Abstract
Aims/Introduction
This study aimed to reveal lifestyle changes and their impact on glycemic control and weight control in patients with diabetes during the coronavirus disease 2019 (COVID‐19) pandemic in Japan.
Materials and Methods
We retrospectively analyzed 1,402 outpatients with diabetes at a clinic in Osaka, Japan, who responded to an interview sheet regarding lifestyle changes during the COVID‐19 pandemic between 28 March and 30 May 2020. The association of lifestyle changes with hemoglobin A1c (HbA1c) and weight changes from February to May 2020 was investigated using the linear regression model. We also investigated the association with clinically important change of HbA1c (by ≥0.3%) and bodyweight (by ≥3%), using the cumulative link model.
Results
Leisure time and other outside physical activities were decreased in one‐quarter of patients during the COVID‐19 pandemic, whereas the amount of meals and snacks was decreased and increased in approximately 10%, respectively. The change in leisure time physical activities was inversely associated with HbA1c and weight changes, whereas the quantitative change of meals with the decline in eating out and that of snacks were positively associated with HbA1c and weight changes (all P < 0.05). The quantitative change of meals without the decline in eating out was also positively associated with weight change (P = 0.012). The cumulative link model for clinically important HbA1c and weight change showed broadly similar associations, except for that between snacks and bodyweight (P = 0.15).
Conclusions
A considerable number of outpatients with diabetes experienced lifestyle changes during the COVID‐19 pandemic. The lifestyle changes were associated with HbA1c and weight changes.
Keywords: Coronavirus disease 2019, Glycemic control, Lifestyle change
We investigated lifestyle changes and their impact on glycemic control and weight control in patients with diabetes during the coronavirus disease 2019 pandemic in Japan. Analyzing 1,402 diabetes patients, we showed that a considerable number of patients with diabetes experienced lifestyle changes during the pandemic. The lifestyle changes were associated with hemoglobin A1c and weight changes.

INTRODUCTION
The coronavirus disease 2019 (COVID‐19) pandemic has substantially affected daily lives of citizens all over the world; Japan is not an exception 1 , 2 . In late February 2020, the Japanese government requested large‐scale events to be canceled, postponed or scaled down, and the government also requested all elementary, junior high and high schools to close from March. In March, the government asked citizens to avoid locations and settings of poor ventilation, congestion with many people and vocalization in close proximity. In early April, the government declared a state of emergency, which lasted until late May. During the emergency, citizens were requested to reduce outings and person‐to‐person contact as thoroughly as possible; the daily lives of citizens were substantially affected.
Daily lifestyle, including both physical activities and dietary habits, can considerably influence glycemic control and weight control in patients with diabetes 3 , 4 , and recent studies analyzing approximately 200 patients suggested the influence of lifestyle changes on glycemic control and weight control during the COVID‐19 pandemic 5 , 6 . However, whether physical activities and dietary habits would be independently associated with these controls remained unknown. The current study, with a larger sample size, aimed to show lifestyles changes and their respective independent impact on glycemic control and weight control in patients with diabetes during the COVID‐19 pandemic in Japan.
MATERIALS AND METHODS
Study population
The current study retrospectively analyzed 1,402 outpatients who were treated for diabetes at Shiraiwa Medical Clinic, Kashiwara City, Osaka Prefecture, Japan, since November 2019 or before (i.e., had been treated for ≥3 months at the time point of February 2020), and responded to an interview sheet about lifestyle changes during the COVID‐19 pandemic between 28 March and 30 May 2020. The participant flow diagram is shown in Figure 1. The interview sheet included questions on whether each of (1) leisure‐time physical activities, (2) other outside physical activities (including walking and cycling during commuting and shopping), (3) the amount of meals and (4) the amount of snacks was (i) decreased, (ii) unchanged or (iii) increased, compared with those before the COVID‐19 pandemic became a problem for society (around February). The interview sheet also asked whether there was a decline in eating out, and whether those lifestyle changes were due to the influence of the COVID‐19 pandemic or not. The clinic started the use of the interview sheet on 28 March 2020. Note that a state of emergency was declared on 7 April 2020 and was lifted on 21 May 2020 in this area. A total of 926 study patients (66.0%) visited the clinic and responded to the interview sheet during a state of emergency (from 7 April to 20 May 2020). Patients responded to the interview sheet during a wait for the results of laboratory examinations at the clinic; their response was therefore completed before they were informed of their hemoglobin A1c (HbA1c) values. In contrast, their bodyweight was measured at the clinic during the wait, regardless of the completion of the interview sheet; therefore, some patients might respond to the interview sheet after the measurement, recognizing their measured bodyweight.
Figure 1.
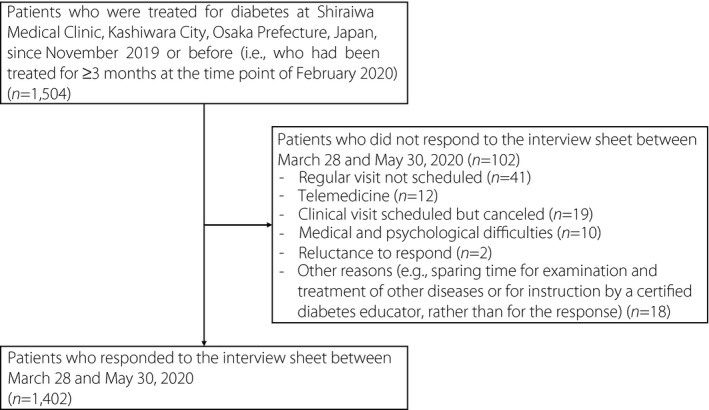
Participant flow diagram.
All data used in the current study were retrospectively derived from medical records. The current study was in accordance with the Declaration of Helsinki, and was approved by the ethics committees of Shiraiwa Medical Clinic (date of approval 2 September 2020; approval number 2020902) and Osaka University Hospital (date of approval 7 September 2020; approval number 15395‐3). As the current study was a retrospective study using existing data, informed consent was exempted, and instead, relevant information regarding the study was open to the public, according to the Ethical Guidelines for Medical and Health Research Involving Human Subjects in Japan.
Definitions
The change of lifestyles (i.e., leisure time physical activities, other outside physical activities, the amount of meals and the amount of snacks) was coded as “decreased” (−1 point), “unchanged” (0 point) and “increased” (1 point). The change of glycemic control under the pandemic was evaluated as the change of HbA1c levels from February to May 2020, which was calculated as HbA1c levels in May 2020 minus those in February 2020. Clinically important change of HbA1c levels was defined as an increase or decrease of ≥0.3%, based on the non‐inferiority margin recommended by the authorities 7 . The threshold of 0.3% was also safely validated by a pooled effect of lifestyle intervention on the HbA1c reduction 8 . The weight change was evaluated as the percentage change of bodyweight from February to May 2020. Clinically important percentage change of bodyweight was defined as a percentage increase or decrease by ≥3% 9 , 10 .
Statistical analysis
Data are presented as the mean ± standard deviation for continuous variables and as frequency (proportion) for discrete variables, unless otherwise mentioned. A P‐value of <0.05 was considered statistically significant, and 95% confidence intervals (CIs) are reported where appropriate. The association of lifestyle changes with the HbA1c change was investigated using the linear regression model, in which the HbA1c change was treated as the dependent continuous variable. In the model, lifestyle changes were treated as continuous variables, after confirmation that there was no significant intercategory difference of regression coefficients (i.e., difference between “increased [1 point] vs unchanged [0 point]” and “unchanged [0 point] vs decreased [−1 point]”). We also checked whether the influence of the COVID‐19 pandemic, the response during the emergency declaration and the decline in eating out had any interaction effect on the association between lifestyle and HbA1c changes, and whether lifestyle changes had any interaction effect with one another. To examine whether lifestyle changes were associated with clinically important change of HbA1c levels, we additionally developed the cumulative link model for ordinal regression in which clinically important change of HbA1c, categorized as decreased (≤−0.3%), unchanged (>−0.3 and <0.3%) and increased (≥0.3%), was set as the dependent ordinal variable. All the regression models were adjusted for medication changes from February to May 2020. The association of lifestyle changes with the percentage change of bodyweight was also similarly investigated. Missing data were addressed by multiple imputation using the chained equations method. In the procedure, we generated five imputed datasets and combined the analytic results based on Rubin’s rule. All statistical analyses were carried out using R version 3.6.0 (R Development Core Team, Vienna, Austria).
RESULTS
Clinical features of the study population, as well as their responses to the interview sheet, are summarized in Table 1. The mean age was 67 ± 13 years, and 61.6% were men. The mean HbA1c levels were 7.2 ± 0.9%, and the mean body mass index was 24.8 ± 4.1 kg/m2. During the COVID‐19 pandemic, leisure‐time and other outside physical activities were decreased in 26.3 and 25.7% of patients, respectively, and the majority of them (79.9 and 86.5%) responded that the decrease was due to the influence of the COVID‐19 pandemic. The amount of meals was decreased and increased in 8.3 and 7.7% of patients, respectively, and 20.0 and 42.1% of patients attributed the change to the COVID‐19 pandemic. The amount of snacks was decreased in 10.1% and increased in 15.8% of patients; 14.8 and 63.8% of patients attributed the change to the pandemic, respectively. Almost half of the patients (44.5%) ate out less, mainly due to the influence of the COVID‐19 pandemic (91.2%). Antihyperglycemic medication regimens were changed from February to May 2020 in 152 patients (10.8%; Table 1).
Table 1.
Clinical features of study participants, who responded to the interview sheet between 28 March and 30 May 2020
| n | 1,402 | |
| Male sex | 863 (61.6%) | |
| Age (years) | 67 ± 13 | |
| Type 1 diabetes | 70 (5.0%) | |
| Duration of diabetes (years) | 14 ± 9 | (Data missing, n = 31) |
| Hypertension | 880 (62.8%) | |
| Dyslipidemia | 1,010 (72.0%) | |
| Weight and glycemic control in February 2020 | ||
| Bodyweight (kg) | 64.9 ± 14.2 | (Data missing, n = 153) |
| Body mass index (kg/m2) | 24.8 ± 4.1 | (Data missing, n = 154) |
| Hemoglobin A1c | 7.2 ± 0.9 | (Data missing, n = 195) |
| Insulin use | 391 (27.9%) | |
| Glucagon‐like peptide‐1 receptor agonist use | 117 (8.3%) | |
| Oral antihyperglycemic agent use | 1,132 (80.7%) | |
| Lifestyle change during COVID‐19 pandemic (response to the interview sheet) | ||
| Response during a state of emergency (from 7 April to 20 May 2020) | 926 (66.0%) | |
| Leisure time physical activities | ||
| Decreased | 369 (26.3%) | |
| Due to COVID‐19 pandemic | 295 (79.9%) | |
| Unchanged | 961 (68.5%) | |
| Increased | 72 (5.1%) | |
| Due to COVID‐19 pandemic | 26 (36.6%) | (Data missing, n = 1) |
| Other physical activities | ||
| Decreased | 360 (25.7%) | |
| Due to COVID‐19 pandemic | 311 (86.4%) | |
| Unchanged | 988 (70.5%) | |
| Increased | 54 (3.9%) | |
| Due to COVID‐19 pandemic | 19 (35.2%) | |
| Amount of meals | ||
| Decreased | 116 (8.3%) | |
| Due to COVID‐19 pandemic | 23 (20.0%) | (Data missing, n = 1) |
| Unchanged | 1,178 (84.0%) | |
| Increased | 108 (7.7%) | |
| Due to COVID‐19 pandemic | 45 (42.1%) | (Data missing, n = 1) |
| Amount of snacks | ||
| Decreased | 142 (10.1%) | |
| Due to COVID‐19 pandemic | 21 (14.8%) | |
| Unchanged | 1,039 (74.1%) | |
| Increased | 221 (15.8%) | |
| Due to COVID‐19 pandemic | 141 (63.8%) | |
| Decline in eating out | 624 (44.5%) | |
| Due to COVID‐19 pandemic | 569 (91.2%) | |
| Change of antihyperglycemic medication regimens from February to May 2020 | 152 (10.8%) | |
| Classified according to intensification of antihyperglycemic effect | ||
| Antihyperglycemic drugs increased | 98 (64.5%) | |
| Antihyperglycemic drugs decreased | 42 (27.6%) | |
| Antihyperglycemic drugs switched | 12 (7.9%) | |
| Classified according to association with weight change † | ||
| Weight‐gaining drugs increased or weight‐reducing drugs decreased | 56 (36.8%) | |
| Weight ‐gaining drugs decreased or weight‐reducing drugs increased | 46 (30.3%) | |
| Both weight‐gaining drugs and weight‐reducing drugs increased or decreased | 6 (3.9%) | |
| Only weight‐neutral drugs changed | 44 (28.9%) |
Data are mean ± standard deviation or frequency (percentage).
COVID‐19, coronavirus disease 2019.
Weight‐gaining drugs include sulfonylureas, glinides, thiazolidinediones and insulin, weight‐reducing drugs include sodium–glucose cotransporter 2 inhibitors and glucagon‐like peptide‐1 receptor agonists, and weight‐neutral drugs include metformin, dipeptidyl peptidase‐4 inhibitors and alpha glucosidase inhibitors, respectively.
The crude mean HbA1c change from February to May 2020 was 0.05% (95% CI 0.02–0.08%). There was a clinically important decrease and increase of HbA1c levels (i.e., by ≥0.3%) in 13.4% (95% CI 11.5–15.2%) and 34.9% (95% CI 32.3–37.4%) of patients, respectively, whereas the HbA1c change was within ±0.2% in the remaining 51.8% (95% CI 49.0–54.5%). Figure 2 shows the crude HbA1c change by lifestyle changes during the COVID‐19 pandemic.
Figure 2.
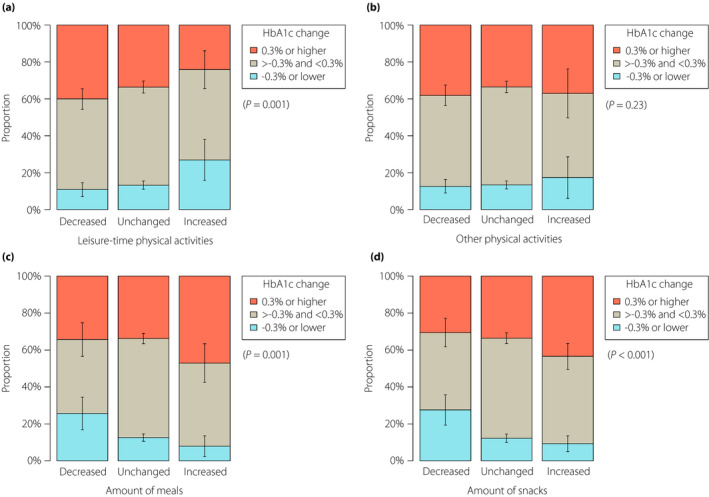
Clinically important hemoglobin A1c (HbA1c) change by lifestyle change during the coronavirus disease 2019 pandemic. Data are proportions and 95% confidence intervals of clinically important change of HbA1c levels (i.e., HbA1c increase or decrease by ≥0.3% between February and May 2020) in subgroups classified according to the change of (a) leisure‐time physical activities, (b) other outside physical activities, (c) the amount of meals and (d) the amount of snacks. P‐values were derived from the cumulative link models.
As shown in Table S1, we confirmed that all lifestyle changes, coded as “decreased” (−1 point), “unchanged” (0 point) and “increased” (1 point), had no significant intercategory difference in the association with the HbA1c change, which justified treating the variables as continuous variables in the subsequent regression models. We also confirmed that the influence of the COVID‐19 pandemic and the response during the emergency declaration had no significant interaction effect on the association of respective lifestyle changes with the HbA1c change in the current study population (all P > 0.05). In contrast, the decline in eating out had a significant interaction effect on the association of the quantitative change of meals with the HbA1c change; the quantitative change of meals accompanied by the decline in eating out was more largely associated with the HbA1c change than that not accompanied by the decline (P = 0.028). Lifestyle changes had no significant interaction effect with one another (all P > 0.05), suggesting their combined effect would be negligible.
Table 2 shows the multivariate regression analyses. We first developed the multivariate regression model in which all kinds of lifestyle changes (i.e., the change of leisure time physical activities, other outside physical activities, the amount of meals and the amount of snacks) and medication changes were entered as the explanatory variables (multivariate model 1). Consequently, the change in leisure time physical activities and snacks, but not other outside physical activities or meals, were significantly associated with the HbA1c change (P = 0.016, 0.001, 0.31 and 0.081, respectively; multivariate linear regression model 1 in Table 2). When the change in the amount of meals was stratified by the decline in eating out (multivariate linear regression model 3 in Table 2), the quantitative change of meals with the decline in eating out, but not that without the decline, was positively associated with the HbA1c change (P = 0.001 and 1.00, respectively). The statistical significance of those associations was unchanged, even after adjustment for patient attributes (multivariate linear regression model 2 and 4 in Table 2). Furthermore, similar findings were observed when clinically important HbA1c change was adopted as the outcome variable, except for the amount of meals in multivariate model 1 and 2, which reached statistical significance (multivariate cumulative link model 1–4 in Table 2). The association of medication changes and HbA1c change during a series of the analyses is summarized in Table S2. There was no significant interaction effect of sex, age or type of diabetes on the association of lifestyle changes with the HbA1c change (all P > 0.05; Table S3).
Table 2.
Adjusted association of lifestyle change with hemoglobin A1c change during the coronavirus disease 2019 pandemic
| Regression coefficient for HbA1c change (as a continuous variable; linear regression model) | Odds ratio for clinically important HbA1c change (cumulative link model) | |
|---|---|---|
| Multivariate model 1 | ||
| Leisure time physical activities | −0.06 [−0.12 to −0.01] (P = 0.016) | 0.71 [0.56 to 0.90] (P = 0.006) |
| Other physical activities | 0.03 [−0.03 to 0.08] (P = 0.31) | 1.02 [0.81 to 1.30] (P = 0.84) |
| Amount of meals | 0.06 [−0.01 to 0.13] (P = 0.081) | 1.40 [1.06 to 1.85] (P = 0.016) |
| Amount of snacks | 0.09 [0.04 to 0.14] (P = 0.001) | 1.44 [1.14 to 1.82] (P = 0.002) |
| Multivariate model 2 (adjusted for patient attributes) | ||
| Leisure time physical activities | −0.06 [−0.11 to −0.01] (P = 0.022) | 0.72 [0.56 to 0.91] (P = 0.007) |
| Other physical activities | 0.03 [−0.02 to 0.08] (P = 0.28) | 1.01 [0.79 to 1.29] (P = 0.95) |
| Amount of meals | 0.06 [−0.01 to 0.13] (P = 0.086) | 1.38 [1.05 to 1.83] (P = 0.021) |
| Amount of snacks | 0.08 [0.03 to 0.14] (P = 0.002) | 1.43 [1.13 to 1.81] (P = 0.003) |
| Multivariate model 3 | ||
| Leisure time physical activities | −0.06 [−0.11 to −0.01] (P = 0.020) | 0.72 [0.57 to 0.91] (P = 0.007) |
| Other physical activities | 0.03 [−0.03 to 0.08] (P = 0.37) | 1.01 [0.79 to 1.29] (P = 0.94) |
| Amount of meals | ||
| With decline in eating out | 0.12 [0.03 to 0.21] (P = 0.011) | 1.79 [1.21 to 2.66] (P = 0.004) |
| Without decline in eating out | 0.00 [−0.09 to 0.09] (P = 1.00) | 1.10 [0.75 to 1.60] (P = 0.63) |
| Amount of snacks | 0.09 [0.04 to 0.14] (P = 0.001) | 1.42 [1.13 to 1.80] (P = 0.003) |
| Multivariate model 4 (adjusted for patient attributes) | ||
| Leisure time physical activities | −0.06 [−0.11 to −0.01] (P = 0.028) | 0.72 [0.57 to 0.92] (P = 0.008) |
| Other physical activities | 0.03 [−0.03 to 0.08] (P = 0.34) | 0.98 [0.77 to 1.26] (P = 0.90) |
| Amount of meals | ||
| With decline in eating out | 0.12 [0.03 to 0.21] (P = 0.012) | 1.78 [1.20 to 2.64] (P = 0.005) |
| Without decline in eating out | 0.00 [−0.09 to 0.09] (P = 0.98) | 1.07 [0.74 to 1.57] (P = 0.71) |
| Amount of snacks | 0.08 [0.03 to 0.13] (P = 0.003) | 1.41 [1.12 to 1.78] (P = 0.004) |
Data are regression coefficients [95% confidence intervals] for the change of hemoglobin A1c (HbA1c) levels (%;as a continuous variable) and odds ratios [95% confidence intervals] for clinically important change of HbA1c (i.e., by ≥0.3%). Each lifestyle change was coded as “decreased” (−1 point), “unchanged” (0 point) and “increased” (1 point). The explanatory variables entered in multivariate models 1 and 3 were lifestyle changes (see below) and medication changes, whereas multivariate models 2 and 4 were further adjusted for patient attributes (sex, age, type of diabetes, duration of diabetes, hypertension, dyslipidemia and medication use). Lifestyle changes entered in multivariate model 1 and 2 were the change of leisure time physical activities, other outside physical activities, the amount of meals and the amount of snacks, whereas in multivariate models 3 and 4, the amount of meals was stratified according to the decline in eating out.
The percentage change of bodyweight from February to May 2020 was 0.46% (95% CI 0.29–0.62%). There was a clinically important percentage decrease and increase of bodyweight (i.e., by ≥3%) in 7.1% (95% CI 5.7–8.6%) and 13.1% (95% CI 10.8–15.3%) of patients, respectively. Figure 3 shows the crude bodyweight change by lifestyle changes during the COVID‐19 pandemic. The subsequent analysis showed that the decline in eating out had a significant interaction effect on the association of the quantitative change of meals with the bodyweight change (Table S4), as with the HbA1c change (Table S1). As shown in Table 3, the change in leisure time physical activities was significantly inversely associated with the percentage change of bodyweight, whereas the quantitative change of meals, regardless of the decline in eating out, and that of snacks were significantly positively associated. In contrast, the quantitative change of snacks lost statistical significance in the association with clinically important percentage change of bodyweight (Table 3). The association of medication changes and bodyweight change during a series of the analyses is summarized in Table S5. Age had a significant interaction effect on the association between the quantitative change of meals without the decline in eating out and clinically important percentage change of bodyweight (P = 0.032; Table S6); patients aged <65 years had a more marked association than those aged ≥65 years (odds ratio 3.58, 95% CI 1.73–7.39 vs 1.06, 95% CI 0.51–2.21).
Figure 3.
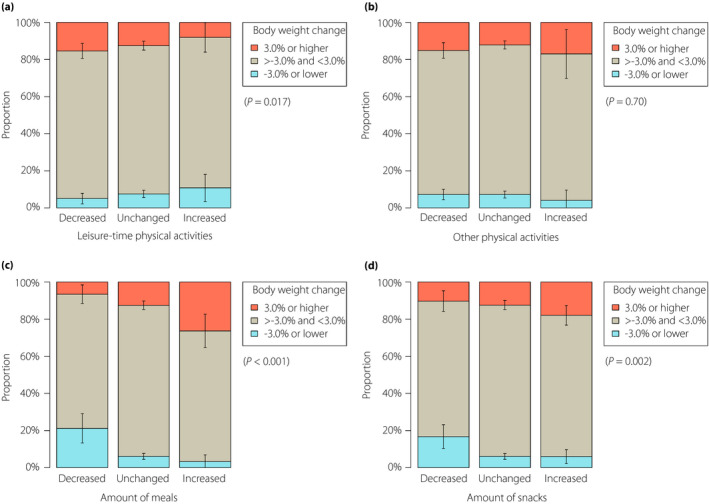
Clinically important bodyweight change by lifestyle change during the coronavirus disease 2019 pandemic. Data are proportions and 95% confidence intervals of clinically important percentage change of bodyweight (i.e., bodyweight increase or decrease by ≥3% between February and May 2020) in subgroups classified according to the change of (a) leisure time physical activities, (b) other outside physical activities, (c) the amount of meals and (d) the amount of snacks. P‐values were derived from the cumulative link models.
Table 3.
Adjusted association of lifestyle change with body weight change during the coronavirus disease 2019 pandemic
| Regression coefficient for bodyweight change (as a continuous variable; linear regression model) | Odds ratio for clinically important bodyweight change (cumulative link model) | |
|---|---|---|
| Multivariate model 1 | ||
| Leisure time physical activities | −0.49 [−0.81 to −0.17] (P = 0.003) | 0.68 [0.51 to 0.90] (P = 0.008) |
| Other physical activities | 0.16 [−0.16 to 0.49] (P = 0.32) | 1.14 [0.86 to 1.50] (P = 0.37) |
| Amount of meals | 1.22 [0.76 to 1.68] (P < 0.001) | 2.64 [1.85 to 3.77] (P < 0.001) |
| Amount of snacks | 0.48 [0.14 to 0.82] (P = 0.007) | 1.32 [0.97 to 1.80] (P = 0.074) |
| Multivariate model 2 (adjusted for patient attributes) | ||
| Leisure time physical activities | −0.47 [−0.78 to −0.15] (P = 0.004) | 0.70 [0.53 to 0.92] (P = 0.012) |
| Other physical activities | 0.22 [−0.10 to 0.54] (P = 0.18) | 1.18 [0.88 to 1.57] (P = 0.26) |
| Amount of meals | 1.23 [0.78 to 1.69] (P < 0.001) | 2.70 [1.89 to 3.85] (P < 0.001) |
| Amount of snacks | 0.42 [0.09 to 0.74] (P = 0.013) | 1.28 [0.94 to 1.73] (P = 0.12) |
| Multivariate model 3 | ||
| Leisure time physical activities | −0.47 [−0.79 to −0.16] (P = 0.004) | 0.68 [0.51 to 0.91] (P = 0.010) |
| Other physical activities | 0.14 [−0.19 to 0.47] (P = 0.41) | 1.08 [0.81 to 1.44] (P = 0.59) |
| Amount of meals | ||
| With decline in eating out | 1.64 [1.08 to 2.20] (P < 0.001) | 3.70 [2.28 to 6.01] (P < 0.001) |
| Without decline in eating out | 0.79 [0.16 to 1.43] (P = 0.016) | 1.79 [1.07 to 2.98] (P = 0.026) |
| Amount of snacks | 0.46 [0.12 to 0.79] (P = 0.009) | 1.30 [0.96 to 1.77] (P = 0.092) |
| Multivariate model 4 (adjusted for patient attributes) | ||
| Leisure time physical activities | −0.46 [−0.78 to −0.14] (P = 0.005) | 0.70 [0.53 to 0.93] (P = 0.014) |
| Other physical activities | 0.17 [−0.15 to 0.50] (P = 0.29) | 1.11 [0.83 to 1.48] (P = 0.50) |
| Amount of meals | ||
| With decline in eating out | 1.64 [1.09 to 2.19] (P < 0.001) | 3.76 [2.30 to 6.16] (P < 0.001) |
| Without decline in eating out | 0.81 [0.19 to 1.44] (P = 0.012) | 1.83 [1.10 to 3.03] (P = 0.020) |
| Amount of snacks | 0.39 [0.07 to 0.71] (P = 0.018) | 1.25 [0.92 to 1.69] (P = 0.15) |
Data are regression coefficients [95% confidence intervals] for the percentage change of bodyweight (%; as a continuous variable) and odds ratios [95% confidence intervals] for clinically important percentage change of bodyweight (i.e., by ≥3%). Each lifestyle change was coded as “decreased” (−1 point), “unchanged” (0 point) and “increased” (1 point). The explanatory variables entered in multivariate models 1 and 3 were lifestyle changes (see below) and medication changes, whereas multivariate models 2 and 4 were further adjusted for patient attributes (sex, age, type of diabetes, duration of diabetes, hypertension, dyslipidemia and medication use). Lifestyle changes entered in multivariate models 1 and 2 were the change of leisure time physical activities, other outside physical activities, the amount of meals and the amount of snacks, whereas in multivariate models 3 and 4, the amount of meals was stratified according to the decline in eating out.
DISCUSSION
The current study showed that a considerable number of outpatients with diabetes experienced lifestyle changes during the COVID‐19 pandemic in Japan. The change in leisure time physical activities was inversely associated with HbA1c and bodyweight changes, whereas the quantitative change of meals accompanied by the decline in eating out and that of snacks were positively associated with them. The quantitative change of meals not accompanied by the decline in eating out was also positively associated with bodyweight change. The cumulative link model for clinically important HbA1c and weight change showed broadly similar associations, except for the association between snacks and bodyweight, which did not reach statistical significance.
Osaka was one of the prefectures at the highest risk of the COVID‐19 pandemic in Japan 1 , 2 , and some institutions in addition to schools announced closures as precautionary measures in early March 2020, in response to the government’s recommendations to avoid crowded places with poor ventilation and close‐distance conversations. In April, Osaka was covered by the first emergency declaration in Japan, which lasted until late May 1 , 2 . The residents were requested to avoid non‐essential outings and to reduce person‐to‐person contact as thoroughly as possible, which considerably affected their daily lives. The current study showed that a considerable number of patients with diabetes experienced lifestyle changes during the pandemic. The observed changes clearly reflected those serious social situations in Osaka Prefecture.
Recent studies suggested the influence of lifestyle changes on glycemic control and weight control during the COVID‐19 pandemic 5 , 6 . Munekawa et al. 5 successfully evaluated the quantitative change of exercise, total diet intake, snack consumption and prepared food intake, as well as stress and sleep time, in 203 patients, using a visual analog scale. They showed that decreased exercise levels and increased snack consumption during the pandemic were correlated with increased bodyweight, and that increased total diet intake was correlated with increased HbA1c levels. However, the correlation remained unadjusted; it was not shown whether those lifestyle changes would be independently associated with HbA1c and weight changes. Kishimoto et al. 6 interviewed 168 patients on lifestyle changes in detail, using some open‐ended questions. They reported the detailed answers descriptively, but did not carry out any statistical tests for the detailed answers. They instead showed that overall changes in physical activity levels (coded as increased, no change and decreased) and those in dietary habits (coded as improved, no change and deteriorated) were associated with the group difference between patients with HbA1c levels elevated by >0.2% and those with HbA1c levels decreased by >0.2%. However, patients with HbA1c levels unchanged or changed by ≤0.2% were ignored. Furthermore, bodyweight changes were not analyzed. We believe that the strengths of the present study were that the sample size was much larger, and that the multivariate analyses were carried out for both HbA1c and weight changes.
We confirmed that more patients responded that physical activities, either leisure time activities or others, were “decreased” under the pandemic than those that responded that they were “increased.” As is well known, reduced physical activities can deteriorate glycemic control, but also promote weight gain 3 , 4 , 10 . Indeed, the change of leisure time physical activities was inversely associated with the HbA1c and weight changes in the current study population, showing that decreased leisure time physical activities were associated with elevated HbA1c levels and increased bodyweight. In contrast, the change of other outside physical activities was not significantly associated with the change of HbA1c levels or bodyweight. One possible explanation is that the decrease of those physical activities might be small in many patients; in other words, the original amount of non‐leisure time outside physical activities before the pandemic might have been so small that their decrease had little effect on metabolic control. Although daily walking and cycling to work or shopping are generally recognized as an effective way to retain physical activity 11 , 12 , only a few Japanese patients might be engaged in the activities as intensively as they could improve glycemic control or weight reduction.
Dietary habits are another key factor that influences glycemic control, as well as weight control; overeating, in addition to sedentariness, will deteriorate their control 3 , 4 , 10 . Although the number of patients with an increased amount of meals and snacks was smaller compared with those with decreased physical activity during the pandemic, it is of note that 141 patients; that is, as many as 10% of the overall population, responded that the amount of snacks was increased due to the influence by the pandemic. The stay‐at‐home request during the pandemic would increase idleness or boredom, which might drive them to snacking 13 . Interestingly, the impact of the quantitative change of meals on glycemic control was more marked in patients with the decline in eating out than in those without the decline. Meals eaten out and those eaten at home might be quite different in quality (e.g., nutritional balances); the decline in eating out might indicate a drastic change in the meal quality. Patients who ate out less might more likely experience a drastic dietary change, which would more markedly affect glycemic control. By contrast, bodyweight was changed regardless of the decline in eating out, implying that total energy intake from meals would be as remarkably changed in patients without the decline in eating out as in those with the decline. The quantitative change of snacks was not associated with clinically important percentage change of bodyweight (by ≥3%), whereas it was associated with the percentage change of bodyweight as a continuous variable. The change of energy intake from snacks would not be so large as that of energy intake from meals, and would be less likely to cause a weight change by ≥3%.
We also showed the association of medication changes with HbA1c and bodyweight change during a series of regression analyses (Tables S2 and S5). These associations shown by the regression models would reflect a sum of causes (i.e., the reason why antihyperglycemic drugs were changed) and results (i.e., the outcome that was achieved by the medication change), or, in other words, a sum of changes before the medication change and those after the medication change. The detailed interpretations are summarized in Appendix S1.
Compared with patients aged ≥65 years, patients aged <65 years had a more marked association between the quantitative change of meals without the decline in eating out and clinically important percentage change of bodyweight. A younger population would include more people who were employed. The pandemic might considerably affect their workstyles and lifestyles, possibly making a more marked dietary change, even though it was not accompanied by the decline in eating out.
Patients responded to the interview sheet between 28 March and 30 May 2020; that is, practically during the emergency declaration with a tolerance of approximately ±10 days. The response during the emergency declaration had no significant interaction effect on the association of lifestyle changes with HbA1c and weight changes in the present study population. The impact of lifestyle changes during the 10 days before and after the emergency declaration would not be so different from that during the emergency declaration.
The current study had some limitations. First, this was a single‐center retrospective study, and a future external validation would be required. The current study did not include patients who did not visit the clinic, which would cause a selection bias of the study. Second, information on lifestyle changes was based on self‐report. Patients completed the interview sheet before they were informed of their HbA1c values, and, in this sense, the respondents were blinded to HbA1c values. In contrast, the sequence of the completion and the bodyweight measurement was not controlled; some patients might respond to the interview sheet recognizing their measured bodyweight, which would affect their responses. Third, patients responded to the interview sheet only once, and the responses were not sequentially collected. In addition, there was no control in the present study. Causal relationships between the COVID‐19 pandemic and observed changes were yet to be scientifically proven. Fourth, lifestyle changes were not quantified, and therefore, we were unable to distinguish the impact on metabolic control between patients who experienced a drastic lifestyle change and those who experienced only a minor change. Fifth, we set the interval of 3 months (i.e., from February to May 2020) simply because HbA1c levels reflect the previous 2–3 months of glycemic control 14 ; however, the responses to the interview sheet were collected only once. Social conditions were drastically altered during the period, and the results might be greatly dependent on the timing of the survey. Although whether patients responded to the interview sheet during the emergency declaration or not had no significant interaction effect in the current study population, further information on social conditions was not available and, therefore, was not included in the analysis. Future studies collecting sequential data on lifestyle changes or those evaluating a shorter‐term glycemic control (e.g., data of continuous glucose monitoring) will be required. Furthermore, more detailed data were not available on lifestyles, including occupation, alcohol consumption, the type, intensity and duration of physical activities, and the volume of foods and nutrients.
In conclusion, a considerable number of outpatients with diabetes experienced lifestyle changes during the COVID‐19 pandemic in Japan. The lifestyle changes were associated with HbA1c and bodyweight changes.
DISCLOSURE
The authors declare no conflict of interest.
Supporting information
Table S1 | Association of lifestyle change with hemoglobin A1c change during the coronavirus disease 2019 pandemic.
Table S2 | Association between the variable of medication changes and hemoglobin A1c change during the coronavirus disease 2019 pandemic.
Table S3 | Interaction effect on association of lifestyle change with hemoglobin A1c change during the coronavirus disease 2019 pandemic.
Table S4 | Association of lifestyle change with weight change during the coronavirus disease 2019 pandemic.
Table S5 | Association between the variable of medication changes and bodyweight change during the coronavirus disease 2019 pandemic.
Table S6 | Interaction effect on association of lifestyle change with weight change during the coronavirus disease 2019 pandemic.
Appendix S1 | Interpretation of the association between the medication change and the hemoglobin A1c and bodyweight change in regression models.
ACKNOWLEDGMENTS
This research did not receive any specific grant from funding agencies in the public, commercial or not‐for‐profit sectors.
J Diabetes Investig 2022; 13: 375–385
REFERENCES
- 1. Japan's Ministry of Health L, and Welfare . Press Conference on COVID‐19. Available from: https://www.mhlw.go.jp/stf/seisakunitsuite/bunya/newpage_00032.html Accessed October 17, 2020.
- 2. Secretariat JGsC . COVID‐19 Information and Resources. Available from: https://corona.go.jp/en/ Accessed October 17, 2020.
- 3. Araki E, Goto A, Kondo T, et al. Japanese clinical practice guideline for diabetes 2019. Diabetol Int 2020; 11: 165–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Araki E, Goto A, Kondo T, et al. Japanese clinical practice guideline for diabetes 2019. J Diabetes Investig 2020; 11: 1020–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Munekawa C, Hosomi Y, Hashimoto Y, et al. Effect of coronavirus disease 2019 pandemic on the lifestyle and glycemic control in patients with type 2 diabetes: a cross‐section and retrospective cohort study. Endocr J 2021; 68: 201–210. [DOI] [PubMed] [Google Scholar]
- 6. Kishimoto M, Ishikawa T, Odawara M. Behavioral changes in patients with diabetes during the COVID‐19 pandemic. Diabetol Int 2021; 12: 241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. European Medicines Agency . Clinical investigation of medicinal products in the treatment or prevention of diabetes mellitus. Available from: https://www.ema.europa.eu/en/clinical‐investigation‐medicinal‐products‐treatment‐prevention‐diabetes‐mellitus Accessed October 17, 2020.
- 8. Chen L, Pei J‐H, Kuang J, et al. Effect of lifestyle intervention in patients with type 2 diabetes: a meta‐analysis. Metabolism 2015; 64: 338–347. [DOI] [PubMed] [Google Scholar]
- 9. Muramoto A, Matsushita M, Kato A, et al. Three percent weight reduction is the minimum requirement to improve health hazards in obese and overweight people in Japan. Obes Res Clin Pract 2014; 8: e466–475. [DOI] [PubMed] [Google Scholar]
- 10. The Japan Society for the Study of Obesity . Guidelines for the Management of Obesity Disease 2016. Tokyo: Life Science Publishing, 2016. (Japanese). [Google Scholar]
- 11. Oja P, Vuori I, Paronen O. Daily walking and cycling to work: their utility as health‐enhancing physical activity. Patient Educ Couns 1998; 33: S87–94. [DOI] [PubMed] [Google Scholar]
- 12. Sahlqvist S, Song Y, Ogilvie D. Is active travel associated with greater physical activity? The contribution of commuting and non‐commuting active travel to total physical activity in adults. Prev Med 2012; 55: 206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moynihan AB, Tilburg WAPV, Igou ER, et al. Eaten up by boredom: consuming food to escape awareness of the bored self. Front Psychol 2015; 6: 369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goldstein DE, Little RR, Lorenz RA, et al. Tests of glycemia in diabetes. Diabetes Care 2003; 26(Suppl 1): S106–108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Association of lifestyle change with hemoglobin A1c change during the coronavirus disease 2019 pandemic.
Table S2 | Association between the variable of medication changes and hemoglobin A1c change during the coronavirus disease 2019 pandemic.
Table S3 | Interaction effect on association of lifestyle change with hemoglobin A1c change during the coronavirus disease 2019 pandemic.
Table S4 | Association of lifestyle change with weight change during the coronavirus disease 2019 pandemic.
Table S5 | Association between the variable of medication changes and bodyweight change during the coronavirus disease 2019 pandemic.
Table S6 | Interaction effect on association of lifestyle change with weight change during the coronavirus disease 2019 pandemic.
Appendix S1 | Interpretation of the association between the medication change and the hemoglobin A1c and bodyweight change in regression models.


