Abstract
Objective
Psychiatric disorders have been associated with unfavourable outcome following respiratory infections. Whether this also applies to coronavirus disease 2019 (COVID‐19) has been scarcely investigated.
Methods
Using the Danish administrative databases, we identified all patients with a positive real‐time reverse transcription‐polymerase chain reaction test for COVID‐19 in Denmark up to and including 2 January 2021. Multivariable cox regression was used to calculate 30‐day absolute risk and average risk ratio (ARR) for the composite end point of death from any cause and severe COVID‐19 associated with psychiatric disorders, defined using both hospital diagnoses and redemption of psychotropic drugs.
Results
We included 144,321 patients with COVID‐19. Compared with patients without psychiatric disorders, the standardized ARR of the composite outcome was significantly increased for patients with severe mental illness including schizophrenia spectrum disorders 2.43 (95% confidence interval [CI], 1.79–3.07), bipolar disorder 2.11 (95% CI, 1.25–2.97), unipolar depression 1.70 (95% CI, 1.38–2.02), and for patients who redeemed psychotropic drugs 1.70 (95% CI, 1.48–1.92). No association was found for patients with other psychiatric disorders 1.13 (95% CI, 0.86–1.38). Similar results were seen with the outcomes of death or severe COVID‐19.
Among the different psychiatric subgroups, patients with schizophrenia spectrum disorders had the highest 30‐day absolute risk for the composite outcome 3.1% (95% CI, 2.3–3.9%), death 1.2% (95% CI, 0.4–2.0%) and severe COVID‐19 2.7% (95% CI, 1.9–3.6%).
Conclusion
Schizophrenia spectrum disorders, bipolar disorder, unipolar depression and psychotropic drug redemption are associated with unfavourable outcomes in patients with COVID‐19.
Keywords: COVID‐19, respiratory infection, schizophrenia, bipolar disorder, depression, unfavourable outcome
Significant outcomes
In this nationwide cohort including 144,321 patients with coronavirus disease 2019 (COVID‐19), we found that a medical history of severe mental illness (ie schizophrenia spectrum disorders, bipolar disorder or unipolar depression) or a psychiatric disorder requiring active medical treatment is associated with an unfavourable outcome in patients with COVID‐19. This was not the case for other psychiatric disorders.
Limitations
Considering its observational nature, the study only reports associations and does not allow conclusions of causality. Likewise, the risk of residual confounding is always a potential limitation in observational studies.
A positive real‐time reverse transcription‐polymerase chain reaction test for COVID‐19 was necessary to be included in the study. As patients with psychiatric disorders often avoid seeking medical help, selection bias may have influenced the results.
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) is caused by the severe acute respiratory syndrome coronavirus 2 (SARS CoV‐2) which has infected more than 126 million people worldwide and claimed more than 2.7 million lives, as of 28 March 2021. 1
Infectious diseases, especially from the respiratory tract system, contribute markedly to the excess mortality of patients with psychiatric disorders compared with the general population. 2 , 3 Notably, previous studies have documented a 2‐to 7‐fold higher mortality following respiratory infections in patients with psychiatric disorders. 4 , 5 , 6 , 7
Several risk factors associated with severe COVID‐19 outcome are common among patients with psychiatric disorders such as chronic obstructive pulmonary disease (COPD), obesity, diabetes, cardiovascular disease, substance abuse and low socioeconomic status. 8 , 9 , 10 Moreover, a previous diagnosis of a mental disorder has been associated with increased risk for COVID‐19 infection. 11
However, few studies have investigated whether different psychiatric disorders are associated with unfavourable COVID‐19 outcomes. 11 , 12 , 13
1.1. Aims of the study
The aim of the study was to evaluate possible differences in the risk of severe coronavirus disease 2019 and death from any cause between patients with and without psychiatric disorders in a large Danish cohort of patients with coronavirus disease 2019. Considering clinical differences among various psychiatric disorders, five subgroups were analysed separately: schizophrenia spectrum disorders, bipolar disorder, unipolar depression (severe mental illness—SMI), other psychiatric disorders and patients who redeemed psychotropic medications within 90 days prior to index date without having a hospital psychiatric diagnosis.
2. METHODS
2.1. Data sources and definitions
In Denmark, nationwide administrative registries hold complete information on various healthcare‐related and administrative variables. The unique and permanent civil registration number allows cross‐linkage of the registries at an individual level. 14 The Danish Civil Registration System includes information on sex, vital status and migration. The Danish National Patient Register holds information on in‐ and outpatient hospital contacts and surgical procedures. 14 The Danish Psychiatric Central Research Register is a nationwide registration of all psychiatric hospitalizations and ambulatory contacts. 15 In these two registries, the diagnoses are recorded according to the International Classification of Diseases (ICD), the 8th revision (ICD‐8) until 1994 and the 10th revision (ICD‐10) from 1994. From the Danish National Prescription Registry, 16 we retrieved data on claimed drug prescriptions from all Danish pharmacies. Medications are classified according to the international Anatomical Therapeutic Chemical Classification System (ATC). Information on patient income and educational status was collected from the Danish registers on personal income and transfer payments and the Danish Education Registers, respectively. 17 , 18
2.2. Study population
We included all patients in Denmark aged between 16 and 80, who were diagnosed with COVID‐19 from 27 February 2020 until 2 January 2021. 19
Diagnosis was confirmed by a positive real‐time reverse transcription‐polymerase chain reaction test for COVID‐19 obtained from the Danish Microbiology Database. 19 , 20 Moreover, for patients diagnosed for COVID‐19 in a hospital setting, we used ICD codes (ICD 10 codes B342A, B972 and B972A) from the Danish National Patient Registry. These codes were created for the COVID‐19 pandemic by the Danish Ministry of Health in accordance with the definition established by the World Health Organization and have been validated with a positive predictive value of 98%. 21
We excluded patients with dementia (previous hospital diagnosis identified by ICD‐10 codes: F00‐F03, G30, G311, G312 or redemption of a prescription for anti‐dementia drugs [N06D] within 180 days prior to index date) and those >80 years of age (Figure 1) as that they are often deemed ineligible for intensive support. 22
FIGURE 1.
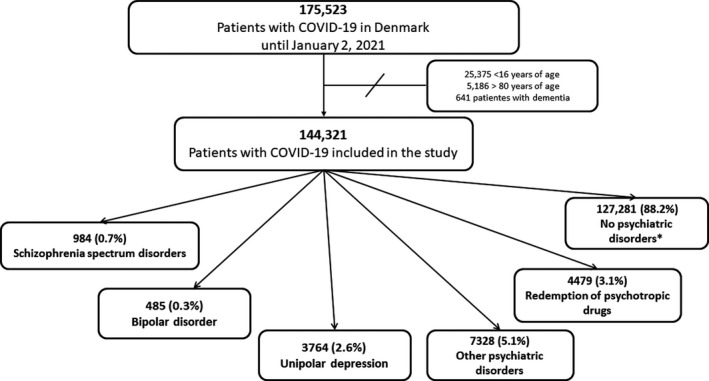
Selection of the study population. COVID‐19, coronavirus disease 2019. *Patients without prior hospital psychiatric diagnoses and who did not redeem psychotropic drugs within 90 days prior to index date
2.3. Exposure of interest
Patients with psychiatric disorders were identified by either an inpatient or an outpatient hospital diagnosis from the Danish Psychiatric Central Research Register any time prior to the day of diagnosis of COVID‐19 (index date), and/or redemption of prescription for a psychotropic drug from the National Prescription Register up to 90 days prior to index date. The following patient groups were identified as follows: schizophrenia spectrum disorders, bipolar disorder, unipolar depression, other psychiatric disorders encompassing psychiatric diagnoses not included in the previous groups; and lastly, patients who redeemed psychotropic medications (antipsychotics, lithium, antidepressants and psychostimulants) within 90 days prior to index date without having a hospital psychiatric diagnosis (ie patients treated for their psychiatric disorder only in the primary sector, not reported in the hospital‐based registries). 23 , 24
If more than one diagnosis was present, the patient was assigned to the most severe group in accordance with the diagnostic hierarchical order in ICD‐10, as done previously. 23 , 25 The ICD and ATC codes used to define psychiatric disorders are listed in Table S1.
2.4. Explanatory covariates
The ICD and ATC codes used to identify baseline comorbidities are listed in Table S2. The use of psychotropic drugs (antipsychotics, lithium, antidepressants and psychostimulants) was defined as the redemption of these medications within 90 days prior to index date. 23 , 24 Socioeconomic status was defined according to the average household income in the year prior to index date and grouped into quartiles. Household income was weighted in accordance with the number of people living in the household by the Organization for Economic Co‐operation and Development—(OECD) modified scale, as done previously. 26 Educational status was defined according to the highest completed educational level in agreement with the International Standard Classification of Education (ISCED). 26
2.5. Outcome
The primary outcome was a composite of death from any cause or severe COVID‐19 (defined as ICD‐10 diagnosis code B972A designating COVID‐19 with SARS or intensive care unit [ICU] admission), as done previously. 21 , 27 Also, the two outcomes were analysed separately. In the analyses where the outcome was severe COVID‐19, death without severe COVID‐19 was considered a competing risk.
2.6. Statistics
We used multivariable cox regression to examine the association between outcomes and the psychiatric subgroups. 21 , 27 The results were presented as 30‐day absolute risks of outcomes standardized to the risk factor distribution of all patients in the sample with related average risk ratios (ARR). Moreover, we calculated unadjusted and adjusted hazard ratios (HRs) with 95% confidence interval (CI). 21
The following variables were included in the models: age, sex, highest obtained education, income, ischaemic heart disease, congestive heart failure, cerebrovascular disease, chronic kidney disease, hypertension, diabetes, asthma, COPD, substance abuse and malignancy.
The following supplementary analyses were performed as follows:
reducing the exposure‐window to one year, that is identifying patients with hospital psychiatric diagnoses (schizophrenia spectrum disorders, bipolar disorder, unipolar depression and other psychiatric disorders) during the year prior to index date;
stratifying patients with a hospital psychiatric diagnosis according to the redemption of psychotropic drugs within 90 days prior to index date (two categories: no redemption or redemption of ≥1 drug);
stratifying the whole psychiatric population by the number of drugs redeemed within 90 days prior to index date, classified as follows: (1) patients not taking psychotropic drugs, (2) taking only one drug, (3) taking two drugs and (4) taking three or more drugs;
including only individuals prescribed psychotropic medications for mental disorders.
Data management and statistical analyses were performed using the SAS Version 9.4 (SAS Institute Inc., Cary, NC, USA) and R version 3.6.1.
2.7. Ethics
In Denmark, registry‐based studies do not require ethical committee approval or patient consent whether the study is conducted for the sole purpose of statistics and scientific research as defined in The Data Protection Act. Approval to use the data sources for research purposes was granted by the data responsible institute in the Capital Region of Denmark in accordance with the General Data Protection Regulation (Approval number: P‐2019–191).
3. RESULTS
We included 144,321 patients with COVID‐19, of these 984 (0.7%) had a schizophrenia spectrum disorder, 485 (0.3%) bipolar disorder, 3764 (2.6%) unipolar depression, 7328 (5.1%) other psychiatric disorders and 4479 (3.1%) were in treatment with psychotropic drugs without having a hospital psychiatric diagnosis (Figure 1). Compared with patients without psychiatric disorders, patients from all five psychiatric groups had a general higher burden of comorbidity, lower income and lower educational level (Table 1). Patients who redeemed psychotropic drugs were older compared with those in the other groups.
TABLE 1.
Baseline characteristics of the study population divided according to the psychiatric disorders
| Characteristics | Patients without psychiatric disorders* | Patients with schizophrenia spectrum disorders | Patients with bipolar disorder | Patients with unipolar depression | Patients with other psychiatric disorders | Patients who redeemed psychotropic drugs |
|---|---|---|---|---|---|---|
| Total number of patients, n (%) | 127,281 (88.2) | 984 (0.7) | 485 (0.3) | 3764 (2.6) | 7328 (5.1) | 4479 (3.1) |
| Median duration of the psychiatric disorder, years (IQR)** | NA | 9.1 (3.8–16.7) | 6.8 (3.2–12.6) | 7.6 (3.7–13.2) | 7.2 (3.3–12.9) | NA |
| Sex | ||||||
| Male, n (%) | 63,241 (49.7) | 480 (48.8) | 181 (37.3) | 1161 (30.8) | 3137 (42.8) | 1714 (38.3) |
| Median age, years (IQR) | 40.5 (26.2, 54.3) | 40 (28.5, 53.6) | 45.3 (31.4, 58.6) | 44 (31.1, 54.8) | 32.6 (23.6, 47.1) | 53.5 (41.5, 64.7) |
| Living alone, n (%) | 38773 (30.5) | 590 (60.0) | 252 (52.0) | 1607 (42.7) | 3347 (45.7) | 1570 (35.1) |
| Ethnic group | ||||||
| Native Danish, n (%) | 95,830 (75.3) | 675 (68.6) | 422 (87.0) | 2,640 (70.1) | 5,488 (74.9) | 3,607 (80.5) |
| Immigrant, n (%) | 23170 (18.2) | 240 (24.4) | 46 (9.5) | 1014 (26.9) | 1345 (18.4) | 784 (17.5) |
| Descendant from immigrant, n (%) | 8281 (6.5) | 69 (7.0) | 17 (3.5) | 110 (2.9) | 495 (6.8) | 88 (2.0) |
| Comorbidities | ||||||
| Ischaemic heart disease***, n (%) | 2240 (1.8) | 19 (1.9) | 9 (1.9) | 120 (3.2) | 132 (1.8) | 178 (4.0) |
| Heart failure, n (%) | 796 (0.6) | 15 (1.5) | 4 (0.8) | 32 (0.9) | 35 (0.5) | 83 (1.9) |
| COPD, n (%) | 7066 (5.6) | 90 (9.1) | 38 (7.8) | 329 (8.7) | 548 (7.5) | 598 (13.4) |
| Asthma, n (%) | 2480 (1.9) | 30 (3.0) | 15 (3.1) | 142 (3.8) | 231 (3.2) | 137 (3.1) |
| Diabetes mellitus, n (%) | 5384 (4.2) | 120 (12.2) | 28 (5.8) | 301 (8.0) | 354 (4.8) | 507 (11.3) |
| Malignancy, n (%) | 3020 (2.4) | 19 (1.9) | 13 (2.7) | 107 (2.8) | 126 (1.7) | 250 (5.6) |
| Cerebrovascular disease, n (%) | 1653 (1.3) | 28 (2.8) | 8 (1.6) | 83 (2.2) | 107 (1.5) | 237 (5.3) |
| Substance abuse, n (%) | 1709 (1.3) | 126 (12.8) | 58 (12.0) | 234 (6.2) | 383 (5.2) | 206 (4.6) |
| Hypertension, n (%) | 10561 (8.3) | 96 (9.8) | 53 (10.9) | 424 (11.3) | 428 (5.8) | 982 (21.9) |
| Chronic kidney disease, n (%) | 1156 (0.9) | 20 (2.0) | 10 (2.1) | 66 (1.8) | 80 (1.1) | 111 (2.5) |
| Number of redeemed psychotropic drugs | ||||||
| 0, n (%) | 127,281 (100.0) | 533 (54.2) | 161 (33.2) | 2375 (63.1) | 5859 (80.0) | 0 (0.0) |
| 1, n (%) | 0 (0.0) | 243 (24.7) | 127 (26.2) | 978 (26.0) | 1170 (16.0) | 3880 (86.6) |
| ≥2, n (%) | 0 (0.0) | 208 (21.1) | 197 (40.6) | 411 (10.9) | 299 (4.1) | 599 (13.4) |
| Socioeconomic status, income quartile | ||||||
| Lowest, n (%) | 17,556 (13.8) | 101 (10.3) | 21 (4.3) | 252 (6.7) | 1264 (17.2) | 187 (4.2) |
| Middle low, n (%) | 33,079 (26.0) | 585 (59.5) | 226 (46.6) | 1481 (39.3) | 3009 (41.1) | 1286 (28.7) |
| Middle high, n (%) | 36,094 (28.4) | 235 (23.9) | 135 (27.8) | 1322 (35.1) | 1964 (26.8) | 1591 (35.5) |
| Highest, n (%) | 40,552 (31.9) | 63 (6.4) | 103 (21.2) | 709 (18.8) | 1091 (14.9) | 1415 (31.6) |
| Highest obtained educational level | ||||||
| ISCED 0–2, n (%) | 33,718 (26.5) | 491 (49.9) | 137 (28.2) | 1172 (31.1) | 3150 (43.0) | 1181 (26.4) |
| ISCED 3, n (%) | 48,153 (37.8) | 296 (30.1) | 179 (36.9) | 1425 (37.9) | 2378 (32.5) | 1791 (40.0) |
| ISCED 5–6, n (%) | 28,853 (22.7) | 124 (12.6) | 109 (22.5) | 829 (22.0) | 1269 (17.3) | 1030 (23.0) |
| ISCED 7–8, n (%) | 13,736 (10.8) | 52 (5.3) | 56 (11.5) | 261 (6.9) | 410 (5.6) | 375 (8.4) |
| Missing, n (%) | 2821 (2.2) | 21 (2.1) | 4 (0.8) | 77 (2.0) | 121 (1.7) | 102 (2.3) |
IQR, interquartile range; ISCED, International Standard Classification of Education.
Patients without prior hospital psychiatric diagnoses and who did not redeem psychotropic drugs within 90 days prior to index date
Defined as the time from the first hospital contact for the specific disorder to index date as a proxy for the duration of the psychiatric disorder
Acute myocardial infarction included.
3.1. Absolute risks and average risk ratio
3.1.1. Composite outcome
By 30 days, 1.2% of COVID‐19 positive patients without psychiatric disorders experienced the composite outcome. In comparison, 4.9% of COVID‐19 positive patients with schizophrenia spectrum disorders, 4.1% of patients with bipolar disorder, 2.1% of patients with unipolar depression, 1.0% of patients with other psychiatric disorders and 5.4% of patients who redeemed psychotropic drugs experienced the composite outcome (Table 2). Patients with schizophrenia spectrum disorders had the highest 30‐day standardized absolute risk (SAR) for the composite outcome: 3.1% (95% CI: 2.3–3.9%).
TABLE 2.
Standardized 30‐Day Absolute Risks and average risk ratio for Composite of Death or Severe COVID‐19 in different subgroups of patients with psychiatric disorders
| Composite outcome | Total number of patients |
Events n (%) |
30‐day absolute risk, % (95% CI) † | Standardized average risk ratio (95% CI) |
|---|---|---|---|---|
| No psychiatric disorders ‡ | 127,281 | 1551 (1.2) | 1.3 (1.2–1.4) | Ref. |
| Schizophrenia spectrum disorders | 984 | 48 (4.9) | 3.1 (2.3–3.9) | 2.43 (1.79–3.07) |
| Bipolar disorder | 485 | 20 (4.1) | 2.7 (1.6–3.8) | 2.11 (1.25–2.97) |
| Unipolar depression | 3764 | 102 (2.7) | 2.2 (1.7–2.6) | 1.70 (1.38–2.02) |
| Other psychiatric disorders | 7328 | 73 (1.0) | 1.4 (1.1–1.8) | 1.13 (0.86–1.38) |
| Psychotropic drug redemption § | 4479 | 242 (5.4) | 2.1 (1.9–2.4) | 1.70 (1.48–1.92) |
COVID‐19, coronavirus disease 2019; CI, confidence interval.
Cox models included age, sex, highest obtained education, income, ischaemic heart disease, congestive heart failure, cerebrovascular disease, chronic kidney disease, hypertension, peripheral artery disease, diabetes, chronic obstructive pulmonary disorder, asthma, substance abuse and malignancy.
Patients without prior psychiatric diagnoses and who did not redeem psychotropic drugs within 90 days prior to index date.
Within 90 days prior to index date without having a hospital psychiatric diagnosis.
The standardized ARR was significantly increased for patients with schizophrenia spectrum disorders 2.43 (95% CI, 1.79–3.07), bipolar disorder 2.11 (95% CI, 1.25–2.97), unipolar depression 1.70 (95% CI, 1.38–2.02) and those who redeemed psychotropic drugs 1.70 (95% CI, 1.38–2.02) (Table 2), but not for patients with other psychiatric disorders 1.13 (95% CI, 0.86–1.38).
3.1.2. Death
Among the different psychiatric subgroups, COVID‐19‐positive patients with schizophrenia spectrum disorders had the highest 30‐day SAR (1.2% [95% CI 0.4–2.0%]). Compared with COVID‐19 positive patients without psychiatric disorders, patients with schizophrenia spectrum disorders (2.29 [95% CI, 1.36–3.22]), bipolar disorder (1.87 [95% CI, 1.12–3.12]), unipolar depression (1.92 [95% CI, 1.39–2.44]) and those who redeemed psychotropic drugs (2.14 [95% CI, 1.77–2.51]) were associated with a significantly increased standardized ARR (Table 3A).
TABLE 3.
Standardized 30‐Day Absolute Risks and average risk ratio for Death and Severe COVID‐19 in different subgroups of psychiatric disorders
| Total number of patients |
Events n (%) |
30‐day absolute risk (95% CI) † | Standardized average risk ratio (95% CI) | |
|---|---|---|---|---|
| A—Death | ||||
| No psychiatric disorders ‡ | 127,281 | 632 (0.5) | 0.5 (0.2–0.8) | Ref. |
| Schizophrenia spectrum disorders | 984 | 20 (2.0) | 1.2 (0.4–2.0) | 2.29 (1.36–3.22) |
| Bipolar disorder | 485 | 8 (1.7) | 1.0 (0.3–1.8) | 1.87 (1.12–3.12) |
| Unipolar depression | 3764 | 49 (1.3) | 1.1 (0.4–1.6) | 1.92 (1.39–2.44) |
| Other psychiatric disorders | 7328 | 28 (0.4) | 0.6 (0.2–1.1) | 1.24 (0.75–1.65) |
| Psychotropic drug redemption § | 4479 | 159 (3.5) | 1.1 (0.5–1.8) | 2.14 (1.77–2.51) |
| B—Severe COVID−19 | ||||
| No psychiatric disorders ‡ | 127,281 | 1197 (0.9) | 0.9 (0.1–1.0) | Ref. |
| Schizophrenia spectrum disorders | 984 | 39 (4.0) | 2.7 (1.9–3.6) | 2.82 (1.96–3.67) |
| Bipolar disorder | 485 | 15 (3.1) | 2.2 (1.1–3.3) | 2.26 (1.16–3.37) |
| Unipolar depression | 3764 | 65 (1.7) | 1.4 (1.1–1.8) | 1.47 (1.11–1.83) |
| Other psychiatric disorders | 7328 | 52 (0.7) | 1.0 (0.7–1.3) | 1.04 (0.76–1.33) |
| Psychotropic drug redemption § | 4479 | 127 (2.8) | 1.3 (1.1–1.5) | 1.27 (0.91–1.52) |
COVID‐19, coronavirus disease 2019; CI, confidence interval.
Cox models included age, sex, highest obtained education, income, ischaemic heart disease, congestive heart failure, cerebrovascular disease, chronic kidney disease, hypertension, peripheral artery disease, diabetes, chronic obstructive pulmonary disorder, asthma, substance abuse and malignancy.
Patients without prior psychiatric diagnoses and who did not redeem psychotropic drugs within 90 days prior to index date.
Within 90 days prior to index date without having a hospital psychiatric diagnosis.
3.1.3. Severe COVID‐19
Among the different psychiatric subgroups, COVID‐19 positive patients with schizophrenia spectrum disorders had the highest 30‐day SAR of severe COVID‐19 disease (2.7% [95% CI, 1.9–3.6%]). Patients with schizophrenia spectrum disorders (2.82 [95% CI, 1.96–3.67]), bipolar disorder (2.26 [95% CI, 1.16–3.37]) and unipolar depression (1.47 [95% CI, 1.11–1.83]) were associated with significantly increased standardized ARR of severe COVID‐19 disease compared with patients without psychiatric disorders (Table 3B).
3.1.4. Hazard ratios
The fully adjusted HRs reflect the results of the main analyses for all three tested outcomes (Table S3). We observed that the adjustment considerably reduced the association between the outcomes and the different psychiatric disorders, especially for the subgroup of patients who redeemed psychotropic drug.
3.1.5. Supplementary analyses
We observed similar results when only including patients with a recent (within past year) diagnosis of a mental disorder (Table S4).
In the analysis stratified by the redemption of psychotropic drugs, for all four considered psychiatric diagnoses (schizophrenia spectrum disorders, bipolar disorder, unipolar depression and other psychiatric disorders), higher estimates were seen for the subgroups receiving treatment with one or more drugs (Table S5). Similarly, when the whole psychiatric population was stratified according to the number of redeemed psychotropic drugs, we observed a trend towards an increasing risk for the composite outcome with increasing number of drugs (Table S6). The adjusted 30‐day SAR went from 1.5% (95% CI, 1.2–1.8%) in patients with psychiatric disorders who did not redeem any drugs to 2.9% (95% CI, 2.4–3.4%) in patients who redeemed ≥2 psychotropic drugs.
The 4479 patients who were receiving treatment with psychotropic drugs but without having a hospital psychiatric diagnosis redeemed in total 8775 prescriptions. Of these, 4476 (51.0%) prescriptions included information on the indication. In 7.8% of the cases (349 out of 4476 prescriptions, corresponding to 231 patients), the indication was not a mental disorder. Repeating the analysis for the composite outcome, excluding these 231 patients, did not change the results: SAR 2.1% (95% CI, 1.8–2.4%) and ARR 1.63 (95% CI, 1.40–1.85), respectively.
4. DISCUSSION
This nationwide study had two major findings: firstly, in COVID‐19 positive patients, schizophrenia spectrum disorders, bipolar disorder, unipolar depression and psychotropic drug redemption—but not other psychiatric disorders—were associated with an increased risk of death and severe COVID‐19; secondly, the number of redeemed psychotropic drugs was (non‐significantly) correlated with an higher risk of death and severe COVID‐19, suggesting an association between illness severity and unfavourable COVID‐19 outcomes.
4.1. Differences in the risk of unfavourable outcome in diverse psychiatric disorders
Two recent studies have suggested an association between psychiatric disorders and severe clinical outcomes in COVID‐19 patients in United States and South Korea. 11 , 13 In a larger and unselected cohort of COVID‐19 patients from Denmark, our study expanded these findings showing differences in the risk of unfavourable outcome associated with diverse mental disorders. Moreover, we showed an increased risk of severe COVID‐19 outcome even in patients not having a hospital psychiatric diagnosis but whose mental disorder is severe enough to require active medical treatment. This implies that thorough monitoring is also needed in COVID‐19 patients with mental disorders handled in the primary sector.
Our data suggested an association between mental illness severity and unfavourable COVID‐19 outcomes. For example, patients with schizophrenia spectrum disorders had the highest 30‐day absolute risk for all the three outcomes. Similarly, in the analysis stratified by the number of redeemed psychotropic drugs, the risk of the composite outcome increased with higher psychotropic drug use.
This may have different explanations. A more severe mental illness often implies a more unhealthy lifestyle and a related higher burden of somatic disease. 2 , 28 Furthermore, SMI per se 29 and different drugs such as alcohol, marijuana and cocaine have been associated with dysregulation of the immune system 30 and may predispose these patients to a more severe infection or bacterial super‐infection.
Cognitive impairment, lack of communication skills, limited comprehension of medical advices and poor self‐awareness typically worsen, as the mental illness progresses. 31 Likewise, stigmatization of mental disorder and ‘diagnostic overshadowing’ may aggravate social isolation of patients with psychiatric disorders and complicate the diagnosis of COVID‐19. 4 , 31 All these factors may lead to a late request for medical advice during the course of the infection, thereby limiting treatment options. 25 , 31
4.2. Factors underlying the association with unfavourable COVID‐19 outcomes
We found an overall higher burden of somatic comorbidity in all psychiatric groups compared with patients without psychiatric disorders. Cardiometabolic and respiratory diseases such as diabetes and COPD are particularly common among patients with psychiatric disorders. 2 These somatic comorbidities have been proven to influence the development of acute respiratory distress syndrome and death in patients with COVID‐19. 9 Therefore, the increased risk of unfavourable outcome that we observed may be explained, at least partly, by a higher comorbidity burden. Correspondingly, adjusting for comorbidities markedly reduced this association.
Both antipsychotics and antidepressants seem to affect the immune system. 32 , 33 , 34 Furthermore, several studies have hypothesized a direct link between antipsychotics use and risk of pneumonia and pneumonia‐related mortality. Sedation, impairment of swallowing and cough reflexes, hypersalivation and changes in pharyngeal and laryngeal muscle tone are among the possible underlying mechanisms. 32 , 33
A low socioeconomic status and educational level were also more common among patients with psychiatric disorders in our cohort. It has been demonstrated that both factors negatively impact the post‐infection course primarily because of an association with unhealthy lifestyle and lower access to medical help. 8 Moreover, as a result of economic and social disadvantage, patients with psychiatric disorders, especially those with more severe disorders, 35 more often live in nursing homes, congregate settings or homeless shelters. The characteristics of these settings such as poor hygienic conditions and overcrowding may pose an increased risk to these patients of being infected by SARS CoV‐2 and exposure to a higher viral load, which correlates with disease severity and mortality. 11
4.3. Clinical implications of the study
The association between psychiatric disorders and unfavourable COVID‐19 outcomes may thus be mediated by somatic comorbidities and severity of the mental disorder. Hence, an important first step in the prevention of unfavourable COVID‐19 outcomes in these patients would be an optimal management of their pulmonary, metabolic and cardiovascular diseases. 8 , 9 However, the management of patients with psychiatric disorders may be particularly challenging in the course of a pandemic, 36 for example because of a low treatment‐adherence. 31 Therefore, during the present recurrence of COVID‐19 outbreak, improvements in management policy including special prevention measures, active surveillance and prompt treatment as well as prioritization in vaccination strategies should be considered in patients with psychiatric disorders to tackle their higher risk of unfavourable COVID‐19 outcome.
5. LIMITATIONS
Firstly, due to the observational nature of the study, no conclusions of causality can be drawn. Secondly, the Danish registries lack important clinical parameters such as smoking habits and BMI, which have been shown to influence outcome in COVID‐19 patients. 8 , 37 As tobacco use and overweight are particularly common among patients with psychiatric disorders, 28 we cannot rule out the possibility that our results were mainly driven by unmeasured confounders.
Thirdly, selection bias must be acknowledged. As patients with psychiatric disorders often seek help late in the course of a disease 25 and considering that this phenomenon would be expected to be proportional to the severity of the mental disorder, 31 it is possible that the association with unfavourable outcomes is the result of a delayed diagnosis of COVID‐19.
Fourthly, information about the indication for drug prescription is poorly reported in the Danish registries. Some psychotropic drugs are prescribed for conditions other than mental disorders. However, repeating the analysis but excluding tricyclic antidepressants—the antidepressants most used in non‐psychiatric condition—and excluding patients with a registered non‐psychiatric indication for their psychotropic drugs did not alter the results.
Fifthly, treatment nonadherence is particularly high among patients with psychiatric disorders, especially among those with SMI. 38 , 39 Therefore, in the analyses based on the number of redeemed drugs, the group of patients without psychotropic drugs may be composed of a mix of patients with a milder disorder, perhaps not requiring treatment, and patients with a more severe disorder, not redeeming well‐indicated drugs. This could have fictively increased the association with unfavourable outcome in this group (and reduced the association in the groups of patients redeeming ≥1 drugs). However, both the analyses based on redeemed drugs and those based on diagnoses were consistent in showing a trend towards an increasing risk of the composite outcome in the patients with a more severe psychiatric disorder.
Lastly, the study was conducted in Denmark and this could weaken the generalizability of our findings, considering differences in healthcare systems, handling of pandemic and screening strategies in different countries.
6.
To conclude, a medical history of schizophrenia spectrum disorders, bipolar disorder or unipolar depression or a psychiatric disorder requiring active medical treatment may represent a substantial risk factor for an unfavourable outcome in patients with COVID‐19.
CONFLICTS OF INTEREST
None of the authors have any conflicts of interest to report.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/acps.13309.
Supporting information
Tables S1‐S6
ACKNOWLEDGEMENTS
The authors wish to express their gratitude to Jørn Korsbø Pedersen and his colleagues at Statistics Denmark for their help regarding data accessibility. Likewise, the authors acknowledge The Danish Departments of Clinical Microbiology and Statens Serum Institut for carrying out laboratory analysis, registration, and release of the national SARS‐CoV‐2 surveillance data for the present study.
Funding information
Departmental funding only.
DATA AVAILABILITY STATEMENT
The data and study materials cannot be made available to other researchers for purposes of reproducing the results or replicating the procedure.
REFERENCES
- 1. Novel Coronavirus (2019‐nCoV) situation reports – World Health Organization (WHO). Available from: https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019/situation‐reports/
- 2. Plana‐Ripoll O, Pedersen CB, Agerbo E, et al. A comprehensive analysis of mortality‐related health metrics associated with mental disorders: a nationwide, register‐based cohort study. Lancet. 2019;394:1827‐1835. [DOI] [PubMed] [Google Scholar]
- 3. Kugathasan P, Stubbs B, Aagaard J, Jensen SE, Munk Laursen T, Nielsen RE. Increased mortality from somatic multimorbidity in patients with schizophrenia: a Danish nationwide cohort study. Acta Psychiatr Scand. 2019;140:340‐348. [DOI] [PubMed] [Google Scholar]
- 4. Ribe AR, Vestergaard M, Katon W, et al. Thirty‐day mortality after infection among persons with severe mental illness: a population‐based cohort study in Denmark. Am J Psychiatry. 2015;172:776‐783. [DOI] [PubMed] [Google Scholar]
- 5. Crump C, Sundquist K, Winkleby MA, Sundquist J. Comorbidities and mortality in bipolar disorder: a Swedish national cohort study. JAMA Psychiatry. 2013;70:931‐939. [DOI] [PubMed] [Google Scholar]
- 6. Crump C, Winkleby MA, Sundquist K, Sundquist J. Comorbidities and mortality in persons with schizophrenia: a Swedish national cohort study. AJP. 2013;170:324‐333. [DOI] [PubMed] [Google Scholar]
- 7. Chen Y‐H, Lin H‐C, Lin H‐C. Poor clinical outcomes among pneumonia patients with Schizophrenia. Schizophr Bull. 2011;37:1088‐1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID‐19‐related death using OpenSAFELY. Nature. 2020;584:430‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guan W, Liang W, Zhao Y, et al. Comorbidity and its impact on 1590 patients with COVID‐19 in China: a nationwide analysis. Eur Respir J. 2020;55:2000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang QQ, Kaelber DC, Xu R, Volkow ND. COVID‐19 risk and outcomes in patients with substance use disorders: analyses from electronic health records in the United States. Mol Psychiatry. 2020. http://www.nature.com/articles/s41380‐020‐00880‐7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang Q, Xu R, Volkow ND. Increased risk of covid ‐19 infection and mortality in people with mental disorders: analysis from electronic health records in the United States. World Psychiatry. 2020; wps.20806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barber S, Reed L, Syam N, Jones N. Severe mental illness and risks from COVID‐19 [Internet]; 2020. Available from: https://www.cebm.net/covid‐19/severe‐mental‐illness‐and‐risks‐from‐covid‐19/#:~:text=People%20with%20severe%20mental%20illness,data%20that%20quantified%20these%20risks
- 13. Lee SW, Yang JM, Moon SY, et al. Association between mental illness and COVID‐19 susceptibility and clinical outcomes in South Korea: a nationwide cohort study. Lancet Psychiatry. 2020;7(12):1025‐1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schmidt M, Schmidt SAJ, Adelborg K, et al. The Danish health care system and epidemiological research: from health care contacts to database records. CLEP. 2019;11:563‐591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mors O, Perto GP, Mortensen PB. The Danish Psychiatric central research register. Scand J Public Health. 2011;39:54‐57. [DOI] [PubMed] [Google Scholar]
- 16. Kildemoes HW, Sørensen HT, Hallas J. The Danish National Prescription Registry. Scand J Public Health. 2011;39:38‐41. [DOI] [PubMed] [Google Scholar]
- 17. Baadsgaard M, Quitzau J. Danish registers on personal income and transfer payments. Scand J Public Health. 2011;39:103‐105. [DOI] [PubMed] [Google Scholar]
- 18. Jensen VM, Rasmussen AW. Danish education registers. Scand J Public Health. 2011;39:91‐94. [DOI] [PubMed] [Google Scholar]
- 19. Registration analyses of Danish Covid‐19 patients. Available from: https://laegemiddelstyrelsen.dk/en/about/danish‐medicines‐agencys‐data‐analytics‐center‐dac/registration‐analyses‐of‐danish‐covid‐19‐patients/
- 20. Voldstedlund M, Haarh M, Mølbak K, the MiBa Board of Representatives . The Danish Microbiology Database (MiBa) 2010 to 2013. Eurosurveillance. 2014;19(1): 10.2807/1560-7917.ES2014.19.1.20667. [DOI] [PubMed] [Google Scholar]
- 21. Fosbøl EL, Butt JH, Østergaard L, et al. Association of angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker use with COVID‐19 diagnosis and mortality. JAMA. 2020;324:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mok VCT, Pendlebury S, Wong A, et al. Tackling challenges in care of Alzheimer’s disease and other dementias amid the COVID‐19 pandemic, now and in the future. Alzheimer’s Dementia. 2020;alz.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barcella CA, Mohr GH, Kragholm K, et al. Out‐of‐hospital cardiac arrest in patients with psychiatric disorders – characteristics and outcomes. Resuscitation. 2020;143:180‐188. [DOI] [PubMed] [Google Scholar]
- 24. Barcella CA, Mohr GH, Kragholm KH, et al. Out‐of‐hospital cardiac arrest in patients with and without psychiatric disorders: differences in use of coronary angiography, coronary revascularization, and implantable cardioverter‐defibrillator and survival. J Am Heart Assoc. 2019;8:e012708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Heiberg IH, Jacobsen BK, Balteskard L, et al. Undiagnosed cardiovascular disease prior to cardiovascular death in individuals with severe mental illness. Acta Psychiatr Scand. 2019;139:558‐571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Møller S, Wissenberg M, Kragholm K, et al. Socioeconomic differences in coronary procedures and survival after out‐of‐hospital cardiac arrest: A nationwide Danish study. Resuscitation. 2020;153:10‐19. [DOI] [PubMed] [Google Scholar]
- 27. Kragholm K, Andersen MP, Gerds TA, et al. Association between male sex and outcomes of Coronavirus Disease 2019 (COVID‐19)—A Danish Nationwide, Register‐based Study. Clin Infect Dis. 2020;ciaa924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leucht S, Burkard T, Henderson J, Maj M, Sartorius N. Physical illness and schizophrenia: a review of the literature. Acta Psychiatr Scand. 2007;116:317‐333. [DOI] [PubMed] [Google Scholar]
- 29. Jacoby AS, Munkholm K, Vinberg M, Pedersen BK, Kessing LV. Cytokines, brain‐derived neurotrophic factor and C‐reactive protein in bipolar I disorder – Results from a prospective study. J Affect Disord. 2016;197:167‐174. [DOI] [PubMed] [Google Scholar]
- 30. Friedman H, Pross S, Klein TW. Addictive drugs and their relationship with infectious diseases. FEMS Immunol Med Microbiol. 2006;47:330‐342. [DOI] [PubMed] [Google Scholar]
- 31. De Hert M, Cohen D, Bobes J, et al. Physical illness in patients with severe mental disorders. II. Barriers to care, monitoring and treatment guidelines, plus recommendations at the system and individual level. World Psychiatry. 2011;10:138‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Correll CU, Detraux J, De Lepeleire J, De Hert M. Effects of antipsychotics, antidepressants and mood stabilizers on risk for physical diseases in people with schizophrenia, depression and bipolar disorder. World Psychiatry. 2015;14:119‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Villasante‐Tezanos AG, Rohde C, Nielsen J, de Leon J. Pneumonia risk: approximately one‐third is due to clozapine and two‐thirds is due to treatment‐resistant schizophrenia. Acta Psychiatr Scand. 2020;142:66‐67. [DOI] [PubMed] [Google Scholar]
- 34. May M, Slitzky M, Rostama B, Barlow D, Houseknecht KL. Antipsychotic‐induced immune dysfunction: a consideration for COVID‐19 risk. Brain Behavior Immunity Health. 2020;6:100097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Folsom DP, Hawthorne W, Lindamer L, et al. Prevalence and risk factors for homelessness and utilization of mental health services among 10,340 patients with serious mental illness in a large public mental health system. Am J Psychiatry. 2005;162:370‐376. [DOI] [PubMed] [Google Scholar]
- 36. Moesmann Madsen M, Dines D, Hieronymus F. Optimizing psychiatric care during the COVID‐19 pandemic. Acta Psychiatr Scand. 2020;142:70‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stefan N, Birkenfeld AL, Schulze MB. Global pandemics interconnected — obesity, impaired metabolic health and COVID‐19. Nat Rev Endocrinol. 2021;17:135‐149. [DOI] [PubMed] [Google Scholar]
- 38. García S, Martínez‐Cengotitabengoa M, López‐Zurbano S, et al. Adherence to antipsychotic medication in bipolar disorder and schizophrenic patients: a systematic review. J Clin Psychopharmacol. 2016;36:355‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lingam R, Scott J. Treatment non‐adherence in affective disorders: Treatment non‐adherence in affective disorders. Acta Psychiatr Scand. 2002;105:164‐172. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1‐S6
Data Availability Statement
The data and study materials cannot be made available to other researchers for purposes of reproducing the results or replicating the procedure.


