Dear Editor, Biologics have become the backbone of treatment for chronic inflammatory diseases, including psoriasis. Postapproval studies allowed for a better understanding of their safety profile, demonstrating a favourable risk–benefit ratio despite an increased risk of infection, and especially an increased risk of bacterial infection with anti‐tumour necrosis factor (TNF) agents.1, 2 In the context of the COVID‐19 pandemic, all experts agree that discontinuing biological therapy is not recommended.3 However, we hypothesized that the pandemic may have modified the first initiation of biological therapy for patients with psoriasis. Indeed, in France, many patients experienced difficulties accessing healthcare during and after the first lockdown (from March to May 2020) owing to the COVID‐19 pandemic.4 Therefore, we studied changes in the dispensation of biologics for psoriasis in France during 2020.
The design of this study has been previously described elsewhere.5 We conducted a French nationwide cohort study based on health administrative data from the French National Health Insurance database (SNDS‐PMSI). The study was approved by the French data protection agency Commission Nationale de l’Informatique et des Libertés (regulatory decision DE‐2015‐165). All adults (aged ≥ 18 years) with psoriasis registered between 1 January 2015 and 31 December 2020 were eligible for inclusion. Psoriasis was defined as having at least two prescriptions of topical vitamin D derivatives (ATC D05AX, the recommended first‐line treatment for psoriasis) within a 2‐year period (a definition commonly used in previous studies).4 All healthcare users who had a prescription for any of the following biological medications for psoriasis were included: etanercept, infliximab, adalimumab, certolizumab (anti‐TNF); ustekinumab [anti‐interleukin (IL)‐12/23]; secukizumab, ixekizumab, and brodalumab (anti IL‐17) or guselkumab (anti IL‐23). New users of biologics were defined as those who had fulfilled a first prescription of any of the available biologics listed above, after 1 year without any biologics. We assessed the number of healthcare users with psoriasis per month who were treated with biologics and the number of new users of biologics per month who initiated treatment with a biologic over time. Lastly, we compared the numbers of both healthcare users with psoriasis treated with biologics and new users of biologics in 2020 with those of the previous years (from 2015 to 2019).
From 2015 to 2020, a total of 45 580 healthcare users with psoriasis were users of biologics [mean (SD) age 44·8 (13·8) years; male patients 52·9%]; 28 441 (62·4%) of these patients were biologic‐naïve and had initiated treatment with a first biologic. From 2015 to 2020, the number of healthcare users with psoriasis treated with biologics was constant over time (Figure 1a), whereas the number of new biologic users dramatically decreased during the first lockdown (from March to May 2020) (Figure 1b). Dispensing of first biologics decreased by up to 57% from March to May compared with 2019. During the rest of 2020, the number of new biologic users remained lower than expected with a second more pronounced decrease during the second lockdown (October to December 2020).
Figure 1.
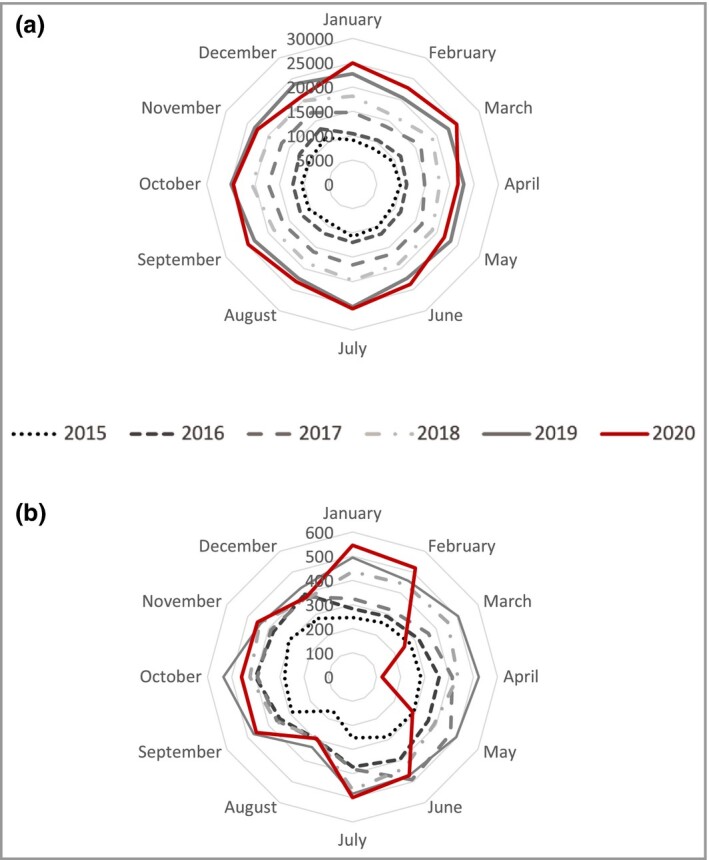
Radar plot. (a) Number of healthcare users with psoriasis treated with biologics per month from 2015 to 2020. (b) Number of new biologic users per month from 2015 to 2020.
This study highlights a marked decrease of up to 57% in the initiation of biologics for healthcare users with psoriasis during the COVID‐19 pandemic in France compared with 2019, which was not compensated for after the lockdown ended, whereas patients with psoriasis who were already being treated with biologics continued to maintain their treatment.
There are several possible explanations for these findings. First prescriptions of biologics for psoriasis in France are hospital‐based prescriptions. Thus, patients may have experienced difficulties accessing biologic prescriber centres during the first lockdown. More generally, patients may have experienced difficulties accessing physicians, as we observed a decrease of up to 48% in the initiation of nonbiological treatments for psoriasis from March to May 2020 (data not shown). These results were also observed for other chronic diseases with a care reduction for newly diagnosed persons (e.g. epilepsy), whereas adherence to treatment remained stable for patients who were already being treated.6 A decrease in the dispensation of systemic anticancer therapy delivery was also observed during the first wave of the pandemic, with a global treatment reduction of 30% (from 20% for breast cancer to 43% for colorectal cancer).7 Another explanation could be that dermatologists prescribed fewer biological treatments because data regarding possible severe COVID‐19 infection in patients treated with biologics were missing at the start of the pandemic. However, reassuring data on the absence of a higher risk of severe COVID‐19 infection for patients receiving biologics became available,8 but we still observed a lower than expected total number of new biologic users initiating a biologic after the first lockdown. On the contrary, the dispensation of systemic anticancer therapy returned to previous levels in the months following May 2020.7 This may have been related to the underlying diseases (oncology vs. inflammatory disorders). Extra data assessing the risks for patients initiating a first biologic could help physicians and patients to continue with the initiation of biologics when needed. Ensuring continuity of psoriasis care should be an important objective in the context of current and future epidemics.
Author Contribution
Laëtitia Penso: Conceptualization (equal); Data curation (lead); Formal analysis (equal); Investigation (equal); Methodology (equal); Validation (equal); Writing‐review & editing (equal). Rosemary Dray‐Spira: Conceptualization (equal); Investigation (equal); Methodology (equal); Validation (equal); Writing‐review & editing (equal). Alain Weill: Conceptualization (equal); Investigation (equal); Methodology (equal); Writing‐review & editing (equal). Mahmoud Zureik: Conceptualization (equal); Methodology (equal); Validation (equal); Writing‐review & editing (equal). Emilie Sbidian: Conceptualization (equal); Formal analysis (equal); Investigation (equal); Methodology (equal); Supervision (lead); Validation (equal); Writing‐original draft (lead).
Contributor Information
L. Penso, GIS‐EPIPHARE, Groupement d’intérêt scientifique Epidémiologie des produits de santé ANSM‐CNAM Paris F‐75020 France Université Paris‐Est Creteil EpiDermE Créteil F‐94010 France.
R. Dray‐Spira, GIS‐EPIPHARE, Groupement d’intérêt scientifique Epidémiologie des produits de santé ANSM‐CNAM Paris F‐75020 France
A. Weill, GIS‐EPIPHARE, Groupement d’intérêt scientifique Epidémiologie des produits de santé ANSM‐CNAM Paris F‐75020 France Caisse Nationale d’assurance Maladie des Travailleurs Salariés (CNAM) Paris F‐75020 France.
M. Zureik, GIS‐EPIPHARE, Groupement d’intérêt scientifique Epidémiologie des produits de santé ANSM‐CNAM Paris F‐75020 France INSERM Echappement aux anti‐infectieux et Pharmacoépidémiologie CESP UVSQ Montigny le Bretonneux F‐78180 France.
E. Sbidian, GIS‐EPIPHARE, Groupement d’intérêt scientifique Epidémiologie des produits de santé ANSM‐CNAM Paris F‐75020 France; Université Paris‐Est Creteil EpiDermE Créteil F‐94010 France; AP‐HP Hôpitaux universitaires Henri Mondor Département de Dermatologie UPEC Créteil F‐94010 France; INSERM Centre d’Investigation Clinique 1430 Créteil F‐94010 France.
References
- Yiu ZZN, Ashcroft DM, Evans I et al. Infliximab is associated with an increased risk of serious infection in patients with psoriasis in the U.K. and Republic of Ireland: results from the British Association of Dermatologists Biologic Interventions Register (BADBIR). Br J Dermatol 2019; 180:329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dávila‐Seijo P, Dauden E, Descalzo MA et al. Infections in moderate to severe psoriasis patients treated with biological drugs compared to classic systemic drugs: findings from the BIOBADADERM registry. J Invest Dermatol 2017; 137:313–21. [DOI] [PubMed] [Google Scholar]
- Lebwohl M, Rivera‐Oyola R, Murrell DF. Should biologics for psoriasis be interrupted in the era of COVID‐19? J Am Acad Dermatol 2020; 82:1217–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPI‐PHARE. Covid‐19: usage des médicaments de ville en France. Available at https://www.epi‐phare.fr/rapports‐detudes‐et‐publications/covid‐19‐usage‐des‐medicaments‐de‐ville‐en‐france‐rapport4/ (last accessed 4 February 2021) (in French).
- Sbidian E, Mezzarobba M, Weill A et al. Persistence of treatment with biologics for patients with psoriasis: a real‐world analysis of 16 545 biologic‐naïve patients from the French National Health Insurance database (SNIIRAM). Br J Dermatol 2019; 180:86–93. [DOI] [PubMed] [Google Scholar]
- Mueller TM, Kostev K, Gollwitzer S et al. The impact of the coronavirus disease (COVID‐19) pandemic on outpatient epilepsy care: an analysis of physician practices in Germany. Epilepsy Behav 2021; 117:107833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter MA, Murphy J, Cameron D et al. The impact of COVID‐19 on systemic anticancer treatment delivery in Scotland. Br J Cancer 2021; 124:1353–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahil SK, Dand N, Mason KJ et al. Factors associated with adverse COVID‐19 outcomes in patients with psoriasis‐insights from a global registry‐based study. J Allergy Clin Immunol 2021; 147:60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]


