving extracorporeal membrane oxygenation. ASAIO J. 2021;67(1):84‐90. [DOI] [PubMed] [Google Scholar]
- 92. Thajudeen B, Kamel M, Arumugam C, et al. Outcome of patients on combined extracorporeal membrane oxygenation and continuous renal replacement therapy: a retrospective study. Int J Artif Organs. 2015;38(3):133‐137. [DOI] [PubMed] [Google Scholar]
- 93. Dado D, Ainsworth C, Thomas S, et al. Outcomes among patients treated with renal replacement therapy during extracorporeal membrane oxygenation: a single‐center retrospective study. Blood Purif. 2020;49(3):341‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Deatrick KB, Mazzeffi MA, Galvagno SM, et al. Breathing life back into the kidney‐continuous renal replacement therapy and veno‐venous extracorporeal membrane oxygenation. ASAIO J. 2021;67(2):208–212. [DOI] [PubMed] [Google Scholar]
- 95. Devasagayaraj R, Cavarocchi NC, Hirose H. Does acute kidney injury affect survival in adults with acute respiratory distress syndrome requiring extracorporeal membrane oxygenation? Perfusion. 2018;33(5):375‐382. [DOI] [PubMed] [Google Scholar]
- 96. Kuo G, Chen S‐W, Fan P‐C, et al. Analysis of survival after initiation of continuous renal replacement therapy in patients with extracorporeal membrane oxygenation. BMC Nephrol. 2019;20(1):318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Schmidt M, Zogheib E, Rozé H, et al. The PRESERVE mortality risk score and analysis of long‐term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Med. 2013;39(10):1704‐1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Schmidt M, Bailey M, Sheldrake J, et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am J Respir Crit Care Med. 2014;189(11):1374‐1382. [DOI] [PubMed] [Google Scholar]
- 99. Schmidt M, Burrell A, Roberts L, et al. Predicting survival after ECMO for refractory cardiogenic shock: the survival after veno‐arterial‐ECMO (SAVE)‐score. Eur Heart J. 2015;36(33):2246‐2256. [DOI] [PubMed] [Google Scholar]
- 100. Muller G, Flecher E, Lebreton G, et al. The ENCOURAGE mortality risk score and analysis of long‐term outcomes after VA‐ECMO for acute myocardial infarction with cardiogenic shock. Intensive Care Med. 2016;42(3):370‐378. [DOI] [PubMed] [Google Scholar]
- 101. Wang L, Yang F, Wang X, et al. Predicting mortality in patients undergoing VA‐ECMO after coronary artery bypass grafting: the REMEMBER score. Crit Care. 2019;23(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Wengenmayer T, Duerschmied D, Graf E, et al. Development and validation of a prognostic model for survival in patients treated with venoarterial extracorporeal membrane oxygenation: the PREDICT VA‐ECMO score. Eur Heart J Acute Cardiovasc Care. 2019;8(4):350‐359. [DOI] [PubMed] [Google Scholar]
- 103. Barbaro RP, MacLaren G, Boonstra PS, et al. Extracorporeal membrane oxygenation support in COVID‐19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet. 2020;396(10257):1071‐1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Baek SD, Kang J‐Y, Shin S, et al. Predictive factors of duration of continuous renal replacement therapy in acute kidney injury survivors. Shock. 2019;52(6):598‐603. [DOI] [PubMed] [Google Scholar]
- 105. Meyer RJ, Brophy PD, Bunchman TE, et al. Survival and renal function in pediatric patients following extracorporeal life support with hemofiltration. Pediatr Crit Care Med. 2001;2(3):238‐242. [DOI] [PubMed] [Google Scholar]
- 106. Paden ML, Warshaw BL, Heard ML, et al. Recovery of renal function and survival after continuous renal replacement therapy during extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2011;12(2):153‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Qiao X, Swain L, Reyelt L, et al. VA‐ECMO increases urinary levels of the biomarker kidney injury marker‐1 (KIM‐1) in a preclinical model of acute myocardial infarction (abstract). Circ Res.. 2019;125(Suppl 1):A586. 10.1161/res.125.suppl_1.586 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.


