Abstract
The aim is to comparatively evaluate the results of simultaneous conjunctiva and oropharynx–nasopharynx (ONP) swabs in patients who had presented to the outpatient department with a suspicion of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). An ONP sample was obtained following bilateral conjunctiva swabs in 85 subjects with a contact history or symptoms but unknown SARS‐CoV‐2 status and with no ocular symptoms or findings. The results were evaluated according to the patient's symptoms and how the swab was taken. The conjunctiva swab was positive in 29 (34.1%) cases and the ONP swab in 20 (23.5%) cases. Both methods produced positive results in 11 (14.1%) cases. The mean cycle threshold (C t) value was 30.15 ± 3.41 in symptomatic cases and 33.62 ± 1.76 in asymptomatic cases (p = .008). The mean C t value was 24.37 ± 3.48 when only the ONP swab was positive and 31.22 ± 1.99 when only the conjunctiva swab was positive. In cases that were positive by both methods, the mean C t value was 25.21 ± 4.94 for the ONP swab and 30.29 ± 5.05 for the conjunctiva swab. We found higher SARS‐CoV‐2 detection rates with the conjunctiva swab than the ONP swab in cases with unknown SARS‐CoV‐2 status in the early period. In addition, the conjunctival viral load seemed to be higher in symptomatic cases than in asymptomatic cases. We, therefore, believe a conjunctiva swab could be an alternative method to detect SARS‐CoV‐2 at the time of the first presentation to the outpatient department.
Keywords: conjunctival swab, COVID‐19, nasopharyngeal swab, oropharyngeal swab, SARS‐CoV‐2, tears
1. INTRODUCTION
The conjunctiva is a mucosal surface directly exposed to air and contains angiotensin‐converting enzyme 2 receptors; it is also connected to the nasal cavity through the nasolacrimal canal, making it an attractive candidate as a route for the spread of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). 1 , 2 There are several reports of SARS‐CoV‐2 detection in conjunctiva swabs. 3 , 4 , 5 However, the relationship between the conjunctival presence of the virus and the pathogenesis is controversial. 6 , 7 Previous studies have been conducted by taking a conjunctiva swab from subjects after the positive status had already been confirmed. This prevents determining the rate at which a positive conjunctiva swab result indicates a new case.
The aim of this study was to evaluate the comparative results of simultaneously obtained conjunctiva and oropharynx–nasopharynx (ONP) swabs in patients presenting to the outpatient department for the first time with unknown SARS‐CoV‐2 status and determine the relationship between the results and the symptoms.
2. MATERIALS AND METHODS
This prospective study was conducted on 85 adult patients who had a contact history or were symptomatic and presented to the Turgut Ozal University Malatya Training and Research Hospital's outpatient department for the first time in December 2020. Simultaneous conjunctiva and ONP swabs were obtained from the patients with unknown SARS‐CoV‐2 infection status who had been randomly selected.
All subjects were queried regarding symptoms related to SARS‐CoV‐2 and a detailed SARS‐CoV‐2 contact history was taken. The medical history was also evaluated regarding ocular surgery or medication use. All subjects were examined with a pen‐light to detect conjunctivitis. Patients with conjunctivitis or conjunctival hyperemia on the eye examination were excluded from the study.
Other exclusion criteria were the use of any ophthalmic medication, ocular surgery within the last 3 months, chronic eye disease, and any anatomical deformity of the conjunctiva or ONP that would present when performing an optimum swab procedure. Patients who could not tolerate the ONP swab procedure were also excluded.
Permission for the study was obtained from the local ethics committee. Written informed consent was obtained from all study subjects. The Helsinki declaration principles were adhered to at every stage of the study.
2.1. Swab procedure
When the patient was looking up with both eyes, the swab for the reverse‐transcription polymerase chain reaction (RT‐PCR) test was obtained from the lower lid conjunctiva after drawing the lower lids slightly downward. The same swab was used to obtain swabs from both eyes in sequence. A topical anesthetic was not administered before the procedure. The technician changed gloves after each patient to avoid cross contamination. Another swab was then used to obtain the ONP swab for the SARS CoV‐2 diagnosis. The samples were then placed in a transfer tube (Bio‐Speedy vNAT, Bioeksen Ar‐Ge Tekn. Ltd. Şti) and the cap closed. The samples were transferred to the laboratory under a temperature of +2°C to 8°C.
2.2. Sample analysis
The ONP and conjunctiva samples from the patients were transferred to the laboratory and simultaneously studied. The tests were conducted within a Level‐2 Biosafety Hood inside a Level‐2 Biosafety Laboratory. The samples were kept for at least 30 min before the study so that the cells could be degraded and the RNA released. The samples were then vortexed for 15–20 s and 100 µl was transferred to an Eppendorf tube. The reaction setup procedure was started and an RT‐PCR kit (Bio‐Speedy SARS CoV‐2 Double Gene RT‐qPCR, Bioeksen Ar‐Ge Tekn. Ltd. Şti) was used. As described in the kit leaflet, 10 µl of 2X prime script mix, 5 µl of CVD Di Oligo Mix, and 5 µl of template nucleic acid were mixed to form a mixture of 20 µl. The ONP and conjunctival samples were then simultaneously placed in the device (Rotor‐Gene Q, Qiagen). Again according to the kit leaflet, the procedure consisted of 1 cycle at 52°C for 5 min, 1 cycle at 95°C for 10 s, and 40 cycles at 95°C for 1 s and at 55°C for 1 s. The reaction curves were interpreted according to the rules of the kit procedure once the test study was completed. Values with a cycle threshold (C t) of less than 38 (C t < 38) were accepted as positive. Internal and external controls were performed to evaluate false positivity and false negativity in our study. There was no adverse situation in these controls. All the stages of the sample analysis were conducted by two microbiologists (Gunduz and Turkoglu) experienced in RT‐PCR.
2.3. Statistical analysis
The SPSS version 25.0 (IBM Corp.) software was used for statistical analysis. The Kolmogorov‐Smirnov test was used to determine whether the measurable variables conformed to a normal distribution. The measurable data are presented as mean ± standard deviation (SD) (minimum – maximum). The Mann–Whitney U test was used for the independent groups and the Wilcoxon signed rank test for the dependent groups when evaluating measurable data. Categorical data were evaluated with Pearson's χ 2 test or Fisher's exact test. Spearman test was used for correlation analysis. A p value of less than .05 was used to indicate statistical significance.
3. RESULTS
The mean age of the study subjects was 38.74 ± 14.36 (18–93) years with 36 (42.4%) females and 49 (57.6%) males. The conjunctiva swab result was positive in 29 (34.1%) cases and the ONP swab in 20 (23.5%) cases. The swab result was negative for SARS‐CoV‐2 with both methods in 47 (55.3%) subjects. There were 18 (21.2%) patients positive on the conjunctiva swab only, 9 (10.6%) patients positive on the ONP swab only, and 11 (12.9%) patients positive on both the conjunctival and ONP swabs.
The mean C t value was 24.83 ± 4.26 (16.68–32.63) for the ONP swabs and 30.87 ± 3.42 (20.34–36.46) for the conjunctiva swabs (p < .001). The mean C t value was 24.37 ± 3.48 (19.54–30.23) in subjects with a positive ONP sample only and 31.22 ± 1.99 (26.57–33.95) in those with a subjects conjunctiva sample only (p < .001). For those who were positive with both methods, the mean C t value was 25.21 ± 4.94 (16.68–32.63) for ONP swabs and 30.29 ± 5.05 (20.34–36.46) for conjunctiva swabs (p = .003) (Table 1). Figure 1 shows the amplification curves of three cases with conjunctiva positive‐ONP negative, conjunctiva positive‐ONP positive, and conjunctiva negative‐ONP positive, respectively.
Table 1.
The mean cycle threshold values of the conjunctiva and oropharynx–nasopharynx samples of the patients
| C(+)/OP(−) | C(+)/OP(+) | C(−)/OP(+) | ||
|---|---|---|---|---|
| C | OP | |||
| C t (mean ± SD) | 31.22 ± 1.99 | 30.29 ± 5.05 | 25.21 ± 4.94 | 24.37 ± 3.48 |
| n (%) | 18 (21.2%) | 11 (12.9%) | 9 (10.6%) | |
Note: There was no statistically significant difference between the mean C t values of the conjunctival swabs of C(+)/OP(−) and C(+)/OP(+) and between the mean C t values of the oropharynx–nasopharynx swabs of C(+)/OP(+) and C(−)/OP(+) (p = 1.000 and p = .603, respectively; Mann–Whitney U).
C(+)/OP(−), conjunctiva swab positive and oropharynx–nasopharynx swab negative.
C(+)/OP(+), Conjunctiva swab positive and oropharynx–nasopharynx swab positive.
C(−)/OP(+), conjunctiva swab negative and oropharynx–nasopharynx swab positive.
Abbreviations: C, conjunctiva, C t, cycle threshold; n, sample size; OP, oropharynx–nasopharynx; SD, standard deviation.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Figure 1.
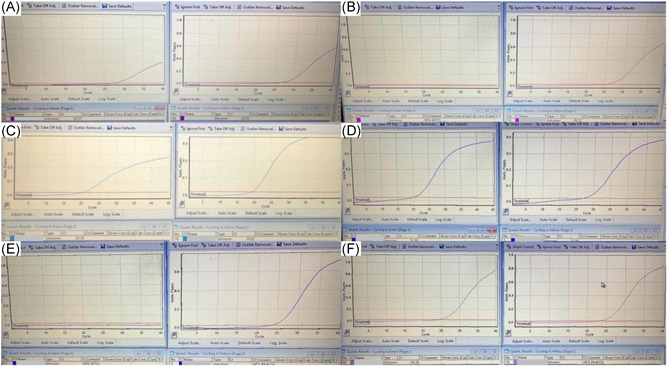
Amplification curves of three cases whose conjunctival and/or oro‐nasopharynx samples were positive by RT‐PCR test. The left amplification curve shows the conjunctiva sample and the right one shows the amplification curve of the oro‐nasopharynx sample of the same patient. Respectively from top to bottom: (A) conjunctiva positive and (B) oro‐nasopharynx negative patient (1st patient in Table 2), (C) conjunctiva positive and (D) oro‐nasopharynx positive patient (19th patient in Table 2) and (E) conjunctiva negative and (F) oro‐nasopharynx positive patient (34th patient in Table 2)
For cases with a positive result from both samples, the C t value for the ONP swab was lower than that for the conjunctiva swab. In addition, there was no relationship between the number of symptoms of the patients and the conjunctiva or ONP swab C t values. Table 2 presents the C t values of the SARS‐CoV‐2 positive subjects according to gender, age, number of symptoms, and the conjunctiva and ONP swab result. There was no significant correlation between the number of symptoms and conjunctival positivity or the number of symptoms and ONP positivity (p = .180 and .148, respectively).
Table 2.
The cycle threshold values of the SARS‐CoV‐2 positive subjects by gender, age, number of symptoms and the conjunctiva and oro‐nasopharynx swab results
| Patient no | Gender | Age | Symptom number | Swab result | Conj. C t (mean ± SD) | ONP C t (mean ± SD) |
|---|---|---|---|---|---|---|
| 1 | F | 36 | 2 | C(+)/OP(−) | 26.57 | ‐ |
| 2 | F | 29 | 2 | C(+)/OP(−) | 27.98 | ‐ |
| 3 | F | 54 | 1 | C(+)/OP(−) | 28.19 | ‐ |
| 4 | F | 19 | 3 | C(+)/OP(−) | 29.96 | ‐ |
| 5 | M | 40 | 4 | C(+)/OP(−) | 30.38 | ‐ |
| 6 | M | 39 | 4 | C(+)/OP(−) | 30.91 | ‐ |
| 7 | M | 36 | 4 | C(+)/OP(−) | 31.15 | ‐ |
| 8 | F | 66 | 4 | C(+)/OP(−) | 31.43 | ‐ |
| 9 | F | 27 | 2 | C(+)/OP(−) | 31.77 | ‐ |
| 10 | F | 31 | 3 | C(+)/OP(−) | 31.77 | ‐ |
| 11 | M | 34 | 0 | C(+)/OP(−) | 31.78 | ‐ |
| 12 | F | 40 | 1 | C(+)/OP(−) | 32.04 | ‐ |
| 13 | F | 24 | 0 | C(+)/OP(−) | 32.25 | ‐ |
| 14 | M | 37 | 2 | C(+)/OP(−) | 32.29 | ‐ |
| 15 | M | 50 | 0 | C(+)/OP(−) | 32.62 | ‐ |
| 16 | M | 41 | 1 | C(+)/OP(−) | 33.13 | ‐ |
| 17 | M | 43 | 1 | C(+)/OP(−) | 33.84 | ‐ |
| 18 | M | 42 | 0 | C(+)/OP(−) | 33.95 | ‐ |
| 19 | F | 44 | 4 | C(+)/OP(+) | 20.34 | 19.90 |
| 20 | F | 29 | 2 | C(+)/OP(+) | 25.94 | 25.14 |
| 21 | M | 41 | 1 | C(+)/OP(+) | 26.38 | 16.68 |
| 22 | M | 50 | 2 | C(+)/OP(+) | 27.19 | 25.22 |
| 23 | M | 35 | 2 | C(+)/OP(+) | 27.80 | 27.70 |
| 24 | F | 21 | 5 | C(+)/OP(+) | 31.63 | 28.85 |
| 25 | F | 30 | 3 | C(+)/OP(+) | 34.13 | 30.32 |
| 26 | F | 64 | 5 | C(+)/OP(+) | 34.16 | 22.91 |
| 27 | F | 38 | 3 | C(+)/OP(+) | 34.55 | 19.90 |
| 28 | M | 38 | 0 | C(+)/OP(+) | 34.66 | 28.13 |
| 29 | F | 93 | 0 | C(+)/OP(+) | 36.46 | 32.63 |
| 30 | M | 28 | 2 | C(−)/OP(+) | ‐ | 19.54 |
| 31 | F | 38 | 0 | C(−)/OP(+) | ‐ | 21.00 |
| 32 | F | 35 | 3 | C(−)/OP(+) | ‐ | 21.89 |
| 33 | F | 42 | 2 | C(−)/OP(+) | ‐ | 23.00 |
| 34 | M | 25 | 1 | C(−)/OP(+) | ‐ | 24.20 |
| 35 | M | 27 | 3 | C(−)/OP(+) | ‐ | 24.67 |
| 36 | M | 22 | 2 | C(−)/OP(+) | ‐ | 26.61 |
| 37 | M | 27 | 2 | C(−)/OP(+) | ‐ | 28.22 |
| 38 | M | 25 | 2 | C(−)/OP(+) | ‐ | 30.23 |
Note: C(+)/OP(−), conjunctiva swab positive and oropharynx–nasopharynx swab negative.
C(+)/OP(+), conjunctiva swab positive and oropharynx–nasopharynx swab positive.
C(−)/OP(+), conjunctiva swab negative and oropharynx–nasopharynx swab positive.
Abbreviations: C t, cycle threshold; Conj., conjunctiva; F, female; M, male; ONP, oropharynx–nasopharynx.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Among those with positive conjunctiva swab results, the mean C t value was 30.15 ± 3.41 (20.34–34.55) in the symptomatic cases (n = 23, 79.3%) and 33.62 ± 1.76 (31.78–36.46) in the asymptomatic cases (n = 6, 20.7%) (p = .008). Similarly, among those with a positive ONP swab result, the mean C t value was 24.41 ± 3.99 (16.68–30.32) in the symptomatic cases (n = 17, 85.0%) and 27.25 ± 5.86 (21.00–32.63) in the asymptomatic cases (n = 3, 15.0%) (p = .416).
The PCR result was positive in 7 (30.4%) of the 23 (27.1%) asymptomatic cases and 31 (50.0%) of the 62 (72.9%) symptomatic cases (p = .107). Among the asymptomatic cases, only the conjunctiva sample was positive in 4 (17.4%), only the ONP sample in 1 (4.3%), and both in 2 cases (8.7%). Among the symptomatic cases, only the conjunctiva sample result was positive in 14 (22.6%), only the ONP sample in 8 (12.9%), and both in 9 cases (14.5%).
Evaluation of the relationship with factors such as gender, age, and the symptoms at presentation revealed that there was a statistically significant increase in only the loss of smell and taste symptom (p = .043) in those with a positive conjunctiva swab result, and the excessive perspiration symptom in those with a positive ONP swab result (p = .039) (Table 3).
Table 3.
The distribution of the gender, age, and symptoms at presentation of the subjects according to the oro‐nasopharyngeal and conjunctival swab results
| Oro‐nasopharynx swab | Conjunctiva swab | |||||
|---|---|---|---|---|---|---|
| (−) | (+) | p 1 | (−) | (+) | p 2 | |
| Age (mean ± SD) | 39.3 ± 13.7 | 37.1 ± 16.4 | .262 * | 37.9 ± 14.1 | 40.4 ± 14.9 | .805 * |
| Gender (F/M) | 26/39 | 10/10 | .429 ** | 20/36 | 16/13 | .085 ** |
| Symptom (−/+) | 20/45 | 3/17 | .165 ** | 17/39 | 6/23 | .342 ** |
| Fever (−/+) | 61/4 | 16/4 | .084 | 52/4 | 25/4 | .436 |
| Weakness (−/+) | 52/13 | 16/4 | 1.000 | 45/11 | 23/6 | .909 ** |
| Backache (−/+) | 55/10 | 16/4 | .731 | 48/8 | 23/6 | .541 |
| Headache (−/+) | 53/12 | 18/2 | .503 | 46/10 | 25/4 | .763 |
| Miyalgia‐arthralgia (−/+) | 54/11 | 17/3 | 1.000 | 50/6 | 21/8 | .065 |
| Vertigo (−/+) | 64/1 | 20/0 | 1.000 | 55/1 | 29/0 | 1.000 |
| Stomach ache (−/+) | 64/1 | 20/0 | 1.000 | 55/1 | 29/0 | 1.000 |
| Chills–shivering (−/+) | 59/6 | 16/4 | .236 | 50/6 | 25/4 | .729 |
| Sore throat (−/+) | 54/11 | 15/5 | .514 | 47/9 | 22/7 | .367 ** |
| Cough (−/+) | 57/8 | 16/4 | .464 | 47/9 | 26/3 | .744 |
| Loss of smell–taste (−/+) | 60/5 | 18/2 | .665 | 54/2 | 24/5 | 0.043 |
| Chest pain(−/+) | 61/4 | 20/0 | .569 | 53/3 | 28/1 | 1.000 |
| Excessive perspiration (−/+) | 64/1 | 17/3 | 0.039 | 54/2 | 27/2 | .603 |
| Diarrhea (−/+) | 63/2 | 19/1 | .558 | 55/1 | 27/2 | .267 |
| Nausea–vomiting (−/+) | 59/6 | 17/3 | .434 | 48/8 | 28/1 | .157 |
| Fainting (−/+) | 64/1 | 20/0 | 1.000 | 56/0 | 28/1 | .341 |
| Runny nose (−/+) | 59/6 | 16/4 | .236 | 50/6 | 25/4 | .729 |
| Hoarseness (−/+) | 64/1 | 20/0 | 1.000 | 56/0 | 28/1 | .341 |
| Dispnea (−/+) | 62/3 | 19/1 | 1.000 | 54/2 | 27/2 | .603 |
Note: All other tests were done with Fisher's exact test. Bold numbers indicate statistically significant ones.
Abbreviations: F, female; M, male; p 1, statistical significance value for oro‐nasopharynx swab; p 2, statistical significance value for conjunctiva swab; SD, standard deviation.
Mann–Whitney U test.
Pearson's χ 2 test.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
4. DISCUSSION
The demonstration of SARS CoV‐2 virus receptors on the conjunctiva and cornea has shown that virus spread through the ocular surface could be possible. 1 In addition, the detection of the virus in conjunctiva swabs and tears has resulted in speculation that the virus could spread with ocular secretions. 6 Another view on such spread via the conjunctiva is that the cause is viruses passing to the nasal mucosa from the conjunctiva through the nasolacrimal canal. 8 , 9 The fact that the virus itself can cause keratoconjunctivitis 10 can be accepted as evidence that it can replicate in the conjunctiva. Detection of the SARS CoV‐2 virus in the conjunctiva of SARS CoV‐2 cases, whether they have conjunctivitis or not, 3 , 4 points towards conjunctival colonization without inflammation in addition to the conjunctival infection.
The conjunctiva swab was positive in 29 (34.1%) cases in our study. Previous studies have reported conjunctiva swab positive rates of 2.23%–28.57%. 3 , 4 , 5 , 11 , 12 One difference in our study was that it was conducted with patients whose SARS CoV‐2 status was unknown and not with subjects who already had a positive nasopharynx swab result. We would not be able to detect whether the conjunctiva swab could detect cases that could not be diagnosed with an ONP swab if we had only obtained conjunctiva swabs from patients who had a former positive ONP swab result. 4 In addition, we would have deducted that the conjunctiva swab could only detect 11 (57.1%) of the cases that were detected with an ONP swab, and 12.9% of all patients. This conclusion would have neglected the assumption that the ONP swab and conjunctiva swab can detect different cases. Our study has shown that the conjunctiva swab could detect 18 (21.2%) cases that the ONP swab could not diagnose in patients without a diagnosis of SARS CoV‐2.
We were able to detect a higher percentage of cases with the conjunctiva swab than the ONP swab in this study. However, the percentage of cases that both the conjunctiva sample and the ONP sample could detect was relatively low. This indicates that different factors are at work for the detection of SARS CoV‐2 by the two methods. One of these factors could be the difference in the period when the virus is present on the ONP and the conjunctiva. There are reports that the conjunctiva samples are more frequently positive in the early period. 13 , 14 In addition, the different peak times of the viral load in oral and nasal swabs could indicate that the conjunctival peak time could differ from the pharyngeal peak time. 6 , 15 Considering that the patients included in our study are early stage patients, the fact that conjunctival samples can detect more cases than the ONP sample may make the conjunctival sample taken in the early period valuable. Another possible cause is the technical difficulties encountered while obtaining an ONP swab. An ONP sample is obtained without seeing the swab's tip and it is possible that the swab tip does not adequately contact the mucosa surface. However, the technician can fully visualize the contact between the swab tip and the mucosa when obtaining the conjunctiva swab.
The nasopharyngeal swab is used as the gold standard in detecting the disease 16 but false‐negative results are also possible. 17 , 18 The test sensitivity can vary depending on the duration since disease onset and the disease severity, necessitating the use of other swab sites. 19 It is obvious that the new site should not make it more difficult to obtain swabs than from an ONP swab. The fact that it is essential to detect asymptomatic cases for the control of the disease could make a conjunctiva swab more desirable in outpatient departments with few resources and in population screening as it is quite easy to take and has a low cost. Obtaining a conjunctiva swab simultaneously with the ONP swab, while using separate swabs, could increase the sensitivity, especially in cases known to have contacted a SARS CoV‐2 patient or who have presented to the outpatient department for the first time.
Obtaining an ONP swab can be difficult as it commonly causes signs of irritation such as coughing and nausea in the patient and can therefore result in false negatives. 20 The patients could show much less resistance to a conjunctiva swab test as it is localized to the eye and causes relatively less irritation. The large number of droplets that are distributed to the environment when obtaining an ONP sample is also important regarding the spread of the disease. 21 Spread via droplets is much less important with a conjunctiva sample. A conjunctiva sample can also be a practical alternative for patients who cannot tolerate the ONP procedure or who have a structural deformity in the airway passage leading to the ONP.
The pulmonary functions have been reported to be better in intensive care patients when both the conjunctiva and ONP samples are negative compared to those when both are positive. 22 The mean C t value was lower with the ONP sample than with the conjunctiva sample in our study. It is understandable that the virus load is higher at the pharynx, one of the main involvement sites of the disease, than at the conjunctiva. The viral load was lower in the conjunctiva and ONP samples of asymptomatic patients in our study. The lack of statistical significance of these results with the ONP sample could have been the result of the low number of asymptomatic patients. The result indicates increased conjunctival viral load and conjunctival replication in the presence of symptoms. In addition, the higher positive rate with both methods in symptomatic cases is another indicator of the relationship between viral load and the presence of symptoms. In cases with positive conjunctival smear and in cases with positive ONP smears, the presence of symptoms was increased, although not significantly, compared to cases with negative results. The increase in the presence of symptoms in cases positive with conjunctival swab indicates that the detection of SARS‐CoV‐2 in the conjunctiva may be a true indicator of positivity. However, the absence of a correlation with the number of symptoms indicates that the presence of symptoms may be more associated with positivity than the number of symptoms. The presence of symptoms in cases that are detected negatively by both methods may be due to the symptoms being secondary to another disease or false negativity.
Positive rates were higher with the conjunctiva swabs in patients with loss of smell and taste and with ONP swabs in patients with excessive perspiration. The fact that the pathological process in patients with loss of smell and taste has not been fully clarified yet makes determining the relationship more difficult. However, this relationship could be a result of both the loss of smell and taste 23 and a positive conjunctiva swab test to be seen at the early stage.
A limitation of our study is that our subjects' symptoms were subjective. However, it is not possible to conduct tests for most symptoms. Another limitation of our study is that the quarantine applied to SARS‐CoV‐2 patients in our country and the outpatient follow‐up of the patients who participated in our study prevented the follow‐up of the patients and the detection of false positivity and false negativity status.
In conclusion, it is possible that the SARS CoV‐2 virus becomes positive in the conjunctiva sample at an earlier stage than the pharynx sample. A conjunctiva swab test can therefore be an alternative method for patients who present to the outpatient department for the first time or in population screening (early stage patients). In addition, it can be an easy, inexpensive, and low‐risk alternative in patients with low tolerance to the ONP swab procedure and whose upper respiratory pathway is not anatomically suitable. We also believe preferring conjunctiva swabs is important as regards decreasing the risk of spread to the health care worker taking the sample. The tests used in this study will need to be performed on subjects of various races and using various devices and test kits to support these results. Studies conducted on larger study populations are also needed.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/jmv.26981
ACKNOWLEDGMENT
We would like to acknowledge the Biyoeksen Ar‐Ge Tekn. Ltd. company due to their RT‐PCR kits donated for our use utilized in this study.
Gunduz A, Firat M, Turkoglu G. Comparison of the simultaneous conjunctiva and oropharynx–nasopharynx swab results in patients applying to the SARS‐CoV‐2 outpatient clinic for the first time. J Med Virol. 2021;93:4516‐4522. 10.1002/jmv.26981
Note: 1st and 3rd places are affiliated hospitals.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Leonardi A, Rosani U, Brun P. Ocular surface expression of SARS‐CoV‐2 receptors. Ocul Immunol Inflamm. 2020;28(5):735‐738. [DOI] [PubMed] [Google Scholar]
- 2. Lu CW, Liu XF, Jia ZF. 2019‐nCoV transmission through the ocular surface must not be ignored. Lancet. 2020;395(10224):e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Atum M, Boz AAE, Çakır B, et al. Evaluation of conjunctival swab PCR results in patients with SARS‐CoV‐2 infection. Ocul Immunol Inflamm. 2020;28(5):745‐748. [DOI] [PubMed] [Google Scholar]
- 4. Kaya H, Çalışkan A, Okul M, Sarı T, Akbudak İH. Detection of SARS‐CoV‐2 in the tears and conjunctival secretions of coronavirus disease 2019 patients. J Infect Dev Countries. 2020;14(9):977‐981. [DOI] [PubMed] [Google Scholar]
- 5. Zhou Y, Duan C, Zeng Y, et al. Ocular findings and proportion with conjunctival SARS‐COV‐2 in COVID‐19 patients. Ophthalmology. 2020;127(7):982‐983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bernabei F, Versura P, Rossini G, Re MC. There is a role in detection of SARS‐CoV‐2 in conjunctiva and tears: a comprehensive review. New Microbiol. 2020;43(4):149‐155. [PubMed] [Google Scholar]
- 7. Seah IYJ, Anderson DE, Kang AEZ, et al. Assessing viral shedding and infectivity of tears in coronavirus disease 2019 (COVID‐19) patients. Ophthalmology. 2020;127(7):977‐979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lange C, Wolf J, Auw‐Haedrich C, et al. What is the significance of the conjunctiva as a potential transmission route for SARS‐CoV‐2 infections? Der Ophthalmologe. 2020;118:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Qing H, Li Z, Yang Z, et al. The possibility of COVID‐19 transmission from eye to nose. Acta Ophthalmol. 2020;98(3):e388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cheema M, Aghazadeh H, Nazarali S, et al. Keratoconjunctivitis as the initial medical presentation of the novel coronavirus disease 2019 (COVID‐19). Can J Ophthalmol. 2020;55(4):e125‐e129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kumar K, Prakash A, Gangasagara S, et al. Presence of viral RNA of SARS‐CoV‐2 in conjunctival swab specimens of COVID‐19 patients. Indian J Ophthalmol. 2020;68(6):1015‐1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mahmoud H, Ammar H, El Rashidy A, Ali AH, Hefny HM, Mounir A. Assessment of coronavirus in the conjunctival tears and secretions in patients with SARS‐CoV‐2 infection in Sohag Province, Egypt. Clin Ophthalmol. 2020;14:2701‐2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Loon SC. The severe acute respiratory syndrome coronavirus in tears. Br J Ophthalmol. 2004;88(7):861‐863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arora R, Goel R, Kumar S, et al. Evaluation of SARS‐CoV‐2 in tears of patients with moderate to severe COVID‐19. Ophthalmology. 2021;128(4):494‐503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zou L, Ruan F, Huang M, et al. SARS‐CoV‐2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177‐1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang W, Xu Y, Gao R, et al. Detection of SARS‐CoV‐2 in different types of clinical specimens. JAMA. 2020;323(18):1843‐1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bullis SSM, Crothers JW, Wayne S, Hale AJ. A cautionary tale of false‐negative nasopharyngeal COVID‐19 testing. IDCases. 2020;20:e00791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gopaul R, Davis J, Gangai L, Goetz L. Practical diagnostic accuracy of nasopharyngeal swab testing for novel coronavirus disease 2019 (COVID‐19). Western J Emergency Med. 2020;21(6):1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Song F, Zhang X, Zha Y, Liu W. COVID‐19: recommended sampling sites at different stages of the disease. J Med Virol. 2020;92(9):1383‐1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kanodia A, Srigyan D, Sikka K, et al. Topical lignocaine anaesthesia for oropharyngeal sampling for COVID‐19. Eur Arch Otorhinolaryngol. 2020:1‐5. 10.1007/s00405-020-06402-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS‐CoV‐2 as compared with SARS‐CoV‐1. N Engl J Med. 2020;382(16):1564‐1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Savastano MC, Gambini G, Savastano A, et al. Evidence‐based of conjunctival COVID‐19 positivity: an Italian experience: Gemelli against COVID Group. Eur J Ophthalmol. 2020:1120672120976548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Klopfenstein T, Kadiane‐Oussou NJ, Toko L, et al. Features of anosmia in COVID‐19. Med Mal Infect. 2020;50(5):436‐439. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


