Abstract
The COVID‐19 pandemic has blurred the traditional distinction between communicable diseases (CD) and noncommunicable diseases (NCDs). The manifestations of COVID‐19 range from an asymptomatic carrier state to fatal multiorgan failure. While initial reports did not report significant effects on the kidneys, it is now well established that kidney involvement (acute kidney injury, urinary abnormalities, tubular function defects) in COVID‐19 is common and it is also associated with poorer outcomes. At the same time, care for patients with existing chronic kidney disease (CKD) has suffered during this pandemic and those with CKD are considered to have higher risk for severity of COVID‐19 symptoms. Widespread lockdowns have affected the delivery of health care to patients with CKD, including those on dialysis or on transplant wait‐lists. The pandemic has reinforced the need for accessible home‐based therapies and highlighted the value of teleconsultation and remote monitoring technologies. COVID‐19 has revealed the poor emergency preparedness by health systems around the world. It has underscored glaring inequities in availability of diagnostic tests and essential medications, including that for dialysis. In response, there has been increasing recognition of the necessity of universal health coverage and in prioritizing vaccine distribution to serve the most vulnerable, including those with kidney failure. The COVID‐19 pandemic has also reaffirmed the role of the environment and eco‐systems contributing to both CDs and NCDs. Attention to universal health coverage through a One Health approach is needed to prevent global health crises and prevent further kidney dysfunction and failure.
Keywords: COVID 19, chronic kidney disease, non‐communicable diseases, universal health care
1. INTRODUCTION
Traditionally, diseases have been categorized as communicable and noncommunicable. While the recognition of health impacts of infectious/communicable diseases (CDs) has been known for centuries, 1 noncommunicable diseases (NCDs) have gained prominence and taken over as the main causes of mortality and morbidity over the last 50 years. Amongst all NCDs, the contribution of chronic kidney disease (CKD) to the increasing global morbidity and mortality has been recognized relatively recently. The risk factors of CKD include other NCDs like diabetes and hypertension, as well as infections and environmental factors. The interaction between these factors is becoming increasingly blurred by climate change and loss of biodiversity which have been implicated in the recent spate of pandemics including COVID‐19.
The long‐term effects of COVID‐19 on various organ systems including kidneys are starting to be recognized. The association between the presence of NCDs (e.g., diabetes, hypertension, coronary heart disease, respiratory disease, cancer, and obesity) with increased risk of developing severe disease and dying was noticed relatively early in the course of the pandemic. However, the toll that COVID‐19 took on the routine care of patients with pre‐existing conditions, including those with kidney disease was not understood immediately. The complete short‐ and long‐term impacts of COVID‐19 on health will take more time to be understood fully.
This manuscript begins by examining the interdependence of CDs and NCD. Then it provides an assessment of CKD prior to COVID‐19. Next, it reviews the clinical evidence of the effects of COVID‐19 on worsening preexisting kidney conditions and creating new ones. Then it examines challenges of delivery of in‐person and remote care, inequity in access to diagnostic tests and therapeutics, and pharmacopolitics. It concludes with lessons for strengthening kidney care through One Health and Universal Health Coverage (UHC) programs.
2. COMMUNICABLE AND NONCOMMUNICABLE DISEASES
The World Health Organization (WHO) Global Health Observatory (GHO) provides global trends of mortality and morbidity through the Global Burden of Disease (GBD) project, which examined disease prevalence from 1990 to 2017 across 195 countries. The GHO shows that the share of global deaths due to NCDs increased from 60% in 2000 to 71% in 2016, 2 while those due to infectious diseases came down from ~30% to ~20% over the same period. 3 The growing impact and awareness of NCDs are reflected in the evolution of the Millennium Development Goals (MDGs) 2000–2015 that did not mention NCDs to the Sustainable Development Goals (SDGs) of 2015–2030 by the WHO and United Nations (UN) which has a specific indicator related to NCDs. 4
It is becoming increasingly evident that the CDs and NCDs are intricately intertwined. NCDs can increase predisposition to infections, worsen the severity of CDs, and increase mortality risk. Particularly devastating is the effect of epidemics and pandemics through their superimposed and overwhelming additional morbidity and mortality burden on those with pre‐existing NCDs. The reverse relationship where CDs can lead to or worsen NCDs also exists. Several observations support this view: The hepatitis and human papilloma viruses are causally linked to cancer, streptococcus, hepatitis and human immunodeficiency virus infections are implicated in kidney disease, hepatitis in liver disease and so forth.
3. PRE‐COVID STATE OF AVAILABILITY AND ACCESSIBILITY OF HEALTH CARE FOR CKD
CKD is characterized by progressive loss of kidney function, from the stage of kidney damage with normal function to the stage of kidney failure that requires treatment by dialysis or transplantation. Other major consequences of CKD include heightened risk for cardiovascular disease and infections. 5
According to GBD estimates from 2017, CKD affects an estimated 700 million people (9.1% of the population) around the world and is the 12th leading cause of death globally. 5 Over 2.6 million people around the world with advanced kidney disease were on kidney replacement therapy (KRT) in 2010 in the form of hemodialysis (HD), peritoneal dialysis (PD), and kidney transplantation (KT). Of greater concern is the fact that at least 2.3 million people died the same year because they could not access KRT with the largest number of people not receiving KRT belonging to low‐income countries in Asia and Africa. 6
The current models of healthcare delivery for patients with CKD, in particular those with advanced stages of the disease, are centered around hospitals, with nephrologists being the main care providers. It has been clear for some time, however, that much of the evaluation and care in early stages of the disease can be effectively delivered in the community by non‐physician health worker teams. Patients need to reach out to health facilities only for specialist decisions and therapies, especially as the disease progresses. The penetration of patient centered home‐based modalities of KRT, such as PD or home HD is relatively poor in the developing world. Recognizing the need to promote cheaper, patient friendly modalities, several countries (Hong Kong, Mexico, Thailand, Indonesia and parts of China) have put in place policies that prioritize home‐based therapies.
The deficiencies and inequities in kidney care worldwide have been widely documented. The Global Kidney Health Atlas (GKHA) project, a multinational, cross‐sectional survey as part of its Closing the Gap initiative of the International Society of Nephrology (ISN) periodically assesses capacity for kidney care around the world. 7 GKHA data from 2019 reveal that over 90% of people ESKD in low and lower‐middle income countries are not receiving KRT. In the GKHA 2017 survey, low‐ and middle‐income countries (LMICs) reported most of the cost for medication for dialysis and transplant coming entirely out of pocket of patients7. The resultant catastrophic out of pocket expenditure often leads in severe financial crisis and premature interruption in care. 8 Across LMICs, about 188 million people experience catastrophic health expenditure annually as a result of kidney diseases, the greatest of any disease group. 9
4. COVID‐19 AND KIDNEYS
Infection with COVID‐19 can range from an asymptomatic carrier state to a fatal multiorgan failure. Although respiratory tract is the main organ system involved, the systemic nature of the disease is reflected by a severe generalized inflammatory state and involvement of other organs.
Initial reports did not identify the kidney involvement in COVID‐19 or notice the impact of pre‐existing kidney disease on COVID‐19 and its outcomes. Subsequent studies, however, established a high frequency of kidney involvement – manifesting as urinary abnormalities and acute kidney injury (AKI). Further, pre‐existing CKD was associated with a high risk of death due to COVID‐19. 10 Amongst the Medicare beneficiaries, those receiving care for ESKD had a 3.5 times increased risk of death compared to non ESKD beneficiaries. Other studies have shown a 25% case fatality rate amongst patients on maintenance dialysis or kidney transplant recipients who developed COVID‐19. 11 , 12
The incidence of AKI in COVID‐19 has been variable. After the first report from Wuhan in which AKI was not reported amongst 116 hospitalized patients, 13 observational studies reported an incidence of 15% to 20%. 14 As the pandemic spread across continents and more data was available, evidence emerged of a higher incidence of AKI, up to 50% amongst critically ill patients. The presence of any degree of acute kidney injury increased the risk of death due to COVID‐19. 11 , 15 An interesting phenomenon was the real‐time dissemination of information through non‐conventional channels – such as social media. It was through Twitter that reports of the high incidence of AKI overwhelming available resources for kidney replacement therapy were first shared, even in high income countries. 16 , 17 , 18
The pathogenesis of COVID‐19 disease involves binding of the corona virus to a partner or receptor on surface of cells called angiotensin converting enzyme 2 receptor (ACE2R), which is commonly expressed in respiratory organs, heart, intestines and kidneys. 19 While lungs are the most profoundly affected, the virus directly enters many other organs including the heart and kidneys. 20 , 21 Cardiac dysfunction secondary to COVID‐19 disease can also predispose to kidney injury. 22 The virus also leads to a dysregulation in the function of cells of the immunological system which leads to a sudden release in a variety of molecules called cytokines. Another common abnormality is the overactivity of coagulation pathways, leading to development of blood clots in many organs. 23 The kidney injury in severe COVID‐19 morphologically resembles that found in other infectious disease states. Other possible mechanisms contributing to AKI include drug induced kidney damage, hypoxia, rhabdomyolysis, microangiopathy, hemophagocytosis, and secondary infections. 24 A direct role of the virus in the genesis of kidney injury has been suggested. In an autopsy series of 26 COVID‐19 patients, virus particles were demonstrated in kidneys on electron microscopy, particularly in renal tubular epithelium and podocytes. 25 Other studies have found viral RNA and proteins in kidney tissue and cells using more sophisticated techniques. 18 , 19
5. CHALLENGES
5.1. Delivery of in‐person and remote care during COVID‐19
Comprehensive management of COVID‐19 and kidney disease requires access to multiple aspects of health care including, functioning health care facilities, availability of specialized healthcare personnel and access to essential medications and diagnostic tests.COVID‐19 has presented daunting challenges for care of people with pre‐existing kidney disease, especially those who need specialized care such as dialysis or kidney transplantation. In the face of large‐scale lockdowns due to COVID‐19 pandemic, access to health care was severely disrupted worldwide. A number of factors contributed to temporary closure and isolation of health care facilities detected with COVID‐19 cases, conversion of existing health care facilities into dedicated COVID care centers, and widespread suspension of non‐emergency procedures and outdoor consultations. The magnitude of morbidity and mortality due to lack of availability of timely health care for other diseases is yet to be truly established. Several reports have documented the rise in non‐COVID deaths during the pandemic, and the WHO has flagged this as an important issue. 26 The effect of the pandemic on patients on maintenance dialysis in India showed that in the first 3 weeks of the lockdown, about 30% missed at least one session of dialysis and about 3% needed emergency dialysis. 27 Shortage of supplies such as personal protective equipment and dialysis consumables due to suboptimal production capacity and interruption of supply chains contributed to care disruption, at least in the early stages. Outpatient care was also severely affected, with about 92% reduction in attendance during the nationwide lockdown. 27
The pandemic has highlighted the advantages of home‐based and remote care. Home‐based care like PD is more sustainable in geographically far‐flung areas and during natural calamities. 28 These therapies also keep the patients safe from infection, limit the stress on facilities and protect healthcare workers. As a consequence of the unprecedented stalling of health care services, telemedicine use rose. 29 , 30 , 31 Telemedicine is beneficial beyond maintenance of social distancing and a way to get medical advice but also cuts down travel expenses, reduces the time spent in travelling and waiting for consultations and the loss of workdays. In resource‐limited countries with significant rural populations, the impact of telemedicine in improving the access and quality of delivered health care to people with chronic conditions, such as CKD, can be particularly significant. Remote technologies also allow efficient pooling of services and utilization of scarce human capital with training in niche areas, such as dieticians, social workers, psychologists, counsellors, etc. 32 The extent to which telemedicine can replace in‐person consultations remains to be established, however.
5.2. Inequity in access to diagnostic tests
COVID has highlighted the unavailability of reliable diagnostic tests in developing countries, both for COVID‐19, 33 , 34 as well as other diseases including kidney diseases. The GKHA has documented that serum creatinine testing and estimated glomerular filtration rate (eGFR), two diagnostic tests to determine the progression of CKD, were available in only 33% and 50% and 0% and 20% of in primary care settings in low‐ and lower middle‐income countries, respectively. 35 These issues translate into delayed diagnosis, loss of opportunity to reduce or prevent disease progression, late referrals to nephrologists, and eventually worse outcomes. Even when they are available, testing methodologies are often not standardized. For example, creatinine testing using measurement procedures traceable to international reference materials and procedures are not universally available. The availability of other more specialized testing for CKD, including kidney ultrasound and biopsies, is even more limited.
5.3. Inequity in availability of therapeutic options
The time taken for a newly discovered effective medicine (drug, technology or vaccine) to become available to all people in need is long and tedious. This gargantuan task requires global strategy, strong political will, and international collaboration and cooperation, 36 as exemplified by eradication of diseases like poliomyelitis 37 and smallpox. 38 Yet, a time lag exists for these measures to reach every individual who might benefit from them. As of now, therapeutic drugs for COVID‐19, such as Remdesivir, have been available in only a few countries. Multiple reports have highlighted the stir created by a lack of transparency in distribution and accessibility of the drug. 39 , 40 Vaccines for protection against Coronavirus infection have just become available at the time of writing, but the extent to which they will be accesseible for people in different countries remains to be seen.
Medication availability for kidney disease is grim in particular. COVID‐19 highlighted several of the fault lines – for example, interruption in supply chains affected the availability of heparin, which is essential for HD. This shortage of heparin was exploited by the suppliers by inflating its price.
5.4. Pharmacopolitics
Pharmacopolitics refers to the interactions between the pharmaceutical industry, government, regulatory bodies, health professionals, and the public. Priority setting in healthcare, including pharmaceuticals, is inherently political. Although primarily the domain of governments and health professionals, health policy priorities can be influenced by values and preferences of the societies, increasingly affected by new sources of information such as social media and the impact of the private healthcare industry, especially the pharmaceutical companies. It is a challenge to find a balance between the competing claims of profit for the pharmaceutical industry and the wider public interest. 41
Long before from the current pandemic, pharmacopolitics affected the care of patients with kidney diseases. Examples include access to technology‐intensive and expensive therapies like dialysis and transplantation, the differential use of HD and PD around the world, and access to new lifesaving therapeutic agents. Several health economic studies have established the advantages to health systems as well as patients associated with PD, including its overall cost‐effectiveness and positive impact on patient associated outcomes. 42 Despite this, PD remains underutilized in large parts of the developing world where it is likely to provide most value for money. At least some of the effect can be attributed to the impact of for‐profit HD industry and nephrologist preference, driven by a differential reimbursement system. 43 Moreover, the global PD market is monopolistic with a couple of manufacturers being able to control pricing and distribution network of fluid bags. Costs in LMICs are elevated due to import and transport tariffs, which are passed on to patients. The importance of local manufacturing in making PD cheaper and more accessible has been repeatedly stressed, 44 yet few countries have taken serious steps in that direction.
Advances in modern medicine are contributing to the ever‐widening therapeutic apartheid. This is particularly applicable to the ever‐expanding list of biological agents, many already in the market already and others in trials for several renal indications. Some companies have chosen not to market their products drugs in poor economies (e.g., eculizumab), perhaps under the fear that the drugs may be reverse engineered or exported back to high income country markets. As these treatment options enter clinical practice guidelines, the disparity in the ability to access best evidence‐based care becomes institutionalized. The generic drug industry levels the playing field to some extent by making medicines available at a lower price, but there are legitimate issues around the quality of products that might actually end up harming the populations that use them. 45
Global organizations, like the WHO, have recognized the need for medicines that satisfy the priority health care needs of the population to be available at all times in adequate amounts, in the appropriate dosage forms, with assured quality and adequate information, and at a price the individual and the communities can afford. 46 However, national regulations, quality control, allocation of funds, and government commitments (or lack thereof) are major determinants of equity around availability of medicines. On the other hand, global pharmaceutical market strategies, competition, quantity and quality of raw material, maintaining chain of supplies with changing rules and regulations and profitability produce supply‐side challenges. 47 , 48 The well‐documented shortage in supply and poor access to essential medicines disproportionately affects the vulnerable population in the developed countries. 49 In practical terms, inter‐governmental organizations like WHO, act as brokers to ensure LMICs do not receive inferior quality. Through the SDGs, they aim to mitigate the influence of pharmaceutical giants, recognize and correct misaligned profit and public good incentives, and establish norms for country‐level health expenditures to promote equity and access to essential medications, including those for NCDs.
Lastly, as COVID‐19 vaccines are now already in circulation in some countries and many more in development, pharmacopolitics are permeating vaccine approval and distribution strategies. Developing rigorous quality control measures, transparent and equitable eligibility criteria for prioritization, and efficient network for implementation are critical. Most LMICs do not have mechanisms for adult vaccinations. Healthcare systems and societies will need to make just and equitable policies to ensure availability, and most importantly, prioritize who will be first in line. Dialysis patients have been shown to be at higher risk of getting SARS‐CoV2 infection and have a higher death risk when they develop COVID‐19. Given their vulnerability, dialysis patients should be prioritized for COVID‐19 vaccines over other high‐risk groups, such as those with obesity, heart disease, diabetes, earlier stages of CKD or smokers, 50 as should healthcare workers managing these patients, such as staff in dialysis units.
6. LESSONS FOR THE FUTURE OF KIDNEY HEALTH
6.1. One health
Sustainable development must consider health holistically, with due consideration to environment and all ecosystems (Figure 1). 51 , 52 The interaction between COVID‐19 and kidney diseases presents strong evidence that CDs and NCDs are closely interlinked. COVID‐19 has highlighted the dangers caused by environmental degradation, with increasing encroachment by humans on spaces occupied by non‐humans. 53 The RNA sequencing of the current SARS Coronavirus has revealed that it is strikingly similar to the strain found in bats, providing strong support to its zoonotic origin. Air pollution, which been associated with increased transmission of COVID‐19, 54 is also emerging as a causative or a strong contributory factor in disease progression of CKD. 55 , 56 Overall, this strong evidence suggests that abnormalities in kidney health may be an early warning in identifying the impact of changes in the planetary health on overall human health. Finally, the social and economic consequences of the pandemic 57 , 58 are likely to further impact the availability and allocation of resources for healthcare, setting up a vicious cycle.
FIGURE 1.
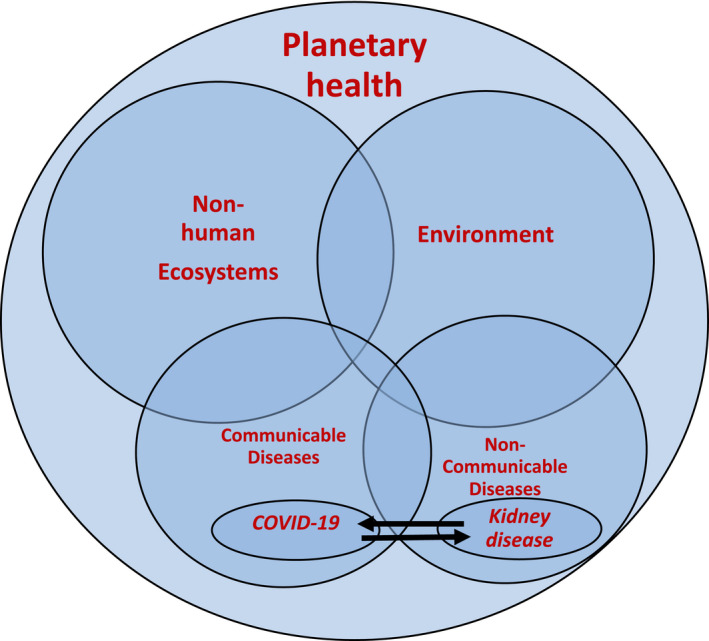
The concept of planetary health in the context of human diseases. The communicable (CD), e.g., Covid‐19 and non‐communicable diseases (NCDs), e.g., Kidney disease are interlinked either by one predisposing/leading to another and/or one influencing the severity of another. Afflictions with both CDs and NCDs are caused or are modified by environment. Non‐human ecosystems affect the environment and CDs. All the entities that affect human health fall under the purview of planetary health. Alterations in any or both the ecosystems and environment is likely to affect both NCDs and CDs
6.2. Universal Health Coverage (UHC)
The necessity of UHC is greater than ever. SDG target 3.8 specifically aims to “by 2030, achieve [UHC], including financial risk protection, access to quality essential health care services, and access to safe, effective, quality, and affordable essential medicines and vaccines for all.” The estimated cumulative financial costs of the COVID‐19 pandemic was estimated at more than $16 trillion in USA. 59 Paying for care of COVID‐19 for people with kidney disease in LMICs has not received sufficient attention. The out‐of‐pocket expenditure for dialysis doubled almost overnight, as many facilities passed on the added costs, such as those for PPEs, directly to patients. This has highlighted the importance of resilient health systems in dealing with the myriad consequences of a pandemic like COVID‐19.
7. CONCLUSION
COVID‐19 has invoked an unprecedented global health care crisis. From a clinician's perspective, the SARS‐CoV2 infection is associated with kidney dysfunction, which in turn increases the risk of death. From the public health perspective, the pandemic has affected health care globally, particularly for people with pre‐existing kidney disease. The pandemic has emphasized the need to move away from physician and health care facility‐based models of health care delivery. There is an urgent need to strengthen primary health care, focusing on patient friendly home‐based therapies and utilizing the potential of teleconsultations to improve outreach. CKD patients – especially those on dialysis – and dialysis care providers should be prioritized, but this will require advocacy from the kidney health community. The current crisis provides an impetus for planning and strategizing towards UHC while integrating it with the wellness of the environment and non‐human ecosystems of the planet.
This article is part of the Global Voices for Prevention of Noncommunicable Diseases Special Collection
REFERENCES
- 1. Brachman PS. Infectious diseases—past, present, and future. Int J Epidemiol. 2003;32(5):684‐686. 10.1093/ije/dyg282 [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization . Noncommunicable Diseases. https://www.who.int/news‐room/fact‐sheets/detail/noncommunicable‐diseases. Accessed 27 July 2020.
- 3. World Health Organization . About The Global Burden Of Disease (GBD) Project. https://www.who.int/healthinfo/global_burden_disease/about/en/. Accessed 27 July 2020.
- 4. World Health Organization . 65th World Health Assembly closes with new global health measures. https://www.who.int/mediacentre/news/releases/2012/wha65_closes_20120526/en/. Published 26 May 2012. Accessed 29 December 2020.
- 5. Cockwell P, Fisher L‐A. The global burden of chronic kidney disease. Lancet. 2020;395(10225):662‐664. 10.1016/S0140-6736(19)32977-0 [DOI] [PubMed] [Google Scholar]
- 6. Liyanage T, Ninomiya T, Jha V, et al. Worldwide access to treatment for end‐stage kidney disease: a systematic review. Lancet. 2015;385(9981):1975‐1982. 10.1016/s0140-6736(14)61601-9 [DOI] [PubMed] [Google Scholar]
- 7. International Society of Nephrology . Global Kidney Health Atlas. https://www.theisn.org/initiatives/global‐kidney‐health‐atlas/. Accessed 28 December 2020.
- 8. Ramachandran R, Jha V. Kidney transplantation is associated with catastrophic out of pocket expenditure in India. PLoS One. 2013;8(7):e67812. 10.1371/journal.pone.0067812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Essue BM, Laba M, Knaul F, et al. Economic burden of chronic Ill health and injuries for households in low‐ and middle‐income countries. In: Jamison DT, Gelband H, Horton S et al. The International Bank for Reconstruction and Development. The World Bank. Published 2017 Nov 27. 10.1596/978-1-4648-0527-1_ch6 [DOI] [PubMed] [Google Scholar]
- 10. Gansevoort RT, Hilbrands LB. CKD is a key risk factor for COVID‐19 mortality. Nat Rev Nephrol. 2020;16:705‐706. 10.1038/s41581-020-00349-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ng JH, Hirsch JS, Wanchoo R, et al. Outcomes of patients with end‐stage kidney disease hospitalized with COVID‐19. Kidney Int. 2020;98(6):1530‐1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jager KJ, Kramer A, Chesnaye NC, et al. Results from the ERA‐EDTA Registry indicate a high mortality due to COVID‐19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int. 2020;98(6):1540‐1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang L, Li X, Chen H, et al. Coronavirus disease 19 infection does not result in acute kidney injury: an analysis of 116 hospitalized patients from Wuhan, China. Am J Nephrol. 2020;51(5):343‐348. 10.1159/000507471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen Y‐T, Shao S‐C, Hsu C‐K, Wu I‐W, Hung M‐J, Chen Y‐C. Incidence of acute kidney injury in COVID‐19 infection: a systematic review and meta‐analysis. Crit Care. 2020;24(1):346. 10.1186/s13054-020-03009-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cheng Y, Luo R, Wang XU, et al. The incidence, risk factors, and prognosis of acute kidney injury in adult patients with coronavirus disease 2019. Clin J Am Soc Nephrol. 2020;15(10):1394‐1402. 10.2215/CJN.04650420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.. Cynamon J. COVID Acute Renal Failure. Asked to place urgent PD. HD limited availability. Easy, Safe and available percutaneous procedure. Placed 10 in 2 weeks. More to come. Published April 15, 2020. Accessed January 3, 2021. https://twitter.com/JacobCynamon/status/1250159005571350528
- 17.. Sethi SS. We are forced to share dialysis circuits given the high percentage of renal failure (these people had normal kidneys before!) Why isn’t there more press for this fact? We need more machines to manage our patients effectively. Published April 12, 2020. Accessed January 3, 2021. https://twitter.com/sanjum/status/1249374584559808515
- 18.. Charytan D. Perspective from NYC #COVID19 1) 20‐30% of icu pts with severe aki 2) lots of rhabdomyolysis 3) filter thrombosis a major issue with ihd/crrt 4) acute PD is a must 5) plan backup plans for your backup coverage 6) national shortages of crrt and PD supplies a@major challenge. Published April 12, 2020. Accessed January 3, 2021. https://twitter.com/DCharytan/status/1249187387080683520
- 19. Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin‐converting enzyme 2 (ACE2) as a SARS‐CoV‐2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(4):586‐590. 10.1007/s00134-020-05985-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Puelles VG, Lütgehetmann M, Lindenmeyer MT, et al. Multiorgan and renal tropism of SARS‐CoV‐2. N Engl J Med. 2020;383(6):590‐592. 10.1056/NEJMc2011400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Braun F, Lütgehetmann M, Pfefferle S, et al. SARS‐CoV‐2 renal tropism associates with acute kidney injury. Lancet. 2020;396(10251):597‐598. 10.1016/s0140-6736(20)31759-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nishiga M, Wang DW, Han Y, Lewis DB, Wu JC. COVID‐19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol. 2020;17(9):543‐558. 10.1038/s41569-020-0413-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cao W, Li T. COVID‐19: towards understanding of pathogenesis. Cell Res. 2020;30:367‐369. 10.1038/s41422-020-0327-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Izzedine H, Jhaveri KD. Acute kidney injury in patients with COVID‐19: an update on the pathophysiology. Nephrol Dial Transplant. 2021;36(2):224‐226. 10.1093/ndt/gfaa184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Su H, Yang M, Wan C, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID‐19 in China. Kidney Internat. 2020;98(1):219‐227. 10.1016/j.kint.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maringe C, Spicer J, Morris M, et al. The impact of the COVID‐19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population‐based, modelling study. Lancet Oncol. 2020;21(8):1023‐1034. 10.1016/S1470-2045(20)30388-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Prasad N, Bhatt M, Agarwal SK, et al. The adverse effect of COVID pandemic on the care of patients with kidney diseases in India. Kidney International Reports. 2020;5(9):1545‐1550. 10.1016/j.ekir.2020.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kumar V, Ramachandran R, Rathi M, Kohli HS, Sakhuja V, Jha V. Peritoneal dialysis: the great savior during disasters. Perit Dial Int. 2013;33(3):327‐329. 10.3747/pdi.2012.00156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Truong T, Dittmar M, Ghaffari A, Lin E. Policy and pandemic: the changing practice of nephrology during the coronavirus disease‐2019 outbreak. Adv Chronic Kidney Dis. 2020;27(5):390‐396. 10.1053/j.ackd.2020.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ngoh CLY, Wong WK, Leo CCH, Choo TT, Khan BA. Rapid transition to a telemedicine service at Singapore community dialysis centers during Covid‐19. NEJM CatalInnov Care Deliv. 2020. 10.1056/CAT.20.0145 [DOI] [Google Scholar]
- 31. Abuzeineh M, Muzaale AD, Crews DC, et al. Telemedicine in the care of kidney transplant recipients with coronavirus disease 2019: case reports. Transpl Proc. 2020;52(9):2620‐2625. 10.1016/j.transproceed.2020.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bello AK, Levin A, Tonelli M, et al. Assessment of global kidney health care status. JAMA. 2017;317(18):1864‐1881. 10.1001/jama.2017.4046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kavanagh MM, Erondu NA, Tomori O, et al. Access to lifesaving medical resources for African countries: COVID‐19 testing and response, ethics, and politics. Lancet. 2020;395(10238):1735‐1738. 10.1016/S0140-6736(20)31093-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McMahon DE, Peters GA, Ivers LC, Freeman EE. Global resource shortages during COVID‐19: bad news for low‐income countries. PLOS Negl Trop Dis. 2020;14(7):e0008412. 10.1371/journal.pntd.0008412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Htay H, Alrukhaimi M, Ashuntantang GE, et al. Global access of patients with kidney disease to health technologies and medications: findings from the Global Kidney Health Atlas project. Kidney Int Suppl. 2018;8(2):64‐73. 10.1016/j.kisu.2017.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Aylward B, Tangermann R. The global polio eradication initiative: lessons learned and prospects for success. Vaccine. 2011;29(Suppl 4):D80‐D85. 10.1016/j.vaccine.2011.10.005 [DOI] [PubMed] [Google Scholar]
- 37. World Health Organization . Polio Eradication Initiative. https://www.who.int/countries/eth/areas/immunization/pei/en/. Accessed 1 August 2020.
- 38. Henderson DA. Principles and lessons from the smallpox eradication programme. Bull World Health Organ. 1987;65(4):535‐546. [PMC free article] [PubMed] [Google Scholar]
- 39. Lupkin S. Remdesivir Distribution Causes Confusion, Leaves Some Hospitals Empty‐Handed. National Public Radio. https://www.npr.org/sections/health‐shots/2020/05/14/855663819/remdesivir‐distribution‐causes‐confusion‐leaves‐some‐hospitals‐empty‐handed. Published 15 May 2020. Accessed 28 October 2020.
- 40. Ison MG, Wolfe C, Boucher HW. Emergency use authorization of remdesivir: the need for a transparent distribution process. JAMA. 2020;323(23):2365‐2366. 10.1001/jama.2020.8863 [DOI] [PubMed] [Google Scholar]
- 41. Keppler H. The Untold AIDS Story: How Access To Antiretroviral Drugs Was Obstructed In Africa. The Einstein Journal of Biology and Medicine. https://theejbm.wordpress.com/2013/10/01/the‐untold‐aids‐story‐how‐access‐to‐antiretroviral‐drugs‐was‐obstructed‐in‐africa/. Published 1 October 2013. Accessed 28 October 2020.
- 42.. Tantivess S, Werayingyong P, Chuengsaman P, Teerawattananon Y. Universal coverage of renal dialysis in Thailand: promise, progress, and prospects. BMJ. 2013;346(jan31 1):f462. 10.1136/bmj.f462 [DOI] [PubMed] [Google Scholar]
- 43. van der Tol A, Lameire N, Morton R, Van Biesen W, Vanholder R. An international analysis of dialysis services reimbursement. Clin J Am Soc Nephrol. 2018;14(1):84‐93. 10.2215/cjn.08150718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zimmerman A. Peritoneal dialysis: increasing global utilization as an option for renal replacement therapy. J Glob Health. 2019;9(2): 10.7189/jogh.09.020316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Praditpornsilpa K, Tiranathanagul K, Kupatawintu P, et al. Biosimilar recombinant human erythropoietin induces the production of neutralizing antibodies. Kidney Int. 2011;80(1):88‐92. 10.1038/ki.2011.68 [DOI] [PubMed] [Google Scholar]
- 46. World Health Organization . Essential medicines. https://www.who.int/topics/essential_medicines/en/. Accessed 4 November 2020.
- 47. World Health Organization . Medicines shortages: global approaches to addressing shortages of essential medicines in health systems. WHO Drug Information. 2016;30(2):180‐185. https://apps.who.int/iris/handle/10665/331028 [Google Scholar]
- 48. Iyengar S, Hedman L, Forte G, Hill S. Medicine shortages: a commentary on causes and mitigation strategies. BMC Medicine. 2016;14(1):124. 10.1186/s12916-016-0674-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mendis S, Fukino K, Cameron A, et al. The availability and affordability of selected essential medicines for chronic diseases in six low‐ and middle‐income countries. Bull World Health Organ. 2007;85(4):279‐288. 10.2471/blt.06.033647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Francis A, Baigent C, Ikizler TA, Cockwell P, Jha V. The urgent need to vaccinate dialysis patients against SARS‐CoV‐2: A call to action. Kidney Int. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Oldekop JA, Horner R, Hulme D, et al. COVID‐19 and the case for global development. World Dev. 2020;134:105044. 10.1016/j.worlddev.2020.105044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Arora NK, Mishra J. COVID‐19 and importance of environmental sustainability. Environmental Sustainability. 2020;3(2):117‐119. 10.1007/s42398-020-00107-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Connolly C, Ali SH, Keil R. On the relationships between COVID‐19 and extended urbanization. Dialogues Human Geography. 2020;10(2):213‐216. 10.1177/2043820620934209 [DOI] [Google Scholar]
- 54. Coccia M. Factors determining the diffusion of COVID‐19 and suggested strategy to prevent future accelerated viral infectivity similar to COVID. Sci Total Environ. 2020;729:138474. 10.1016/j.scitotenv.2020.138474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bowe B, Xie Y, Li T, Yan Y, Xian H, Al‐Aly Z. Particulate matter air pollution and the risk of incident CKD and progression to ESRD. J Am Soc Nephrol. 2018;29(1):218‐230. 10.1681/ASN.2017030253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Afsar B, ElsurerAfsar R, Kanbay A, Covic A, Ortiz A, Kanbay M. Air pollution and kidney disease: review of current evidence. Clin Kidney J. 2019;12(1):19‐32. 10.1093/ckj/sfy111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rastandeh A, Jarchow M. Urbanization and biodiversity loss in the post‐COVID‐19 era: complex challenges and possible solutions. Cities & Health. 2020;1‐4. 10.1080/23748834.2020.1788322 [DOI] [Google Scholar]
- 58. Nicola M, Alsafi Z, Sohrabi C, et al. The socio‐economic implications of the coronavirus pandemic (COVID‐19): a review. Int J Surg. 2020;78:185‐193. 10.1016/j.ijsu.2020.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cutler DM, Summers LH. The COVID‐19 pandemic and the $16 trillion virus. JAMA. 2020;324(15):1495. 10.1001/jama.2020.19759 [DOI] [PMC free article] [PubMed] [Google Scholar]


