ETHICAL PUBLICATION STATEMENT
We confirm that we have read the Journals' position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
We describe the case of a 57‐y‐old female who presented 1 week after receiving the second dose of the Pfizer coronavirus disease 2019 (COVID‐19) vaccine with subacute onset of intense burning dysesthesias in the feet, gradually spreading to the calves and minimally into the hands, unaccompanied by other neurological or constitutional symptoms. There was no known prior COVID‐19 exposure; a COVID‐19 reverse‐transcriptase‐polymerase‐chain‐reaction test 9 mo before this presentation was negative. She was not on any medications and denied the use of alcohol. Besides the distal loss of pinprick and cold sensations in the feet, her examination was unremarkable.
An electrodiagnostic study performed on the day of presentation (including left median, peroneal, and tibial motor nerves with F‐waves; left median, sural, and superficial peroneal sensory nerves; and needle examination of the left tibialis anterior, gastrocnemius, vastus medialis, iliopsoas, and lower lumbar paraspinal muscles), was normal. Skin biopsies showed multifocal involvement 1 (Figure 1). Hematoxylin and eosin and CD3 staining showed no histologic abnormalities.
FIGURE 1.
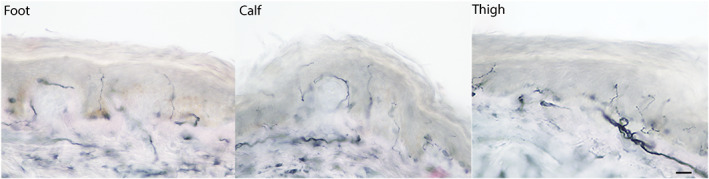
Three mm punch biopsies of skin undertaken confirmed multifocal small fiber neuropathy, suggested by loss of protein gene product 9.5 immunolabeled nerve fibers in the dorsum of left foot, over the extensor digitorum brevis muscle (2.1 fibers/mm of skin; normal >3.0) and left upper thigh at the level of ischial tuberosity (3.8 fibers/mm of skin; normal >6.0), and low normal intra‐epidermal nerve fiber density (IENFD) in the calf segment, 10 cm above the lateral malleolus (4.7 fibers/mm of skin; normal >4.3). Scale bar represents 20 μm. Interpretation of IENFD was done by Corinthian Reference Laboratory, Benbrook, TX [Color figure can be viewed at wileyonlinelibrary.com]
The following laboratory tests were normal or negative: complete blood count, comprehensive metabolic profile, thyroid‐stimulating hormone, methylmalonic acid, folic acid, thiamine, pyridoxine, hemoglobin A1c, serum and urine electrophoresis with immunofixation, serum‐free light chains, HIV antibody, Lyme antibody, sedimentation rate, anti‐double‐stranded DNA antibody, rheumatoid factor, anti‐Smith antibody, antineutrophil cytoplasmic antibodies, anti‐SSA/SSB antibodies, complement levels, and a serum paraneoplastic antibody profile. The severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) antibody profile was consistent with a post‐vaccination state and ruled out previous asymptomatic COVID‐19 exposure.
Treatment with gabapentin provided symptomatic improvement. At 2 wk follow‐up, she reported complete resolution of neuropathic pain and successfully discontinued gabapentin therapy, although stocking‐type small fiber modality loss persisted on examination.
We have identified a case of biopsy‐proven small fiber neuropathy as a post‐vaccination complication. The SARS‐CoV‐2 antibody profile was consistent with a post‐vaccination state but ruled out previous asymptomatic COVID‐19 exposure, which could have resulted in a robust immune response. The development of our patient's presentation soon after the vaccination, and exclusion of other known etiologies, support a possible causal association. A proposed pathophysiologic mechanism for vaccine‐associated polyneuropathy is an immune‐mediated hypersensitivity to the solvent/adjuvant (polyethylene glycol). 2
Concerns regarding neurologic complications of vaccination are not new and include the development of Guillain‐Barré syndrome (GBS); however, the associated risk is minute – on the scale of one to two GBS cases per million doses of influenza vaccine administered, favoring a recommendation for flu vaccination. 3 Although rare, a small number of case reports of small fiber neuropathy have been described in the literature following various vaccinations. 3
With regard to SARS‐CoV‐2, a recent study from the United Kingdom found no association between COVID‐19 and GBS. 4 Furthermore, the lack of significant homology between any SARS‐CoV‐2 genetic or linear protein structure and human linear protein structures in this study make molecular mimicry as a cause less likely, thus minimizing the concerns for COVID‐19 vaccination‐associated GBS. 4
Rare cases of Bell palsy were noted in phase III trials with both COVID‐19 vaccines (Moderna: three in the vaccine and one in the placebo group; Pfizer: four in the vaccine and zero in the placebo group); however, the observed frequency rates did not exceed the background rate found in the general population. 5
A temporary pause after rare reports of transverse myelitis in other COVID‐19 vaccination trials, by AstraZeneca and Sinopharm, was removed after documentation of safety and lack of causal relationship. 6
Adverse neuromuscular events following immunization against SARS‐CoV‐2 were not seen in the pivotal vaccine trials; however, vaccine safety monitoring is an ongoing process. As of February 19, 2021, 28 cases of GBS and no case of Bell palsy have been reported to the Vaccine Adverse Event Reporting System (VAERS) following the COVID‐19 vaccination. 7 In addition to our biopsy‐proven report of small fiber neuropathy, VAERS has received additional reports: 2 of acute motor‐sensory axonal neuropathy, 27 of peripheral neuropathy, 1 of mononeuropathy, 1 of peripheral sensorimotor neuropathy, and 3 of polyneuropathy. New‐onset neuropathies following inoculation against SARS‐CoV‐2 remain rare, with incidence rates between 0.01% and 0.13%. 7
In summary, rare occurrences of COVID‐19 vaccine‐related neuromuscular complications are indeed possible. Although infrequent, any serious adverse reaction to vaccines must be sought, reported, and rigorously investigated to facilitate ongoing safety evaluation and to minimize vaccine hesitancy among the general population. Further studies are warranted to better understand the spectrum of neurological complications after COVID‐19 vaccination and to determine whether a causal relationship exists between these vaccines and neurological sequelae, including small fiber neuropathy.
CONFLICT OF INTEREST
None of the authors have any conflicts of interest to disclose.
ACKNOWLEDGMENTS
We thank Ms. Molly Partelow for manuscript formatting, figure editing, and proofreading of the article. The authors are grateful for the insightful comments and assistance with obtaining the skin biopsy figures by Todd D. Levine, MD.
Abbreviations: COVID‐19, coronavirus disease 2019; GBS, Guillain‐Barré syndrome; IENFD, intra‐epidermal nerve fiber density; S1, Subunit 1; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; VAERS, Vaccine Adverse Event Reporting System.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study
REFERENCES
- 1. Levine T. A proposed taxonomy of isolated small fiber neuropathy. RRNMF Neuromuscular J. 2020;1(4):18‐23. 10.17161/rrnmf.v1i4.13837. [DOI] [Google Scholar]
- 2. Kafaie J, Kim M, Krause E. Small fiber neuropathy following vaccination. J Clin Neuromuscul Dis. 2016;18:37‐40. [DOI] [PubMed] [Google Scholar]
- 3. Salmon DA, Dudley MZ, Carleton BC. Guillain‐Barré syndrome following influenza vaccines affords opportunity to improve vaccine confidence. J Infect. 2021;223(3):355‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Keddie S, Pakpoor J, Mousele C, et al. Epidemiological and cohort study finds no association between COVID‐19 and Guillain‐Barré syndrome. Brain. 2020;144(2):682‐693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shimabukuro T. COVID‐19 vaccine safety update. Advisory Committee on Immunization Practices (ACIP) meeting, January 27, 2021. https://www‐cdc‐gov.ezproxy.uvm.edu/vaccines/acip/meetings/downloads/slides‐2021‐01/06‐COVID‐Shimabukuro.pdf. Accessed January 28, 2021
- 6. Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV‐19 vaccine (AZD1222) against SARS‐CoV‐2: an interim analysis of four randomized controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. US Centers for Disease Control and Prevention Vaccine Adverse Event Reporting System, https://wonder.cdc.gov/vaers.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study


