Abstract
The hypercoagulable state observed in COVID‐19 could be responsible for morbidity and mortality. In this retrospective study we investigated whether therapeutic anticoagulation prior to infection has a beneficial effect in hospitalized COVID‐19 patients. This study included 1154 COVID‐19 patients admitted to 6 hospitals in the Netherlands between March and May 2020. We applied 1:3 propensity score matching to evaluate the association between prior therapeutic anticoagulation use and clinical outcome, with in hospital mortality as primary endpoint. In total, 190 (16%) patients used therapeutic anticoagulation prior to admission. In the propensity score matched analyses, we observed no associations between prior use of therapeutic anticoagulation and overall mortality (risk ratio 1.02 [95% confidence interval; 0.80–1.30]) or length of hospital stay (7.0 [4–12] vs. 7.0 [4–12] days, P = .69), although we observed a lower risk of pulmonary embolism (0.19 [0.05–0.80]). This study shows that prior use of therapeutic anticoagulation is not associated with improved clinical outcome in hospitalized COVID‐19 patients.
Keywords: anticoagulation, corona virus disease 2019, direct oral anticoagulants, pulmonary embolism, thromboprophylaxis, thrombosis, vitamin K antagonist
What is already known about this subject
Patients with COVID‐19 have a hypercoagulable state with increased risk of thrombotic events.
Several studies investigated the association between therapeutic anticoagulation prior to hospitalization and mortality with ambivalent results, probably due to methodological limitations.
What this study adds
A rigorous statistical analysis with thorough adjustment for confounding to properly investigate the treatment effect of prior therapeutic anticoagulation on different clinically relevant outcomes in a large cohort of hospitalized COVID‐19 patients.
This study provides convincing evidence that therapeutic anticoagulation used prior to infection is associated with a decreased risk of pulmonary embolism, but not with mortality and other disease severity parameters.
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) is an infectious disease caused by the severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2). Besides respiratory failure, COVID‐19 also has high rates of thromboembolic complications as seen in multiple retrospective studies. 1 , 2 , 3 , 4 In accordance, autopsy studies showed high incidences of macro‐ and microembolism in patients who were infected by SARS‐CoV‐2. 5 , 6 The exact underlying pathophysiology of COVID‐19 related thrombotic complications remains unknown, but excessive inflammation, hypoxia, immobilization, thrombotic microangiopathy, diffuse intravascular coagulation and complement activation probably play a role. 7 Importantly, low‐molecular‐weight heparin (LMWH) thromboprophylaxis seems to decrease mortality in patients with COVID‐19. 8
Because of the overt COVID‐19 associated coagulopathy, anticoagulation in COVID‐19 receives much attention. Consequently, all guidelines recommend administration of prophylactic LMWH for all hospitalized patients with COVID‐19. 9 , 10 Nevertheless, recent reports show that despite the use of apparently adequate thrombosis prophylaxis, the incidence of venous thromboembolism (VTE) in COVID‐19 patients who were admitted to a hospital ward appears to be much higher compared to patients with other infectious diseases. 2 , 3 , 4 , 11 Higher dose of anticoagulation is especially interesting in patients with history of cardiovascular disease who are at higher risk for adverse events in COVID‐19. 12 However, there are no rigorous trials on therapeutic anticoagulation, and retrospective studies that address the effect of therapeutic anticoagulation prior to or at hospital admission on clinical outcomes show conflicting results, and often compare very heterogeneous cohorts that lack statistical power to draw firm conclusions (summarized in Table 1).
TABLE 1.
Summary of published studies on the effect of prior anticoagulation on COVID‐19 outcome
| Author, date of publication | Design, methods | Primary outcome | OAC | Patients | Findings |
|---|---|---|---|---|---|
| Tremblay et al., July 2020 13 | Retrospective cohort study, PSM & Cox proportional‐hazard models | All‐cause mortality | Prior DOAC + VKA before admission |
Hospitalized patients ‐ OAC n = 139 ‐ control n = 417 |
HR 1.208 (95% CI, 0.750–1.946) |
| Rossi, July 2020 14 | Retrospective cohort study, Cox proportional‐hazards models | All‐cause mortality | Prior DOAC before admission |
Nonhospital patients aged >60 y ‐ DOAC n = 26 ‐ control n = 44 |
HR 0.38 (CI 95%, 0.17–0.58) |
| Lachant, Oct 2020 15 | Retrospective cohort study without correction for confounders | Thrombotic events | Prior OAC before admission |
Hospitalized and nonhospitalized patients ‐ OAC n = 107 |
0 thrombotic events in OAC group |
| Rivera‐Caravaca, Oct 2020 16 | Retrospective cohort study, PSM & Cox proportional‐hazard models | All‐cause mortality | Prior DOAC + VKA before admission |
Hospitalized patients: ‐ OAC n = 109 ‐ control n = 109 |
HR 1.53 (95% CI, 1.08–2.16) |
| Fröhlich et al., Jan 2021 17 | Retrospective cohort study, multivariable logistic regression model | In‐hospital all‐cause mortality or need for invasive or noninvasive ventilation or ECMO implant | Prior DOAC + VKA before admission |
Hospitalized patients registered in health insurance company AOK ‐ VKA n = 223 ‐ DOAC n = 508 ‐ control n = 5059 |
VKA: OR 0.57 (95% CI, 0.40–0.83) DOAC: OR 0.71 (95%, CI 0.56–0.91) |
| Harrison et al., Jan 2021 18 | Retrospective cohort study, multivariable logistic regression model with IPTW on propensity score | In‐hospital mortality at 21 days from the first test | DOAC + VKA before and during admission |
Hospitalized patients ‐ DOAC n = 104 ‐ warfarin n = 28 ‐ control n = 894 |
DOAC: OR 0.44 (95% CI, 0.20–0.90) warfarin: OR, 0.29 (95% CI, 0.02–1.62) |
| Schiavone et al., Jan 2021 19 | Retrospective cohort study, multivariable logistic regression | Mortality | Prior DOAC + VKA before admission |
Hospitalized patients ‐ OAC n = 65 ‐ control n = 779 |
No association with the use of OACs |
| Chocron et al., February 2021 20 | Retrospective cohort study, Cox proportional‐hazard model | ICU admission and composite outcome for ICU admission and/or death | Prior DOAC + VKA before admission |
Hospitalized patients ‐ OAC n = 382 ‐ control n = 1528 |
ICU admission: HR 0.43 (95% CI 0.29–0.63) ICU & death: HR 0.76 (95% CI 0.61–0.98) |
| Flam et al., March 2021 21 | Retrospective cohort study, Cox proportional‐hazards regression | Hospital admission and composite outcome for ICU admission and/or death | Prior DOAC before admission |
Non‐hospitalized patients with AF registered in the national patient register of Sweden ‐ DOAC n = 103 703 ‐ control n = 36 875 |
Hospital admission: HR 1.00 (CI 95%, 0.75–1.33) ICU admission or death: HR 0.76 (CI 95%, 0.51–1.12) |
PSM, propensity score matching; ICU, intensive care unit; IPTW, inverse probability of treatment weighting; OAC, oral anticoagulant; DOAC, direct oral anticoagulant; VKA, vitamin K antagonist; AF, atrial fibrillation; ECMO, extracorporeal membrane oxygenation.
The aim of this study was to investigate the effect of therapeutic anticoagulation used prior to hospitalization on morbidity and mortality in a large cohort of hospitalized COVID‐19 patients.
2. METHODS
2.1. Patients
We included all patients aged ≥18 years with confirmed COVID‐19 admitted to 1 of the 6 participating hospitals in the Netherlands (one academic hospital [Radboudumc, Nijmegen], and 5 teaching hospitals [Canisius Wilhelmina Hospital, Nijmegen; Sint Maartenskliniek, Nijmegen; Rijnstate, Arnhem; Bernhoven, Uden; Jeroen Bosch Hospital, ‘s‐Hertogenbosch]) between 1 March and 31 May 2020. The diagnosis COVID‐19 was made with an in‐house real‐time reverse transcriptase–polymerase chain reaction (PCR) positive for SARS‐CoV‐2 on a deep naso‐oropharyngeal swab. In addition, patients with negative PCR but with clinical symptoms consistent with COVID‐19 and a computed tomography (CT) scan of the chest showing a very high suspicion of typical pulmonary involvement of COVID‐19 (COVID‐19 reporting and data system score of 5 defined by the Dutch Radiology Society) were included. 13 Patients were excluded when COVID‐19 was not PCR or radiographically confirmed, or when patients had insufficient clinical documentation because they were transferred to or from another hospital due to capacity constraints.
The index date was the day of hospital admission. Patients were followed until hospital discharge or death. Data on the occurrence of thrombotic events, length of hospital stay, intensive care unit (ICU) admission, type of oxygen ventilation, and mortality were obtained from the patients' records (EPIC, EPIC Systems Corporation, Verona, WI, USA; HiX, ChipSoft, Amsterdam, The Netherlands; xCare EPD, NEXUS, Nieuwegein, The Netherlands) and recorded in our database using a standardized case report form in the good clinical practice‐compliant data management system Castor (Castor Electronic Data Collection, Amsterdam, the Netherlands). CT pulmonary angiograms were performed at the discretion of the treating clinician. Common indications for CT pulmonary angiogram included high D‐dimer levels and/or progressive hypoxemia. The study was carried out in the Netherlands in accordance with the applicable rules concerning the review of research ethics committees and informed consent. The Institutional Review Boards of the participating hospitals waived the need for informed consent due to the observational nature of this study.
2.2. Outcomes
The primary outcome was all‐cause in hospital mortality. Secondary outcomes included admission to the ICU, need for invasive mechanical ventilation, critical respiratory status (defined as a composite endpoint of the need for invasive mechanical ventilation and/or need of venturi mask and/or nonrebreathing mask), imaging‐proved pulmonary embolism (PE) and length of hospital stay.
2.3. Potential confounders
We identified potential confounders a priori by performing a literature review. Directed acyclic graphs were subsequently drawn to visualize causal assumptions to identify confounders (Figure S1 in the online supplement). Age, sex, body mass index (BMI), medical history of chronic pulmonary disease, diabetes mellitus, active malignancy, hypertension, obstructive coronary heart disease, myocardial infarction, nonischaemic cardiomyopathy, heart failure, previous heart surgery, electronic heart device, cerebrovascular accidents, and/or peripheral artery disease, use of immunosuppressive medication, and no‐ICU policy were identified as confounders that were available in our database.
2.4. Statistical analysis
Descriptive statistics were used to compare the patients with and without prior therapeutic anticoagulation and to estimate the prevalence of the outcomes. Categorical parameters were presented as counts with percentages, continuous parameters with medians and interquartile ranges, based on their non‐normal distribution tested with the Shapiro–Wilk test. Comparisons were performed using Mann–Whitney U test or χ2 test as appropriate. To compare the outcomes while adjusted for potential confounding resulting from the nonrandomized design of our observational study, we applied propensity score‐matching methods. In our database, 174 (15%) patients had missing information for BMI. Therefore, we first imputed BMI values with single imputation using predicted values from multivariable models including age, sex, hypertension, diabetes mellitus and the outcome mortality. Propensity scores were generated using a multivariable logistic regression model with prior therapeutic anticoagulation use as outcome and the 17 variables previously stated as predictors. The patients with therapeutic anticoagulation were subsequently matched in a 1:3 ratio with patients without therapeutic anticoagulation prior to admission on these propensity scores with Nearest Neighbor Matching techniques without replacement and a calliper width of 0.1 of the standard deviation of the logit of the propensity score. To evaluate the balance of measured confounders between exposed and unexposed groups, we calculated the standardized mean difference. A standardized mean difference <0.25 indicated balance of matched cohorts. 22
Associations between prior therapeutic use of anticoagulants and the outcomes overall in hospital mortality, admission to ICU, occurrence of PE, critical respiratory state and the need for invasive mechanical ventilation were estimated as risk ratios with 95% confidence intervals. Estimating the differences in length of hospital stay between the 2 groups was performed using Mann–Whitney U test stratified by mortality.
In our secondary analysis, we compared vitamin K antagonists (VKAs) vs. no therapeutic anticoagulation, direct oral anticoagulants (DOACs) vs. no therapeutic anticoagulation and VKA vs. DOAC on the before mentioned outcome parameters. Matching was performed using the R‐software/studio Version 1.3.1093 and statistical analysis were performed using STATA/SE 16.0 (Stata Corp, TX, USA).
3. RESULTS
Of the 1316 patients who were hospitalized with proven COVID‐19 between 1 March and 31 May 2020, 239 patients were excluded because of reasons depicted in Figure 1. Of the 1154 patients included in this study, 92 (8%) used VKA and 98 (8%) used DOAC, and 964 (84%) patients did not use therapeutic anticoagulation prior to COVID‐19 diagnosis; 76% of patients used OAC because of atrial fibrillation, 13% because of VTE in history, 4% because of mechanical valve replacement, 2 patients because of a cardiac arrest in history and, in 5% of patients, the reason of chronic OAC use was unknown. All patients in the exposed group were continued on therapeutic anticoagulation during hospitalization. Among patients who did not use therapeutic anticoagulation prior to admission, 856 (89%) received prophylactic LMWH during hospitalization. COVID‐19 was confirmed by a positive PCR test in 1124 (97%) patients or considered confirmed by clinical features in combination with a CT scan with a very high level of suspicion (CO‐RADS 5) in 30 (3%) patients.
FIGURE 1.
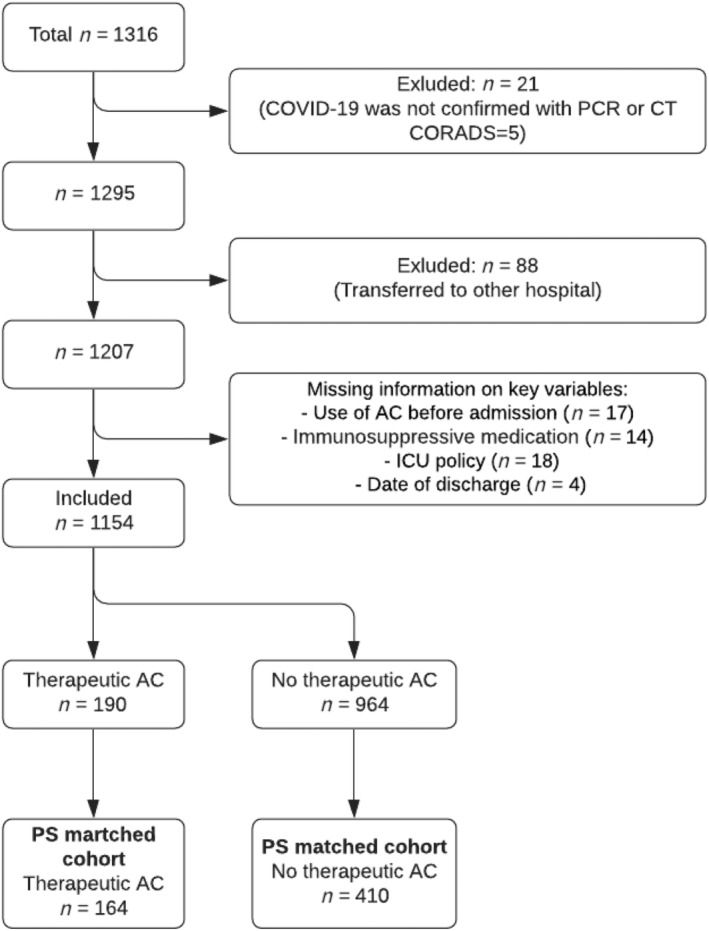
Flow chart displaying included database sample matched by propensity‐score. PCR, reverse transcriptase polymerase chain reaction; CORADS, COVID‐19 reporting and data system score; AC, anticoagulation; ICU, intensive care unit; PS, propensity score; CT, computed tomography
Baseline patient characteristics are shown in Table 2. Patients who used therapeutic anticoagulation prior to hospitalization were older, more likely to be male, and more likely to have cardiovascular comorbidities or a no‐ICU policy compared to patients who did not use prior therapeutic anticoagulation. Subsequent propensity score matching retained 164 (86%) patients who used prior therapeutic anticoagulation and 410 unexposed patients. The main covariates were balanced between the groups after the propensity score matching (Table 2).
TABLE 2.
Characteristic of the total cohort and the study population matched on propensity score for patients with therapeutic anticoagulation use and unexposed patients
| Total cohort | Propensity score‐matched cohort | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Therapeutic AC use (n = 190) | No therapeutic AC use (n = 964) | Therapeutic AC use (n = 164) | No therapeutic AC use (n = 410) | |||||||||
| Characteristic | n | (%) | n | (%) | P‐value | St. Diff. 1 | n | (%) | n | (%) | P‐value | St. Diff. 1 |
| Age, median (IQR), y | 76 | (72–82) | 68 | (57–76) | <.001 | 0.89a | 76 | (71‐82) | 76 | (70–82) | .70 | 0.035 |
| Females | 55 | (29) | 364 | (38) | .02 | 0.19 | 53 | (32) | 132 | (32) | .98 | 0.0026 |
| BMI, median (IQR), kg/m2 | 27.6 | (24.0–31.1) | 27.6 | (25.0–30.8) | .73 | 0.03 | 27.6 | (24.4–31.7) | 27.7 | (24.8–30.8) | .91 | 0.010 |
| Cardiovascular disease | ||||||||||||
| Hypertension | 112 | (59) | 341 | (35) | <.001 | 0.49a | 96 | (58) | 213 | (52) | .15 | 0.13 |
| Obstructive CAD | 52 | (27) | 96 | (10) | <.001 | 0.46a | 42 | (26) | 83 | (20) | .16 | 0.13 |
| Myocardial infarction | 30 | (16) | 80 | (8) | <.001 | 0.23 | 22 | (13) | 59 | (14) | .76 | 0.028 |
| Heart failure | 40 | (21) | 17 | (2) | <.001 | 0.64a | 17 | (10) | 17 | (4) | .004 | 0.24 |
| Nonischaemic cardiomyopathy | 12 | (6) | 15 | (2) | <.001 | 0.25a | 8 | (5) | 14 | (3) | .41 | 0.073 |
| Previous heart surgery | 18 | (9) | 25 | (3) | <.001 | 0.29a | 13 | (8) | 24 | (6) | .36 | 0.082 |
| Electronic heart device | 13 | (7) | 12 | (12) | <.001 | 0.29a | 7 | (4) | 11 | (3) | .33 | 0.086 |
| CVA | 37 | (19) | 99 | (10) | <.001 | 0.26a | 34 | (21) | 73 | (18) | .42 | 0.074 |
| Peripheral artery disease | 20 | (11) | 41 | (4) | <.001 | 0.24 | 18 | (11) | 33 | (8) | .27 | 0.10 |
| Diabetes mellitus | 52 | (27) | 210 | (22) | .09 | 0.13 | 47 | (29) | 110 | (27) | .66 | 0.041 |
| Chronic pulmonary disease | 53 | (28) | 225 | (23) | .18 | 0.10 | 43 | (26) | 107 | (26) | .98 | 0.0028 |
| Active malignancy | 43 | (23) | 169 | (18) | .10 | 0.13 | 38 | (23) | 91 | (22) | .80 | 0.023 |
| Immunosuppressant use | 23 | (12) | 102 | (11) | .54 | 0.05 | 19 | (12) | 55 | (13) | .56 | 0.055 |
| No ICU policy | 114 | (60) | 268 | (28) | <.001 | 0.69a | 92 | (56) | 218 | (53) | .53 | 0.059 |
AC, anticoagulation; BMI, body mass index; CAD, coronary artery disease; CVA, cerebrovascular accident; ICU, intensive care unit; IQR, interquartile range.
Standardized differences to compare the distribution of covariates between the exposure groups.
St. Diff >0.25, the covariate is not balanced between the 2 groups.
The results from the total cohort and the propensity score‐matched analysis on the associations between therapeutic anticoagulation use and the dichotomous outcomes are presented in Table 3A. In the crude total cohort analysis, therapeutic anticoagulation use was associated with an increased risk of mortality and decreased risks of ICU admission, mechanical ventilation and PE. In the propensity score‐matched analyses, however, no associations between therapeutic anticoagulation use and the outcomes were observed, except for a decreased risk of PE among patient with therapeutic anticoagulation (risk ratio 0.19 [95% confidence interval 0.05– 0.80]; Table 3, Figure S2). In addition, therapeutic anticoagulation use was not associated with length of hospital stay (Table 3B).
TABLE 3A.
Risk estimates for COVID‐19 outcome associated with therapeutic anticoagulation vs. no therapeutic anticoagulation in the total cohort and the propensity score (PS)‐matched cohort
| Total cohort | PS‐matched cohort | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Therapeutic AC use (n = 190) | No therapeutic AC use (n = 964) | Therapeutic AC use (n = 164) | No therapeutic AC use (n = 410) | |||||||
| Outcome | n | (%) | n | (%) | Crude RR (95% CI) | n | (%) | n | (%) | PS‐matched RR (95% CI)a |
| Deceased | 75 | (39) | 207 | (22) | 1.84 (1.48–2.28) | 60 | (37) | 147 | (36) | 1.02 (0.80–1.30) |
| ICU admission | 20 | (11) | 193 | (20) | 0.53 (0.34–0.81) | 20 | (12) | 61 | (15) | 0.82 (0.51–1.31) |
| Mechanical ventilation | 16 | (8) | 134 | (14) | 0.61 (0.37–0.99) | 16 | (10) | 42 | (10) | 0.95 (0.55–1.65) |
| Critical respiratory state | 83 | (44) | 362 | (38) | 1.16 (0.97–1.39) | 68 | (41) | 176 | (43) | 0.97 (0.78–1.20) |
| Pulmonary embolism | 2 | (1) | 77 | (8) | 0.13 (0.03–0.53) | 2 | (1) | 26 | (6) | 0.19 (0.05–0.80) |
AC = anticoagulation, CI = confidence interval, ICU = intensive care unit, RR = relative risk.
The propensity scores included the following characteristics: age, sex, body mass index, active malignancy, chronic pulmonary disease, diabetes mellitus, hypertension, obstructive coronary artery disease, myocardial infarction, heart failure, nonischaemic cardiomyopathy, previous heart surgery, electronic heart device, cerebrovascular accident, peripheral artery disease, immunosuppressive medication, no ICU policy.
TABLE 3B.
Associations between therapeutic AC use and length of hospital stay in the total cohort and the propensity score (PS)‐matched cohort
| Total cohort | PS‐matched cohort | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Therapeutic AC use (n = 190) | No therapeutic AC use (n = 964) | Therapeutic AC use (n = 164) | No therapeutic AC use (n = 410) | |||||||
| Length of hospital stay | Days | (IQR) | Days | (IQR) | P‐value | Days | (IQR) | Days | (IQR) | P‐value |
| All patients | 7.0 | (4.0–11.0) | 7.0 | (4.0–13.0) | .47 | 7.0 | (4.3–11.8) | 7.0 | (4.0–12.0) | .69 |
| Deceased | ||||||||||
| Yes | 6.0 | (4.0–9.0) | 7.0 | (5.0–12.0) | .06 | 6.5 | (4.0–10.8) | 7.0 | (4.0–10.0) | .61 |
| No | 7.0 | (5.0–13.0) | 7.0 | (4.0–13.0) | .51 | 7.0 | (5.0–12.8) | 7.0 | (4.0–12.0) | .40 |
AC = anticoagulation, IQR = interquartile range.
The propensity scores included the following characteristics: age, sex, body mass index, active malignancy, chronic pulmonary disease, diabetes mellitus, hypertension, obstructive coronary artery disease, myocardial infarction, heart failure, nonischaemic cardiomyopathy, previous heart surgery, electronic heart device, cerebrovascular accident, peripheral artery disease, immunosuppressive medication, no ICU policy.
Similar to the primary analysis, no associations were found between VKA or DOAC use prior to admission and COVID‐19 related clinical outcome parameters after propensity score matching (see Tables S1–S6).
4. DISCUSSION
The main findings of this study are that therapeutic anticoagulation used prior to SARS‐CoV‐2 infection is associated with a lower risk for PE but is not associated with a decreased risk of other COVID‐19 related outcomes in hospitalized COVID‐19 patients, including in‐hospital mortality. In addition, we did not observe differences in outcomes between DOAC or VKA‐treated subgroups.
The acute inflammatory phenomenon in COVID‐19 amplifies hypercoagulability and increases the risk of thrombosis even under prophylaxis of LMWH. 3 , 4 It has been hypothesized that therapeutic anticoagulation used prior to infection could improve the prognosis of COVID‐19 by hampering coagulation activation. Indeed, a previous study showed that the use of therapeutic anticoagulation at hospital admission resulted in a much lower incidence of VTE compared to thromboprophylaxis alone. 15 Other studies, however, showed ambivalent results on COVID‐19 severity and mortality due to comparison of dissimilar cohorts and lack of proper statistical adjustments for imbalances in baseline characteristics including comorbidities (Table 1). Tremblay et al. also used a propensity score‐matched comparison and found no statistically significant difference in mortality, time to mechanical ventilation, or hospitalization when comparing patients with and without therapeutic oral anticoagulation prior to SARS‐CoV‐2 infection. 23 However, they included both ambulatory and hospitalized patients, and only adjusted for age, sex, race, Charlson comorbidity index and obesity in their propensity‐score analysis while we illustrated that adjustment for more potential confounders is relevant. Moreover, they did not include thrombotic complications as an outcome parameter whereas our study showed a benefit on PE incidence, but not on other clinical endpoints.
This is also the first study to investigate the effects of therapeutic anticoagulation subgroups, ie vitamin K antagonists (VKAs) and direct oral anticoagulants (DOACs), in hospitalized COVID‐19 patients. There are different hypotheses as to why VKA could have unfavourable effects, and DOACs, by contrast, could be of benefit in COVID‐19. Dofferhof et al. detected reduced extrahepatic vitamin K status in patients with COVID‐19 and showed that low vitamin K status was related to poor prognosis in these patients. 24 VKAs are evident causes of reduced vitamin K status, but the relationship between VKA vs. other therapeutic anticoagulation on the prognosis of COVID‐19 patients has never been studied before.
Our study has several limitations that need to be addressed. Most importantly, its observational and retrospective nature limit causal inference, although the propensity score matching increases the credibility of our observations. Noteworthy, propensity score matching is often criticized because of its dependence on the included covariates. Confounders not included in the propensity score could lead to significant bias. However, in our study, the prior visualization of relevant covariates in the directed acyclic graph and the subsequent high and relevant number of included covariates in the propensity score matching, make this a valid approach. It is possible that a history of atrial fibrillation (AF) is a potential confounder since AF is the most important indication for prescribing therapeutic oral anticoagulation. However, patients with AF are at higher risk for poor prognosis as they are older and more likely to have other cardiovascular risk factors. 25 In our study we have thoroughly corrected for these confounders and thus reduced the risk for residual confounding. Although our database represents 1 of the largest matched cohorts of anticoagulation users in the literature (Table 1), the relatively small sample size could potentially lead to a type II error. However, in our study, we found no suggestion of an effect on mortality with a risk ratio close to 1. Furthermore, the increased risk observed for PE is in line with previous studies. Nevertheless, our study population might have been too small to detect small differences in clinical outcomes between the exposure groups. Other limitations are that there was no routine screening for PE, which may have resulted in underdiagnosis of this outcome.
Strengths of our study include the rigorous statistical analysis with thorough adjustment for confounding to properly investigate the treatment effect of prior therapeutic anticoagulation on different clinically relevant outcomes in a large cohort of hospitalized COVID‐19 patients. Furthermore, we are the first to investigate the effect of therapeutic anticoagulation subgroups, i.e. vitamin K antagonists and DOACs.
In summary, although prior therapeutic anticoagulation use is associated with reduced PE occurrence, it is not associated with better outcome parameters in hospitalized COVID‐19 patients in terms of all‐cause mortality, ICU admittance, need for mechanical ventilation and length of hospital stay. Secondary analyses between subgroups also showed no differences in clinical outcomes between VKA‐ and DOAC‐treated patients.
ACKNOWLEDGEMENT
The study was internally funded by the participating departments.
COMPETING INTERESTS
R. Janssen discloses application of a patent on vitamin K in COVID‐19. R. Janssen and A.S.M. Dofferhoff have a scientific collaboration with Kappa Bioscience AS, a manufacturer of vitamin K2 (MK‐7). All other authors declare no conflict of interest or competing interest.
CONTRIBUTORS
J.L. is the principal investigator. J.S., M.G., M.M., M.H., A.E., A.D., R.J., C.K. and J.L. designed the study. A.D., J.v.d.M., J.H., N.J, M.v.A., A.K., K.V., M.B. and R.J.H. were responsible for sample collection and laboratory processing. J.S., A.D., R.J., J.v.d.M., J.H., N.J., M.v.A., A.K., K.V., M.B., R.J.H. and J.L. were responsible for data collection and management. J.S., M.G., C.K. and J.L. performed the statistical analyses and drafted the manuscript. M.M., M.H., A.E., A.D., R.J., J.v.d.M., J.H., N.J., M.v.A., A.K., K.V., M.B. and R.J.H. critically revised the manuscript. All authors read and approved the final manuscript.
ETHICS APPROVAL
The study was approved by the local ethics committee of the Radboud University Medical Centre (number 2020–6344) and carried out in the Netherlands in accordance with the applicable rules concerning the review of research ethics committees and informed consent.
Supporting information
FIGURE S1 A directed acyclic graph for therapeutic anticoagulation exposure prior to COVID‐19 infection and the association with overall mortality. Short title: Directed Acyclic Graph
FIGURE S2 Risk ratios with 95% CI for COVID‐19 outcome associated with therapeutic anticoagulation vs. no therapeutic anticoagulation in the propensity score matched cohort.
TABLE S1 Risk estimates for COVID‐19 outcome associated with VKA vs. no therapeutic anticoagulation in the total cohort and the propensity score matched cohort.
TABLE S2 Associations between VKA use and length of hospital stay in the total cohort and the propensity score matched cohort.
TABLE S3 Risk estimates for COVID‐19 outcome associated with DOAC vs. no therapeutic anticoagulation in the total cohort and the propensity score matched cohort.
TABLE S4 Associations between DOAC use and length of hospital stay in the total cohort and the propensity score matched cohort.
TABLE S5 Risk estimates for COVID‐19 outcome associated with VKA vs. DOAC in the total cohort and the propensity score matched cohort.
TABLE S6 Associations between VKA vs. DOAC use and length of hospital stay in the total cohort and the propensity score matched cohort.
Spiegelenberg JP, van Gelder MMHJ, Maas ML, et al. Prior use of therapeutic anticoagulation does not protect against COVID‐19 related clinical outcomes in hospitalized patients: A propensity score‐matched cohort study. Br J Clin Pharmacol. 2021;87(12):4839–4847. 10.1111/bcp.14877
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Poissy J, Goutay J, Caplan M, et al. Pulmonary Embolism in Patients With COVID‐19: Awareness of an Increased Prevalence. Circulation. 2020;142(2):184‐186. [DOI] [PubMed] [Google Scholar]
- 2. Lodigiani C, Iapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID‐19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Middeldorp S, Coppens M, van Haaps TF, et al. Incidence of venous thromboembolism in hospitalized patients with COVID‐19. J Thromb Haemost. 2020;18(8):1995‐2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Klok FA, Kruip M, van der Meer NJM, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID‐19: An updated analysis. Thromb Res. 2020;191:148‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dolhnikoff M, Duarte‐Neto AN, de Almeida Monteiro RA, et al. Pathological evidence of pulmonary thrombotic phenomena in severe COVID‐19. J Thromb Haemost. 2020;18(6):1517‐1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wichmann D, Sperhake JP, Lutgehetmann M, et al. Autopsy Findings and Venous Thromboembolism in Patients With COVID‐19: A Prospective Cohort Study. Ann Intern Med. 2020;173(4):268‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Belen‐Apak FB, Sarialioglu F. Pulmonary intravascular coagulation in COVID‐19: possible pathogenesis and recommendations on anticoagulant/thrombolytic therapy. J Thromb Thrombolysis. 2020;50(2):278‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thachil J. The versatile heparin in COVID‐19. J Thromb Haemost. 2020;18(5):1020‐1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moores LK, Tritschler T, Brosnahan S, et al. Prevention, Diagnosis, and Treatment of VTE in Patients With Coronavirus Disease 2019: CHEST Guideline and Expert Panel Report. Chest. 2020;158(3):1143‐1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Spyropoulos AC, Levy JH, Ageno W, et al. Scientific and Standardization Committee communication: Clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID‐19. J Thromb Haemost. 2020;18(8):1859‐1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Artifoni M, Danic G, Gautier G, et al. Systematic assessment of venous thromboembolism in COVID‐19 patients receiving thromboprophylaxis: incidence and role of D‐dimer as predictive factors. J Thromb Thrombolysis. 2020;50(1):211‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gerotziafas GT, Catalano M, Colgan MP, et al. Guidance for the Management of Patients with Vascular Disease or Cardiovascular Risk Factors and COVID‐19: Position Paper from VAS‐European Independent Foundation in Angiology/Vascular Medicine. Thromb Haemost. 2020;120(12):1597‐1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. De Smet K, De Smet D, Ryckaert T, et al. Diagnostic Performance of Chest CT for SARS‐CoV‐2 Infection in Individuals with or without COVID‐19 Symptoms. Radiology. 2021;298:E30‐E37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rossi R, Coppi F, Talarico M, Boriani G. Protective role of chronic treatment with direct oral anticoagulants in elderly patients affected by interstitial pneumonia in COVID‐19 era. Eur J Intern Med. 2020;77:158‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lachant DJ, Lachant NA, Kouides P, Rappaport S, Prasad P, White RJ. Chronic therapeutic anticoagulation is associated with decreased thrombotic complications in SARS‐CoV‐2 infection. J Thromb Haemost. 2020;18(10):2640‐2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rivera‐Caravaca JM, Nunez‐Gil IJ, Vivas D, et al. Clinical profile and prognosis in patients on oral anticoagulation before admission for COVID‐19. Eur J Clin Invest. 2020;51:e13436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Frohlich GM, Jeschke E, Eichler U, et al. Impact of oral anticoagulation on clinical outcomes of COVID‐19: a nationwide cohort study of hospitalized patients in Germany. Clin Res Cardiol. 2021. 10.1007/s00392-020-01783-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harrison RF, Forte K, Buscher MG Jr, et al. The Association of Preinfection Daily Oral Anticoagulation Use and All‐Cause in Hospital Mortality From Novel Coronavirus 2019 at 21 Days: A Retrospective Cohort Study. Crit Care Explor. 2021;3(1):e0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schiavone M, Gasperetti A, Mancone M, et al. Oral anticoagulation and clinical outcomes in COVID‐19: An Italian multicenter experience. Int J Cardiol. 2021;323:276‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chocron R, Galand V, Cellier J, et al. Anticoagulation prior to hospitalization is a potential protective factor for COVID‐19: insight from a French multicenter cohort study. J Am Heart Assoc. 2021;e018288. 10.1161/JAHA.120.018624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Flam B, Wintzell V, Ludvigsson JF, Martensson J, Pasternak B. Direct oral anticoagulant use and risk of severe COVID‐19. J Intern Med. 2021;289(3):411‐419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stuart EA, Lee BK, Leacy FP. Prognostic score‐based balance measures can be a useful diagnostic for propensity score methods in comparative effectiveness research. J Clin Epidemiol. 2013;66(8 Suppl):S84‐S90 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tremblay D, van Gerwen M, Alsen M, et al. Impact of anticoagulation prior to COVID‐19 infection: a propensity score‐matched cohort study. Blood. 2020;136(1):144‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dofferhoff ASM, Piscaer I, Schurgers LJ, et al. Reduced vitamin K status as a potentially modifiable risk factor of severe COVID‐19. Clin Infect Dis. 2020. 10.1093/cid/ciaa1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Denas G, Fedeli U, Gennaro N, Ferroni E, Corti MC, Pengo V. Death rates and causes in anticoagulated atrial fibrillation patients: a population‐based study. J Cardiovasc Med (Hagerstown). 2020;21(6):415‐419. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 A directed acyclic graph for therapeutic anticoagulation exposure prior to COVID‐19 infection and the association with overall mortality. Short title: Directed Acyclic Graph
FIGURE S2 Risk ratios with 95% CI for COVID‐19 outcome associated with therapeutic anticoagulation vs. no therapeutic anticoagulation in the propensity score matched cohort.
TABLE S1 Risk estimates for COVID‐19 outcome associated with VKA vs. no therapeutic anticoagulation in the total cohort and the propensity score matched cohort.
TABLE S2 Associations between VKA use and length of hospital stay in the total cohort and the propensity score matched cohort.
TABLE S3 Risk estimates for COVID‐19 outcome associated with DOAC vs. no therapeutic anticoagulation in the total cohort and the propensity score matched cohort.
TABLE S4 Associations between DOAC use and length of hospital stay in the total cohort and the propensity score matched cohort.
TABLE S5 Risk estimates for COVID‐19 outcome associated with VKA vs. DOAC in the total cohort and the propensity score matched cohort.
TABLE S6 Associations between VKA vs. DOAC use and length of hospital stay in the total cohort and the propensity score matched cohort.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.


