Abstract
SARS-CoV-2 infection has produced high mortality in kidney transplant (KT) recipients, especially in the elderly. Until December 2020, 1011 KT with COVID-19 have been prospectively included in the Spanish Registry and followed until recovery or death. In multivariable analysis, age, pneumonia, and KT performed ≤6 months before COVID-19 were predictors of death, whereas gastrointestinal symptoms were protective. Survival analysis showed significant increasing mortality risk in four subgroups according to recipient age and time after KT (age <65 years and posttransplant time >6 months, age <65 and time ≤6, age ≥65 and time >6 and age ≥65 and time ≤6): mortality rates were, respectively, 11.3%, 24.5%, 35.4%, and 54.5% (p < .001). Patients were significantly younger, presented less pneumonia, and received less frequently specific anti-COVID-19 treatment in the second wave (July–December) than in the first one (March–June). Overall mortality was lower in the second wave (15.1 vs. 27.4%, p < .001) but similar in critical patients (66.7% vs. 58.1%, p = .29). The interaction between age and time post-KT should be considered when selecting recipients for transplantation in the COVID-19 pandemic. Advanced age and a recent KT should foster strict protective measures, including vaccination.
KEYWORDS: clinical research/practice, infection and infectious agents – viral, kidney transplantation/nephrology, patient survival
Abbreviations: ACEIs, angiotensin converting enzyme inhibitors; ARBs, angiotensin receptor blockers; CI, confidence interval; DM, diabetes mellitus; HR, hazard ratio; ICU, intensive care units; IQR, interquartile range; KT, kidney transplant; RT-PCR, positive reverse-transcriptase–polymerase-chain-reaction; S.E.N., Spanish Society of Nephrology
1. INTRODUCTION
COVID-19 is a global pandemic that has affected more than 66 million people and caused more than 1.5 million of deaths all over the world.1 This rapid expansion led to the collapse of healthcare systems, negatively affecting transplant programs.2 , 3 Since the first confirmed case was isolated in Spain on January 31, 2020, the curve of new infections increased, reaching the peak of highest incidence at the end of March 2020.4 As a result, there was a dramatic decrease of transplantation activity during the critical early weeks of the outbreak.5 To better know the impact of SARS-CoV-2 infection in kidney transplant (KT) patients, the Spanish Society of Nephrology (S.E.N.) set up a voluntary registry in March 2020.6 The analysis of data registered until May 2020 showed that COVID-19 has a high mortality in KT patients, especially in elderly recipients and in the early post-KT period.7, 8, 9 In this scenario, recommendations on preventive strategies in waitlisted and solid organ transplant patients emerged, and transplant activity progressively recovered over the next weeks.10
Starting mid-July and peaking in October, a second wave of COVID-19 was documented in Spain and many other regions.4 Several differences have been reported between the first and the second wave in the general population, with a lower proportion of severe cases and younger patients in the second phase.11, 12, 13 This evolution allowed us to continue with the transplant activity, so in this context it is crucial to clarify the pre-infection risk factors in KT patients to assess properly the benefit of the procedure. Likewise, data comparing the characteristics of the infection in KT patients between both epidemic phases are scarce. Herein, we present the predictors of severe COVID-19 and the differences between the first and second phase of the pandemic in a Spanish multicenter KT recipient cohort.
2. METHODS
A registry regarding dialysis and KT patients with COVID-19 in Spain started in 03/18/2020 promoted by the S.E.N. (www.senefro.org). Of the 39 existing KT centers in Spain, 38 of them (97.4%) participated. The participating hospitals perform more than 99% of KT in Spain each year.14 Only cases diagnosed with positive reverse-transcriptase–polymerase-chain-reaction (RT-PCR) assay of a specimen collected on a nasopharyngeal swab or bronchoalveolar lavage were included. In Spain, COVID-19 cases are collected in the regional registries and then the regional authorities notify them to the Ministry of Health. The dialysis and KT COVID-19 registry is voluntary, but it is likely that most cases are included.
2.1. Variables collected and definitions
The characteristics of the S.E.N. COVID-19 registry and the variables included have been previously reported ( Table 1).7 The first epidemic wave was considered to end in June 2020, when the infection rate in Spain fell below 10 cases/100,000 inhabitants, and the second wave started July 202015 ( Figure 1).
TABLE 1.
Characteristics of all kidney transplant patients included in the COVID-19 Spanish Registry
| Variables | All (n = 1011) | Dead (n = 220) | Recovered (n = 791) | p value |
|---|---|---|---|---|
| Waves | ||||
| 1st wave, n (%) | 548 (54.2) | 150 (27.4) | 398 (72.6) | <.001 |
| 2nd wave, n (%) | 463 (45.8) | 70 (15.1) | 393 (84.9) | |
| Baseline characteristics | ||||
| Males, n (%) | 635 (62.8) | 144 (66.5) | 491 (62.1) | .35 |
| Recipient age (years), median [IQR]a | 60 [50–69] | 70.5 [61–75] | 57 [48–67] | <.001 |
| Age <65 years, n (%)b | 628 (62.1) | 78 (12.4) | 550 (87.6) | <.001 |
| Age ≥65 years, n (%)b | 383 (37.9) | 142 (37.1) | 241 (62.9) | |
| DM as cause of kidney disease, n (%) | 131 (13) | 35 (17.2) | 96 (12.5) | .08 |
| ACEIs treatment, n (%) | 144 (14.2) | 31 (14.1) | 113 (14.3) | .94 |
| ARBs treatment, n (%) | 274 (27.1) | 54 (24.5) | 220 (27.8) | .33 |
| Time from KT to COVID–19 (months), median [IQR]a | 72 [30–140] | 72 [21–157] | 72 [31–139] | .74 |
| Time from KT ≤6 months, n (%)c | 86 (8.5) | 31 (36) | 55 (64) | .001 |
| Time from KT >6 months, n (%)c | 925 (91.5) | 189 (20.4) | 736 (79.6) | |
| Immunosuppressive therapy at COVID–19 diagnosisd | ||||
| Prednisone, n (%) | 777 (76.9) | 171 (83) | 606 (79.2) | .22 |
| Tacrolimus, n (%) | 829 (82) | 168 (81.6) | 661 (86.4) | .08 |
| Mycophenolate, n (%) | 733 (72.5) | 158 (76.7) | 575 (75.2) | .64 |
| mTOR inhibitors, n (%) | 174 (17.2) | 31 (15) | 143 (18.7) | .22 |
| Clinical features at COVID–19 diagnosis | ||||
| Asymptomatic, n (%) | 99 (9.8) | 0 (0) | 99 (12.5) | … |
| Fever, n (%) | 742 (73.4) | 177 (79.5) | 567 (71.7) | .02 |
| Cough, expectoration, and/or rhinorrhea, n (%) | 682 (67.5) | 173 (78.6) | 509 (64.3) | <.001 |
| Gastrointestinal symptoms, n (%) | 323 (31.9) | 56 (25.5) | 267 (33.8) | .02 |
| Pneumonia, n (%) | 671 (66.4) | 204 (92.7) | 467 (59) | <.001 |
| Lymphopenia, n (%) | 690 (68.2) | 190 (86.4) | 500 (63.2) | <.001 |
| COVID–19 outcomes and treatment | ||||
| Hospitalized, n (%) | 791 (78.2) | 214 (97.3) | 577 (72.9) | <.001 |
| Ventilator support, n (%) | 155 (15.3) | 125 (56.8) | 30 (3.8) | <.001 |
| ICU admission, n (%) | 140 (13.8) | 87 (39.5) | 53 (6.7) | <.001 |
| Hydroxychloroquine, n (%) | 480 (47.5) | 126 (57.3) | 354 (44.8) | .001 |
| Azithromycin, n (%) | 280 (27.7) | 66 (30) | 214 (27.1) | .38 |
| Glucocorticoids, n (%) | 494 (48.9) | 143 (65) | 351 (44.4) | <.001 |
| Lopinavir/ritonavir, n (%) | 184 (18.2) | 69 (31.4) | 115 (14.5) | <.001 |
| Tocilizumab, n (%) | 141 (13.9) | 50 (22.7) | 92 (11.5) | <.001 |
| Remdesivir, n (%) | 26 (2.6) | 4 (1.8) | 22 (2.8) | .42 |
| Non anti-COVID–19 therapy, n (%)e | 242 (23.9) | 19 (8.6) | 223 (28.2) | <.001 |
| Length of COVID–19 episode (days), median [IQR]a | 15 [9–22] | 10 [5–20] | 15 [10–23] | <.001 |
Abbreviations: ACEI, angiotensin converting enzyme inhibitors; ARBs, angiotensin receptor blockers; DM, diabetes mellitus; IQR, interquartile range; KT, kidney transplantation.
Mood’s median test according to order of appearance in the table: p < .001, p = .99, p < .001, respectively.
Percentage within age < or ≥65 years.
Percentage within time from KT ≤ or >6 months.
Data of 971 patients: 765 survivors and 206 nonsurvivors.
Non-anti-COVID-19 therapy includes the cases that have not received specific drugs against SARS-CoV-2 or against the inflammatory response due to COVID-19.
FIGURE 1.
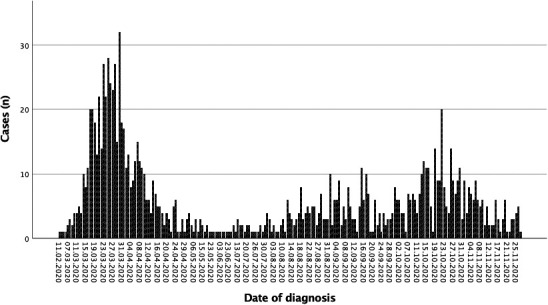
Epidemic curve of COVID-19 cases in kidney transplant recipients. First wave (until June 2020) and second wave (from July 2020)
Outcomes were assessed as COVID-19-related mortality or recovery until December 5, 2020. The immunosuppression collected corresponds to that received at the time of COVID-19 diagnosis. Length of the COVID-19 episode was defined as days from COVID-19 diagnosis to death or recovery and recovery as clinical improvement with negative RT-PCR and/or SARS-CoV-2 positive IgG serology.16
The study was conducted according to the guidelines dictated by the Declaration of Helsinki. All data were recorded anonymously.
2.2. Statistical analysis
Categorical variables were expressed as counts and percentages and continuous variables as the mean and standard deviation or median with interquartile range. All categorical variables were compared using Fisher exact test or Chi-square test, and continuous variables were compared with t test or Mann-Whitney U test, according to variables normality. Mood’s median test was performed to compare median scores.
Survival curves were plotted using the Kaplan-Meier method and compared between patients according to their age (< or ≥65 years) and time post-KT (≤ or >6 months after KT) by log-rank test. Kaplan-Meier survival analysis was also performed to compare patients infected in the first and second epidemic waves. In addition, the crossing survival curves were compared using Gehan-Wilcoxon-Breslow test.
Univariable and multivariable Cox proportional hazard regression analyses were assessed for independent risk factors of COVID-19-related death. In the multivariable analysis, demographic variables and those covariates with a p value < .1 were included. Proportionality assumption in the model was assessed by visual inspection of the log-log survival plots. Covariates included in the model did not violate the proportionality assumption. Differences in anti-COVID-19 therapy were not considered for multivariable analysis as they were consequences and not factors causing the severity of the infection. Survival time was considered until death or 90 days after COVID-19 episode for both the Cox and the Kaplan Meier analyses. This 90-day period was chosen because those cases with a new positive RT-PCR that had a confirmed SARS-CoV-2 infection more than 90 days ago are considered reinfection.16 Results are expressed as hazard ratio with their 95% confidence intervals. Statistical analyses were performed with SPSS V 22.0 (SPSS Inc.). A p value < .05 was considered statistically significant.
3. RESULTS
3.1. Characteristics of kidney transplant patients with COVID-19
From March 18, 2020, to December 5, 2020, 1178 KT recipients with COVID-19 were reported to the registry, 585 until June 30th and 576 from July 1st. Recipients with incomplete data (n = 17) and those whose had not reported an outcome for the episode (n = 150) were excluded. Finally, 1011 fully documented patients had a final outcome, recovery or death, and were included in the study.
Table 1 shows characteristics of the entire cohort, survivors (n = 791) and nonsurvivors (n = 220). Median age was 60 years. Tacrolimus-based immunosuppression was the most common therapy. More data on immunosuppressive regimens are reported in Tables S1 and S2. Median time from KT to the COVID-19 episode was 6 years and 8.5% had been transplanted within the last 6 months. Fever (73.4%), respiratory symptoms (67.5%), and pneumonia (66.4%) were the most frequent COVID-19-related clinical features. Ninety-nine patients (9.8%) were asymptomatic (tests performed due to contact with positive cases). Hospitalization was required in 791 patients and 14% were admitted to intensive care units (ICU). The overall mortality rate was 21.7%.
Patients who died were older and, although KT vintage was similar, the percentage of recent transplants was significantly higher in this group. We found no differences neither in terms of immunosuppressive therapy nor in renin-angiotensin-blocker treatment between survivors and nonsurvivors. Pneumonia was present in 92.7% of patients who died and they needed intensive care (39.5%) and ventilator support (56.8%) more frequently than survivors. Gastrointestinal symptoms (diarrhea or vomiting) appeared more frequently in patients who recovered (33.8%). Nonsurvivors were more frequently treated with glucocorticoids, hydroxychloroquine, lopinavir-ritonavir, or tocilizumab.
3.2. Univariable and multivariable Cox regression analysis for the relationship between risk factors and outcomes
In univariable analysis, infection during the first wave, age (continuous), age ≥65 years, time after KT to COVID-19 diagnosis less than 6 months, fever, respiratory symptoms, pneumonia and lymphopenia were significantly associated with mortality ( Table 2). Gastrointestinal symptoms were a protective factor for death.
TABLE 2.
Univariable and multivariable Cox regression analyses for death after COVID-19 in all kidney transplant recipients included in the COVID-19 Spanish Registry
| Univariable analysis |
Multivariable analysis |
|||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| 1st wave | 1.93 (1.45–2.57) | .000 | 1.10 (0.82–1.50) | .503 |
| Age (years) | 1.07 (1.06–1.09) | .000 | 1.06 (1.05–1.08) | .000 |
| DMa | 1.37 (0.96–1.99) | .090 | 1.02 (0.69–1.50) | .913 |
| Time from KT ≤6 months | 1.79 (1.22–2.63) | .003 | 1.64 (1.07–2.50) | .021 |
| Fever | 1.43 (1.03–1.99) | .031 | 1.00 (0.69–1.46) | .974 |
| Gastrointestinal symptoms | 0.68 (0.50–0.93) | .016 | 0.66 (0.48–0.90) | .011 |
| Respiratory symptomsb | 1.89 (1.37–2.61) | .000 | 1.29 (0.90–1.83) | .154 |
| Pneumonia | 7.31 (4.39–12.16) | .000 | 5.04 (2.81–9.05) | .000 |
| Lymphopenia | 3.13 (2.13–4.60) | .000 | 1.38 (0.89–2.11) | .144 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
DM: Diabetes mellitus as kidney disease.
Cough, expectoration, and/or rhinorrhea.
In multivariable Cox regression analysis, pneumonia and KT within the last 6 months remained as independent predictors of death while gastrointestinal symptoms were associated with better survival. The development of the disease during one or another phase of the pandemic was not an independent risk factor for COVID-19-related mortality. Similar results were observed when age was analyzed as a categorical variable, the models were adjusted for sex, or respiratory symptoms were excluded.
3.3. Comparison between patients regarding independent risk factors prior to SARS-CoV-2 infection
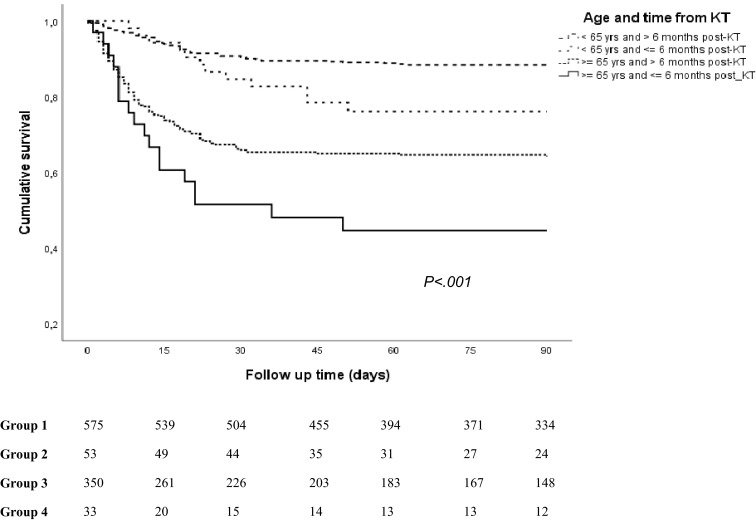
In Table 3, we have distributed the patients in four subgroups according to age and post-KT period: Group 1, age <65 years old and post-KT time to the COVID-19 episode >6 months (n = 575); Group 2, age <65 years old and post-KT time ≤6 months (n = 53); Group 3, age ≥65 years and post-KT time >6 months (n = 350); and Group 4, age ≥65 years and post-KT time ≤6 months (n = 33). Group 4 (elderly and recent KT) developed a greater clinical severity, with 81.4% of patients presenting pneumonia. Most patients in group 4 required hospitalization and almost a third ventilator support. On the other hand, there was a higher rate of asymptomatic patients in Group 1 (11.8%) and they presented more frequently gastrointestinal symptoms (35.1%). We also observed changes in immunosuppression in relation to time after transplantation, especially with steroids and tacrolimus, which were more frequent in recent transplants. Survival analysis showed a significant increasing mortality: Group 1, 11.3%; Group 2, 24.5%; Group 3, 35.4%; Group 4, 54.5% ( Figure 2).
TABLE 3.
Clinical characteristics of kidney transplant patients according to their age and the time from kidney transplant to SARS-CoV-2 infection
| Age <65 years and time post-KT >6 months | Age <65 years and time post-KT ≤6 months | Age ≥65 years and time post-KT >6 months | Age ≥65 years and time post-KT ≤6 months | p value | |
|---|---|---|---|---|---|
| Patients, n | 575 | 53 | 350 | 33 | |
| Prednisone, n (%)a,b | 431 (78.4) | 50 (98) | 267 (78.5) | 29 (96.7) | .001 |
| Tacrolimus, n (%)a,b | 471 (85.6) | 51 (100) | 278 (81.8) | 29 (96.7) | .002 |
| Mycophenolate, n (%)a,b | 409 (74.4) | 47 (92.2) | 252 (74.1) | 25 (83.3) | .02 |
| mTOR inhibitors, n (%)a,b | 108 (19.6) | 2 (3.9) | 61 (17.9) | 3 (10) | .02 |
| Asymptomatic, n (%) | 68 (11.8) | 8 (15.1) | 21 (6) | 2 (6.1) | .01 |
| Gastrointestinal symptoms, n (%) | 202 (35.1) | 14 (26.4) | 101 (28.9) | 6 (18.2) | .04 |
| Pneumonia, n (%) | 348 (60.5) | 34 (64.2) | 262 (74.9) | 27 (81.8) | <.001 |
| Lymphopenia, n (%) | 359 (62.4) | 43 (81.1) | 258 (73.7) | 30 (90.9) | <.001 |
| Hospitalized, n (%) | 417 (72.5) | 45 (84.9) | 299 (85.4) | 30 (90.9) | <.001 |
| Ventilator support, n (%) | 67 (11.7) | 12 (22.6) | 67 (19.1) | 9 (27.3) | .001 |
| Non anti-COVID–19 therapy, n (%)c | 170 (29.6) | 11 (20.8) | 58 (16.6) | 3 (9.1) | <.001 |
| COVID–19 pharmacological treatment | |||||
| Hydroxychloroquine, n (%) | 250 (43.5) | 23 (43.4) | 184 (52.6) | 23 (69.7) | .003 |
| Glucocorticoids, n (%) | 259 (45) | 33 (62.3) | 188 (53.7) | 14 (42.4) | .01 |
| Tocilizumab, n (%) | 74 (12.9) | 14 (26.4) | 44 (12.6) | 9 (27.3) | .005 |
| Dead, n (%) | 65 (11.3) | 13 (24.5) | 124 (35.4) | 18 (54.5) | <.001 |
Note: All variables of the study were compared between the four groups, showing only those with p < .05.
Abbreviation: KT, kidney transplantation.
As baseline immunosuppressive treatment at the time of COVID-19 diagnosis.
Data of 971 patients: 550, 51, 340, and 30 patients from the 1st to the 4th group, respectively.
Non-anti-COVID-19 therapy includes the cases that have not received specific drugs against SARS-CoV-2 or against the inflammatory response due to COVID-19.
FIGURE 2.
Survival function for death according to age and time from kidney transplant (KT) to SARS-CoV-2 infection
3.4. Characteristics and outcomes of kidney transplant patients with COVID-19 during the different epidemic waves
Of the 1011 cases included, 548 correspond to the first wave and 463 to the second wave (93.6% and 80.4% respectively of the total of patients collected in the registry) ( Table 4). In the second wave, KT recipients were younger, 18.4% were asymptomatic and presented less pneumonia (49.7% vs. 80.5%). Fever, lymphopenia and respiratory symptoms were also less frequent. Treatment changed, with more use of remdesivir and steroids. Ritonavir/lopinavir, hydroxychloroquine, and azithromycin were hardly used. A higher percentage of patients did not receive any specific drugs against SARS-CoV-2 or against the COVID-19-associated cytokine storm in the second wave (44.3% vs. 6.8% in the first one). Hospitalization decreased (63.3% vs. 90%), but more KT recipients were admitted to ICU when we analyzed only hospitalized patients.
TABLE 4.
Characteristics of all kidney transplant patients included in the first and the second waves
| Variables | 1st wave (n = 548) | 2nd wave (n = 463) | P value |
|---|---|---|---|
| Baseline characteristics | |||
| Males, n (%) | 356 (65) | 279 (60.3) | .12 |
| Recipient age (years), median [IQR]a | 62 [52–72] | 57 [47–67] | <.001 |
| Age ≥65 years, n (%) | 238 (43.4) | 145 (31.3) | <.001 |
| DM as cause of kidney disease, n (%) | 76 (14.7) | 55 (12.1) | .23 |
| ACEIs treatment, n (%) | 76 (13.9) | 68 (14.7) | .71 |
| ARBs treatment, n (%) | 144 (26.3) | 130 (28.1) | .52 |
| Tacrolimus-based immunosuppressive therapyb | 434 (83.5) | 395 (87.6) | .07 |
| Time from KT to COVID–19 (months), median [IQR]a | 68.5 [29–140] | 76 [31–140] | .45 |
| Time from KT to COVID–19 ≤6 months, n (%) | 54 (9.9) | 32 (6.9) | .09 |
| Clinical features at COVID–19 diagnosis | |||
| Known risk contact, n (%) | 142 (25.8) | 213 (45.1) | <.001 |
| Asymptomatic, n (%) | 14 (2.6) | 85 (18.4) | <.001 |
| Fever, n (%) | 445 (81.2) | 297 (64.1) | <.001 |
| Cough, expectoration, and/or rhinorrhea, n (%) | 406 (74.1) | 276 (59.6) | <.001 |
| Gastrointestinal symptoms, n (%) | 193 (35.2) | 130 (28.1) | .01 |
| Pneumonia, n (%) | 441 (80.5) | 230 (49.7) | <.001 |
| Lymphopenia, n (%) | 445 (81.2) | 245 (52.9) | <.001 |
| COVID–19 outcomes and treatment | |||
| Hospitalized, n (%) | 498 (90.9) | 293 (63.3) | <.001 |
| Ventilator support, n (%) | 102 (18.6) | 53 (11.4) | .002 |
| ICU admission, n (%) | 74 (13.5) | 66 (14.3) | .73 |
| ICU admission only hospitalized, n (%) | 74 (14.9) | 67 (22.5) | .006 |
| Hydroxychloroquine, n (%) | 479 (87.4) | 1 (0.2) | <.001 |
| Azithromycin, n (%) | 266 (48.5) | 14 (3.0) | <.001 |
| Glucocorticoids, n (%) | 259 (47.3) | 235 (50.8) | .26 |
| Lopinavir/ritonavir, n (%) | 182 (33.2) | 2 (0.4) | <.001 |
| Tocilizumab, n (%) | 101 (18.4) | 40 (8.6) | <.001 |
| Remdesivir, n (%) | 2 (0.4) | 24 (5.2) | <.001 |
| Non anti-COVID–19 therapy, n (%)c | 37 (6.8) | 205 (44.3) | <.001 |
| Length of COVID–19 episode (days), median [IQR]a | 14 [8–21] | 16 [10–23] | <.001 |
| Dead, n (%) | 150 (27.4) | 73 (15.1) | <.001 |
| Dead only hospitalized patients, n (%) | 147 (29.5) | 67 (22.9) | .04 |
| Dead only patients admitted to ICU, n (%) | 43 (58.1) | 44 (66.7) | .29 |
Abbreviations: ACEI, angiotensin converting enzyme inhibitors; ARBs, angiotensin receptor blockers; DM, diabetes mellitus; IQR, interquartile range; KT, kidney transplantation.
Mood’s median test according to order of appearance in the table: p < .001, p = .24, p = .001, respectively.
Data of 971 patients: 520 in the 1st wave and 451 in the 2nd wave.
Non-anti-COVID-19 therapy includes the cases that have not received specific drugs against SARS-CoV-2 or against the inflammatory response due to COVID-19.
When we excluded asymptomatic patients from the analysis, the differences between both phases in age (63 vs. 58 years, p < .001) and pneumonia (82.4% vs. 60.6%, p < .001) remained. There were also no differences in the rest of the variables except in respiratory and gastrointestinal symptoms, which presented a similar incidence in the two waves (Table S3).
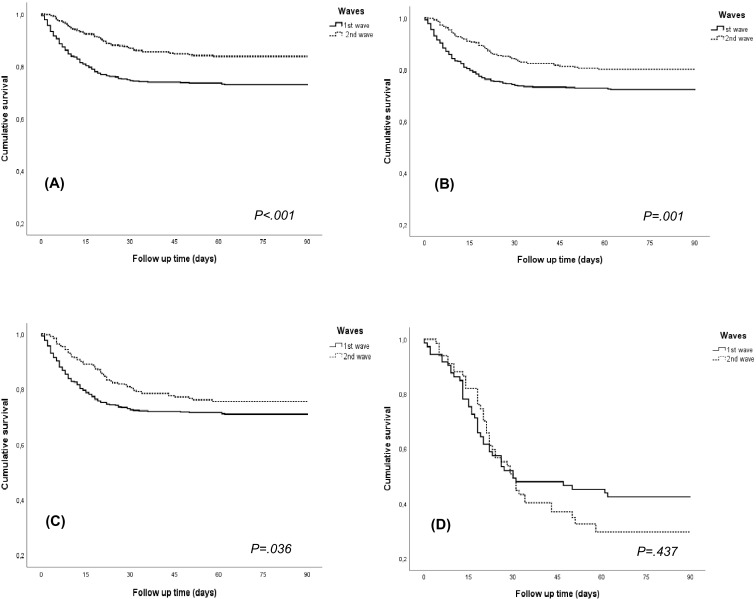
Overall mortality was lower (even excluding asymptomatic cases) during the second wave (15.1%). Mortality in hospitalized patients was also lower (22.9%). However, in critical KT recipients, mortality was 66.7%, not significantly different to that reported in the first wave (58.1%) ( Figure 3).
FIGURE 3.
Survival function for death in the first and second waves. (A) All kidney transplant patients, (B) asymptomatic patients excluded, (C) only hospitalized patients, (D) only patients admitted to ICU
4. DISCUSSION
Our study evaluates simultaneously age and time after transplantation as predictors of severe COVID-19 in KT patients. As in our previous analysis of a smaller number of patients, we can confirm that in the KT population, age and pneumonia are risk factors for death, while gastrointestinal symptoms are associated with a better prognosis.7 Otherwise, patients transplanted recently before the COVID-19 episode also have a higher mortality. We have identified a group of patients, the oldest and recently transplanted, with a higher fatality rate compared to the youngest and with a longer time post-KT, who have the best prognosis. Furthermore, this is the first report that compares the first and the second epidemic waves in KT patients, confirming that clinical severity and anti-COVID-19 therapy have changed.
Advanced age is considered the main risk factor for COVID-19-associated severity and death due to the greater prevalence of chronic conditions in older patients.17 Moreover, in the elderly, alveolar macrophages increase and convert to a pro-inflammatory state that could accelerate COVID-19 in its early stages.18 In our series, median age was 60 years and more than a third of the patients were ≥65 years old, with a mortality rate of almost 40%.
Several registries have analyzed the impact of time after transplantation on the outcomes of COVID-19, but none of them found significant differences.19, 20, 21, 22 In these reports, the time from KT to COVID-19 was analyzed globally or in time periods around the first year after KT. We have focused on the first 6 months after transplantation, finding a higher mortality during this earlier period. The maximum effect of immunosuppression is exerted in the first months after transplantation and recipients are at maximum risk of infection and severity by viral pathogens in this period.23 Consequently, infections are the main cause of death with a functioning graft in the first year after KT.24
In our registry, age and early post-KT period were the baseline conditions prior to infection related to death. The combination of these two variables allowed us to stratify the risk of our COVID-19 KT patients. Recipients ≥65 years diagnosed with COVID-19 in the first 6 months after transplantation presented the highest risk for a fatal outcome, with more than half of the patients deceased. Younger patients infected over 6 months after KT showed a similar mortality to that of the Spanish general population with COVID-19.15 Based on our findings, we consider that we should be very cautious when selecting older patients for KT during the COVID-19 pandemic. Furthermore, as we already have vaccines with proven efficacy,25 patients on the waiting list, especially older patients, should be a priority group in the access to these vaccines.
The evolution of the pandemic in KT recipients is quite similar to the observed in the general population.11, 12, 13 In the second wave, KT patients infected are younger. According to the reports of the Spanish Ministry of Health, the median age of cases in Spain have decreased from 60 years in May to 41 years in December 2020.4 In our registry, these differences are not so remarkable, probably due to a high incidence of COVID-19 in our cohort of elderly KT recipients.4 , 10 Apparently, the severity of COVID-19 in KT patients is also lower in the second wave. We found a higher proportion of asymptomatic cases, so the global mortality rate could be underestimated. These changes may be due to the greater availability of testing, as in most countries, during this period. However, when asymptomatic cases were excluded, we still found clinical and epidemiological differences, a decrease of hospitalized patients and a lower mortality rate in the second wave. When we analyzed only patients who required hospitalization, death rate between both periods also decreased. However, during this second wave, half of our patients developed pneumonia, which is a serious risk factor for death in our population. Further, if we take into account only critical patients, the fatality rate during the second wave has been 66.7%, without significant differences in survival between both epidemic phases. These data show that the mortality due to COVID-19 in KT patients is still markedly higher than in the general population, especially in critical cases. In addition, during the last months of the pandemic, more hospitalized KT patients were admitted to ICU, although probably it is not due to a greater severity of COVID-19, but to the availability of a better prepared healthcare system.
COVID-19 management in KT patients has also changed during the two consecutive waves. Several treatments were used during the first months, but subsequently most of them were deemed inefficient.26, 27, 28, 29, 30 Thus, we have documented an important decrease in the use of hydroxychloroquine, azithromycin, lopinavir/ritonavir and tocilizumab, while glucocorticoids and remdesivir prescriptions have increased in this second wave. Additionally, a higher number of patients did not receive any COVID-19 specific treatment, related to the lower severity of symptoms observed in our cohort and parallel to the change in the specific management of the infection.
Our study presents several limitations. First, the registry is voluntary, and this may induce some underreporting, and decrease estimated incidence. Likewise, the high rate of hospitalized patients may suggest that milder cases were not detected or reported. Second, the difference in the availability of testing may influence the comparison between both waves. Considering this limitation, we compared the clinical characteristics of COVID-19 patients excluding asymptomatic patients and carried out several survival sub-analyses (no asymptomatic cases, only hospitalized patients, those requiring ICU admission). This approach may reduce this patient selection bias. Third, the restricted number of variables collected made the registry an easy tool but limited the analysis of some characteristics evaluated in other studies. We do not have information about comorbidities such as cardiovascular risk factors or renal function and we are missing most laboratory data. In addition, we have assessed only baseline immunosuppression but not possible changes during infection which may have influenced the final outcome. Furthermore, in those patients infected in an early post-KT period, we did not document if they received immunosuppressive induction treatment or if they had a recent diagnosis of acute rejection and subsequent increase in immunosuppression. Notwithstanding, it is a multicenter study which includes the COVID-19 cases identified in KT patients in almost all Spanish KT centers for 9 months, even mild or asymptomatic cases. In consequence, we have recruited a considerable number of patients, quite representative of the global burden of KT recipients in Spain.
In conclusion, over a thousand KT have suffered COVID-19 in Spain with a high mortality rate in the first and second waves. Both advanced age and an early post-KT period were related to a higher mortality rate. Thus, in our opinion, the interaction between age and time after transplant has to be considered when selecting recipients during the COVID-19 pandemic and these older patients should access vaccination as soon as possible. Epidemiological aspects of SARS-CoV-2 have changed in this second wave, affecting predominantly younger people with a less serious clinical picture. However, mortality rate remains similar in severe cases. Likewise, we have documented the change in the COVID-19 specific management during these months.
ACKNOWLEDGMENTS
We are indebted to the many physicians and nurses who take care of these patients and are facing the COVID-19 pandemic in our country.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, A.M., upon reasonable request.
Funding information The Spanish COVID-19 renal registry is supported by the Spanish Society of Nephrology.
Footnotes
Florentino Villanego and Auxiliadora Mazuecos share cofirst authorship. Marta Crespo and Julio Pascual share cosenior authorship.
Members of the Spanish Society of Nephrology COVID-19 Group listed in the supporting information.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
Supplementary Material
REFERENCES
- 1.World Health Organization. Coronavirus disease (COVID-19) weekly epidemiological update and weekly operational update. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports. Published 2020. Accessed December 9, 2020.
- 2.Ahmed O, Brockmeier D, Lee K, Chapman WC, Doyle MBM. Organ donation during the COVID-19 pandemic. Am J Transplant. 2020;20(11):3081–3088. doi: 10.1111/ajt.16199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loupy A, Aubert O, Reese PP, Bastien O, Bayer F, Jacquelinet C. Organ procurement and transplantation during the COVID-19 pandemic. Lancet. 2020;395(10237):e95–e96. doi: 10.1016/S0140-6736(20)31040-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlos III Health Institute. COVID-19 reports. https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Paginas/InformesCOVID-19.aspx. Published 2020. Accessed December 9, 2020.
- 5.Domínguez-Gil B, Coll E, Fernández-Ruiz M, et al. COVID-19 in Spain: transplantation in the midst of the pandemic. Am J Transplant. 2020;20(9):2593–2598. doi: 10.1111/ajt.15983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sánchez-Álvarez JE, Pérez-Fontán M, Jiménez-Martín C, et al. SARS-CoV-2 infection in patients on renal replacement therapy. Report of the COVID-19 Registry of the Spanish Society of Nephrology (SEN) Nefrologia. 2020;40(3):272–278. doi: 10.1016/j.nefro.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crespo M, Mazuecos A, Rodrigo E, et al. Respiratory and gastrointestinal COVID-19 phenotypes in kidney transplant recipients. Transplant. 2020;104(11):2225–2233. doi: 10.1097/TP.0000000000003413. [DOI] [PubMed] [Google Scholar]
- 8.Pascual J, Melilli E, Jiménez-Martín C, et al. COVID-19-related mortality during the first 60 days after kidney transplantation. Eur Urol. 2020;78(4):641–643. doi: 10.1016/j.eururo.2020.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coll E, Fernández-Ruiz M, Sánchez-Álvarez JE, et al. COVID-19 in transplant recipients: the Spanish experience. Am J Transplant. 2020. doi: 10.1111/ajt.16369. Online ahead of print. [DOI] [PMC free article] [PubMed]
- 10.Domínguez-Gil B, Fernández-Ruiz M, Hernández D, et al. Organ donation and transplantation during the COVID-19 pandemic: a summary of the Spanish experience. Transplant. 2021;105(1):29–36. doi: 10.1097/TP.0000000000003528. [DOI] [PubMed] [Google Scholar]
- 11.Fan G, Yang Z, Lin Q, et al. Decreased case fatality rate of COVID-19 in the second wave: a study in 53 countries or regions. Transbound Emerg Dis. 2020;00:1–3. doi: 10.1111/tbed.13819. [DOI] [PubMed] [Google Scholar]
- 12.Vahidy FS, Drews AL, Masud FN, et al. Characteristics and outcomes of COVID-19 patients during initial peak and resurgence in the Houston Metropolitan Area. JAMA. 2020;324(10):998–1000. doi: 10.1001/jama.2020.15301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saito S, Asai Y, Matsunaga N, et al. First and second COVID-19 waves in Japan: a comparison of disease severity and characteristics. J Infect. 2020;2:30693–30699. doi: 10.1016/j.jinf.2020.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Transplant Organization. Donation and transplant activity reports. http://www.ont.es/infesp/Paginas/DatosdeDonacionyTrasplante.aspx. Published 2020. Accessed February 18, 2021.
- 15.Ministry of Health. Government of Spain. Update nº 143. Coronavirus disease (COVID-19). https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/Actualizacion_143_COVID-19.pdf. Published 2020. Accessed January 2, 2021.
- 16.Ministry of Health. Government of Spain. Strategy for early detection, surveillance and COVID-19 control. Updated. December 18, 2020 [Internet]. https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/COVID19_Estrategia_vigilancia_y_control_e_indicadores.pdf. Published 2020. Accessed February 18, 2021.
- 17.Crespo M, Pérez-Sáez MJ, Redondo-Pachón D, et al. COVID-19 in elderly kidney transplant recipients. Am J Transplant. 2020;20(10):2883–2889. doi: 10.1111/ajt.16096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lian J, Jin XI, Hao S, et al. Analysis of epidemiological and clinical features in older patients with coronavirus disease 2019 (COVID-19) outside Wuhan. Clin Infect Dis. 2020;71(15):740–747. doi: 10.1093/cid/ciaa242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caillard S, Anglicheau D, Matignon M, et al. An initial report from the French SOT COVID Registry suggests high mortality due to COVID-19 in recipients of kidney transplants. Kidney Int. 2020;98(6):1549–1558. doi: 10.1016/j.kint.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Azzi Y, Parides M, Alani O, et al. COVID-19 infection in kidney transplant recipients at the epicenter of pandemics. Kidney Int. 2020;98(6):1559–1567. doi: 10.1016/j.kint.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jager KJ, Kramer A, Chesnaye NC, et al. Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int. 2020;98(6):1540–1548. doi: 10.1016/j.kint.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cravedi P, Mothi SS, Azzi Y, et al. COVID-19 and kidney transplantation: results from the TANGO international transplant consortium. Am J Transplant. 2020;20(11):3140–3148. doi: 10.1111/ajt.16185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357:2601–2614. doi: 10.1056/NEJMra064928. [DOI] [PubMed] [Google Scholar]
- 24.Boenink R, Stel VS, Waldum-Grevbo BE, et al. Data from the ERA-EDTA Registry were examined for trends in excess mortality in European adults on kidney replacement therapy. Kidney Int. 2020;98(4):999–1008. doi: 10.1016/j.kint.2020.05.039. [DOI] [PubMed] [Google Scholar]
- 25.Sánchez-Álvarez E, Quiroga B., De Sequera P on behalf of the Spanish Society of Nephrology Position statement of the Spanish Society of Nephrology on the SARS-CoV-2 vaccines. Nefrologia. 2021;S0211-S6995(20):30211–30213. doi: 10.1016/j.nefro.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stone JH, Frigault MJ, Serling-Boyd NJ, et al. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383(24):2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.RECOVERY Collaborative Group. Horby P, Mafham M, et al. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;383(21):2030–2040. doi: 10.1056/NEJMoa2022926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cavalcanti AB, Zampieri FG, Rosa RG, et al. Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19. N Engl J Med. 2020;383(21):2041–2052. doi: 10.1056/NEJMoa2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.RECOVERY Collaborative Group. Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19 - Preliminary report. N Engl J Med. 2020;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 - Final report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, A.M., upon reasonable request.