Abstract
Objective
The COVID‐19 pandemic has led to significant public health measures that have resulted in decreased acute pediatric care utilization. We evaluated whether the rate of severe presentations of new onset type 1 diabetes (DM1), such as, diabetic ketoacidosis (DKA) has changed since the COVID‐19 public health measures were enacted.
Research Design and Methods
A retrospective chart review of children less than 18 years of age presenting with new onset DM1 during the pandemic period of March 17, 2020 to August 31, 2020 was conducted at two tertiary care pediatric hospitals in Alberta, Canada. Rates of DKA and severe DKA were compared to the same time period in the year 2019 (pre‐pandemic control).
Results
The number of children presenting with newly diagnosed DM1 was similar during the pandemic year of 2020 compared with 2019 (107 children in 2020 vs. 114 in 2019). The frequency of DKA at DM1 onset was significantly higher in the pandemic period (68.2% vs 45.6%; p < 0.001) and incidence of severe DKA was also higher (27.1% in 2020 vs 13.2% in 2019; p = 0.01).
Conclusions
There was a significant increase in DKA and severe DKA in children presenting with new onset DM1 during the COVID‐19 pandemic period. This emphasizes the need for educating health care professionals and families to be aware of the symptoms of hyperglycemia and the importance of early diagnosis and treatment even during public health measures for COVID‐19.
Keywords: Children, COVID‐19, diabetes, diabetic ketoacidosis, pandemic
1. INTRODUCTION
The SARS‐Coronavirus‐2 pandemic (COVID‐19) is a serious health crisis with severe morbidity and mortality worldwide. COVID‐19 was labeled a global pandemic by the World Health Organization on March 11, 2020 with a call for countries to make crucial and timely changes to control global virus transmission and prevent overburdening of the healthcare system. 1 COVID‐19 was declared a public health emergency in Alberta, Canada on March 17, 2020 with enactment of school closures and public policies aimed at preventing the spread of the virus through social distancing and isolation. 2 Stay‐at‐home recommendations were made to reduce healthcare demand on emergency and intensive care services, with an increased reliance on virtual care appointments throughout Alberta. Health care utilization and pediatric emergency care visits were reportedly decreased during the pandemic, 3 , 4 raising concerns about the possible under diagnosis of other medical conditions.
Diabetic ketoacidosis (DKA) is a potentially severe, life‐threatening presentation of diabetes that can lead to significant morbidity in children; it is also largely preventable. The prevalence of ketoacidosis at diabetes diagnosis in children between the years 2008 and 2017 in the United States has increased from 31% to 58%, respectively. 5 , 6 Canadian data suggest recent prevalence rates of 18.6% to 25.6%. 7 , 8 Healthcare provider perceptions suggest a delay in diagnosis of children with new onset DM1 during the COVID‐19 pandemic due to possible fears around virus transmission during healthcare appointments and under‐recognition of symptoms secondary to reduced face‐to‐face interactions with medical providers. 9 Recent evidence has emerged showing an increase in severe ketoacidosis at diabetes diagnosis in children in Germany, Italy, and Australia. 10 , 11 , 12 We hypothesized that the COVID‐19 pandemic would have an effect on the rate of severe presentations of new onset type 1 diabetes (DM1), such as, DKA. We report the frequency of DKA presentations at onset of type 1 diabetes diagnosis in the pediatric population in Alberta, Canada during the COVID‐19 pandemic compared to pre‐pandemic control rates in 2019.
2. METHODS
The study design was a multi‐center, retrospective chart review. The study population included children less than 18 years of age presenting to the Stollery Children's Hospital (Edmonton, Alberta) and Alberta Children's Hospital (Calgary, Alberta) with a new diagnosis of DM1. The COVID‐19 pandemic group comprised those presenting with new onset DM1 from March 17, 2020 to August 31, 2020. The control group included those newly diagnosed with DM1 from March 17, 2019 to August 31, 2019.
The diagnosis of DM1 was established by clinical parameters as defined by the 2018 Diabetes Canada Clinical Practice Guidelines. 13 Patients with non‐DM1 and patients with new onset diabetes for whom type of diabetes is unclear were excluded. Data collected from patient charts and electronic medical records included clinical presentation (DKA at diabetes onset, pediatric intensive care unit (PICU) admission, DKA with coma, cerebral edema) and patient history (self‐reported symptoms and duration, first degree relative with diabetes, use of home glucose testing and visit to a healthcare facility in the 2 weeks prior to diagnosis). DKA was defined 14 , 15 as hyperglycemia (blood glucose >11 mmol/L) and blood pH <7.3 or bicarbonate <15 mmol/L and ketonemia or ketonuria (based on beta‐hydroxybutyrate level or presence of urinary/serum ketones). Severe DKA was defined as pH<7.1. 15
Ethics approval was obtained from the University of Alberta Research Ethics Board and the University of Calgary Conjoint Health Research Ethics Board.
2.1. Statistical analysis
The incidence of DKA at DM1 onset before and during COVID‐19 public health restrictions was reported. Means, medians and interquartile ranges were used to describe age at diagnosis and duration of symptoms. The difference in incidence rate of DM1 diagnosis between 2019 and 2020 periods was calculated with the chi‐square approximation for incidence rates using child population data from the Government of Alberta. 16 Additional comparisons, such as, the severity of DKA, were analyzed using the chi‐square test or Fisher's exact test where the assumptions of the chi‐square test are not met. A p‐value of <0.05 was used to determine statistical significance. Statistical analysis completed using the R Version 3.5.2 (R Core Team, 2018).
3. RESULTS
A total of 107 children presented with newly diagnosed DM1 from the period of March 17, 2020 to August 31, 2020, compared with 114 children during the same time period in 2019. The incidence rate of diagnosis of DM1 in the 2019 period was 10.64 per 100,000 children (19 years and younger) in Alberta and 9.907 per 100,000 in the 2020 period, which was not statistically different (p = 0.5959). The baseline demographics and characteristics at diagnosis are outlined in Table 1. The mean age at presentation, gender distribution and duration of self‐reported symptoms were similar between the two periods. Abdominal pain and weight loss were reported more frequently during the pandemic period compared to control (41.1% in 2020 vs 25.4% in 2019, and 67.2% in 2020 vs 52.6% in 2019, respectively). The mean bicarbonate level at presentation was significantly lower during the pandemic period compared to control (11.3 mmol/L (SD = 7.1) in 2020 vs 14.1 mmol/L (SD = 7.8) in 2019, p = 0.008).
TABLE 1.
Demographics and clinical characteristics at diagnosis of DM1 from March 17, 2019 to August 31, 2019 (control period) and March 17, 2020 to August 31, 2020 (pandemic period)
| Characteristic | 2020 | 2019 | p | |
|---|---|---|---|---|
| Total diagnosed | Total | 107 | 114 | ‐ |
| Age at diagnosis (years) | Mean | 9.62 | 9.43 | ‐ |
| Median | 10.04 | 9.72 | ‐ | |
| [Q1, Q3], IQR | [6.69, 12.8], 6.06 | [6.11, 13.4], 7.29 | ‐ | |
| Age group | <6 years | 23 (21.5%) | 28 (24.6%) | ‐ |
| 6–11 years | 51 (46.7%) | 48 (42.1%) | ‐ | |
| 12–18 years | 33 (30.8%) | 38 (33.3%) | ‐ | |
| Sex | Male | 46 (43.0%) | 47 (41.2%) | ‐ |
| Female | 61 (57%) | 67 (58.8%) | ||
| Location a | Rural | 17 (15.9%) | 11 (9.7%) | ‐ |
| Urban | 90 (84.1%) | 103 (90.4%) | ||
| Duration of Symptoms b (days) | Mean | 36.85 | 29.85 | ‐ |
|
Median [Q1, Q3], IQR |
14 [7.0, 30.0], 23.0 |
14 [8.5, 30], 21.5 |
||
| Symptoms b | Polyuria | 101 (94.3%) | 101 (88.6%) | ‐ |
| Polydipsia | 100 (93.5%) | 103 (90.4%) | ‐ | |
| Weight loss | 72 (67.2%) | 60 (52.6%) | * | |
| Blurred vision | 6 (5.6%) | 2 (1.8%) | ‐ | |
| UTI | 1 (0.9%) | 1 (0.8%) | ‐ | |
| Yeast infection | 4 (3.7%) | 5 (4.4%) | ‐ | |
| Abdominal pain | 44 (41.1%) | 29 (25.4%) | * | |
| Nausea/vomiting | 48 (44.9%) | 40 (35.1%) | ‐ | |
Note: *p < 0.05; **p < 0.01; −− p ≥ 0.05.
Location based on patient address postal code.
Self‐reported. Result of Test of Association (t test for age at diagnosis, Chi‐square or Fisher' exact test).
During the COVID‐19 pandemic period, the number of children presenting with DKA at onset of DM1 diagnosis was significantly higher compared to 2019 (68.2% in 2020 vs 45.6% in 2019; p < 0.001), representing an absolute increase of 22.6% (Figure 1). The prevalence of severe DKA at DM1 onset was also significantly higher during the pandemic period compared to control (27.1% in 2020 vs 13.2% in 2019, p < 0.01), representing an absolute increase of 13.9% (Figure 1). Of those children presenting in DKA, a similar proportion presented in severe DKA at onset of DM1 during the two observation periods (Figure 2). However, there was a significant increase in admission to the PICU during the COVID‐19 pandemic (17.8% in 2020 vs. 7.69% in 2019, p < 0.001). Of those children presenting with DKA, there were no significant differences in Glasgow Coma Scale (GCS), use of neurologic imaging or treatment of cerebral edema with 3% saline and/or mannitol (Table 2).
FIGURE 1.
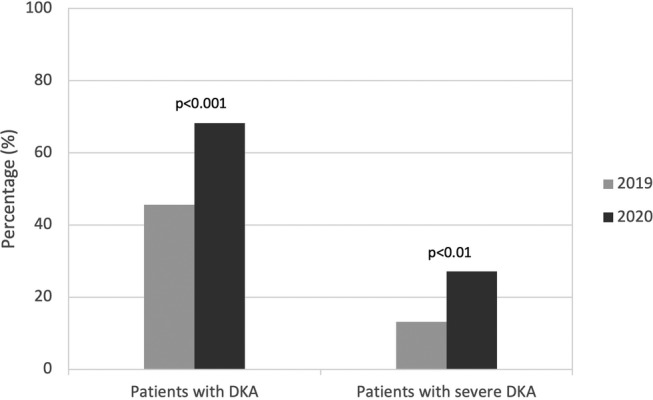
Diabetic ketoacidosis (DKA) at presentation: percentage of all patients with DKA and with severe DKA at onset of type 1 diabetes during the control period (March 17, 2019 to August 31, 2019; N = 114) and pandemic period (March 17, 2020 to August 31, 2020; N = 107), p < 0.05 significant
FIGURE 2.
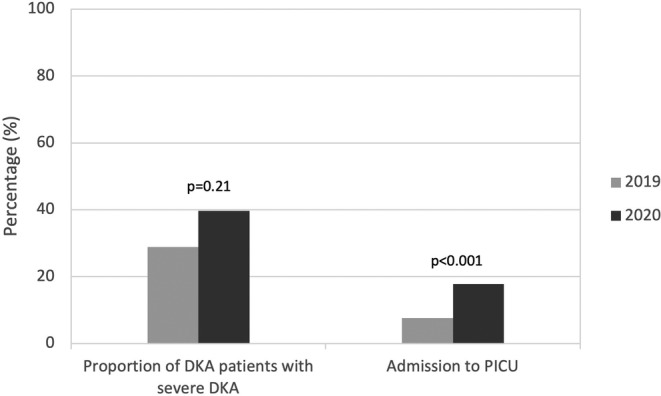
Severity of diabetic ketoacidosis (DKA) at presentation: difference between the control period (March 17, 2019 to August 31, 2019; N = 52) and pandemic period (March 17, 2020 to August 31, 2020; N = 73) in frequency of severe DKA and Pediatric Intensive Care Unit (PICU) admission for those who presented with DKA, p < 0.05 significant
TABLE 2.
Patients with altered Glasgow Coma Scale, diagnostic imaging and treatment for cerebral edema at diagnosis of DM1 from March 17, 2019 to August 31, 2019 (control period) and March 17, 2020 to August 31, 2020 (pandemic period)
| 2020 | 2019 | p | |
|---|---|---|---|
| New diagnosis type 1 diabetes | 107 | 114 | |
| Patients presenting with DKA | 73 (68.2%) | 52 (45.6%) | <0.001 |
| GCS | |||
| Mild (14‐15) | 65 | 49 | 0.36 |
| Moderate to Severe (≤13) | 8 | 3 | |
| Patient with DKA at diagnosis who had a head CT | 3 | 1 | 0.64 |
| Patient with DKA at diagnosis who received 3% saline and/or mannitol | 5 | 0 | 0.075 |
Abbreviations: DKA, diabetic ketoacidosis; DM1, type 1 diabetes; GCS, Glasgow Coma scale.
During the COVID‐19 pandemic period, there was a significantly higher rate of DKA amongst those without a first‐degree relative with diabetes mellitus compared to those with a first degree relative with diabetes (75.2% vs 33.3%, a difference of 35.2%, p = 0.03). For children without a first degree relative with diabetes, the rate of DKA at presentation was significantly higher during the pandemic period compared to the pre‐pandemic control period (75.2% in 2020 vs 46.9% in 2019, p = 0.01). In contrast, we did not observe a difference in DKA rate at presentation for children with a first degree relative with diabetes (33.3% in 2020 vs 37.5% in 2019, p = 0.84). During the pandemic period, 25% of children presenting with DKA had checked glucose at home by capillary blood glucose monitoring or home glucose urine dip sticks, which was similar to the pre‐pandemic period (22% in 2019, p = 0.87). There was a significantly higher rate of DKA in children who did not measure glucose at home compared to those who had utilized home glucose monitoring during the pandemic period (75.8% vs 25%, a difference of 50.8%, p = 0.001). A considerable proportion (20.2%) of the children presenting in DKA at DM1 onset during the pandemic period had been seen at a healthcare facility in the 2 weeks prior to their admission for DKA, similar to the pre‐pandemic control period in 2019 (24.3%, p = 0.4608).
4. DISCUSSION
Recent studies investigating the incidence of DM1 during the COVID‐19 pandemic have revealed mixed results. Tittel et al. demonstrate an increasing incidence of DM1 in Germany since the year 2011; however, do not show any pandemic related short‐term changes in DM1 incidence between 2019 and 2020. 17 Unsworth et al. establish an increase in new DM1 cases between March 23, 2020 and June 4, 2020 when compared to estimates of previous DM1 cases in the 5 years prior. However, this study is limited by its sample size and follow up timeframe. 18 Our study demonstrates that the incidence of type 1 diabetes did not differ significantly during the COVID‐19 pandemic compared to the pre‐pandemic control in Alberta, Canada. Longer term studies will be needed to determine if the incidence remains stable as the pandemic continues.
This study found a significant increase in the incidence of DKA at onset of DM1 in children during the COVID‐19 pandemic. The reason for this increase is unknown and requires further study. We hypothesize that public health measures enacted during the pandemic resulted in delayed diabetes presentation. From a family perspective, recommendations to decrease contact with other individuals may have contributed to increased parental fear and decreased utilization of medical services. 19 Increased virtual visits resulted in reduced face‐to‐face contact with healthcare providers and may have contributed to the under‐recognition of the severity of illness. Some health care providers may have been re‐deployed or had decreased office hours, which would have made it challenging for families to connect with their usual care team. Clinicians may have been more sensitized to assess for infectious and respiratory symptoms to diagnose possible COVID‐19 cases rather than considering new onset of DM1. With laboratory resources being targeted to COVID‐19 diagnosis, clinicians may have been reluctant to pursue lab testing for blood glucose or urinalysis. Further, requirements to prebook laboratory services appointments and lack availability for those appointments may have also contributed to delays in access to care. Studies are needed to quantify health care utilization during the pandemic period and can be conducted through administrative physician billing data available through provincial claims databases. Patient perceived barriers to care can also be studied through future qualitative research assessing patient values and beliefs around health care access during the pandemic.
Protective factors associated with reduced rates of DKA at onset of DM1 diagnosis were the presence of a first degree relative with diabetes and the ability to check glucose at home. The protective effect of a family history of type 1 diabetes is consistent with previous studies; 20 however, we report that 33% to 38% of children with a first degree relative still presented in DKA, showing a concerning under‐recognition of symptoms. Early symptoms of diabetes include polyuria, polydipsia, nocturia, enuresis, and weight loss. Polyuria and polydipsia symptoms were present in >90% of children presenting with new onset of diabetes. Early recognition of these symptoms by families and healthcare providers can facilitate prompt referral to pediatric diabetes services and early initiation of outpatient diabetes therapy. Nausea, vomiting and abdominal pain are markers of ketosis and represent metabolic decompensation concerning for DKA. Children with these symptoms should be directed to the emergency department immediately for further evaluation. DKA is the leading cause of premature mortality in individuals with DM1 20 and is a predictor of poor long term glycemic control. Duca et al reported that children presenting with DKA at diagnosis tracked along a higher hemoglobin A1c for up to 15 years compared with those presenting without DKA. 21 Children who present in severe DKA are at increased risk of cerebral edema, a serious neurologic complication of DKA that has an incidence of 0.5% to 0.9% and a high mortality rate of 21% to 24%. 20 Severe DKA also results in increased intensive care utilization during a time when pandemic efforts were underway to reduce PICU demand.
Over time, the literature shows that the rates of DM1 have been increasing. In a study in British Columbia, Canada, the rate of new onset DM1 increased from 23 per 100,000 in 2002 to 2003 up to 27 per 100,000 in 2012 to 2013. 22 DKA at initial diagnosis of DM1 is estimated to range from 13% to 80% in various countries. 20 This current study shows that Alberta has a high baseline rate of DKA at presentation of 45.6% in 2019 and up to 68.2% in 2020 during the pandemic. This is concerning due to the associated morbidity and mortality with DKA as well as the significant health care costs of treating DKA and long‐term risks of poor glycemic control.
Prevention of DKA at diagnosis relies on early identification of cases and can help to avoid costly hospital admissions as the rates of DM1 continue to increase. Public health campaigns aimed at community and provider education have been studied in the past and demonstrated a decrease in DKA rate at DM1presentation in Italy 23 and Australia. 24 The provincial scalability and sustainability of targeted awareness campaigns remains to be studied, particularly with regards to their long‐term impact on patient, family and provider recognition of early signs and symptoms of DM1.
Twenty percent of children in our study had presented to a health care facility in the 2 weeks prior to diagnosis with DM1. This indicates missed opportunities for early recognition of symptoms of new onset DM1 and chances for early diagnosis and treatment. Further studies are needed to understand the individual and healthcare system factors contributing to this missed diagnosis in order to inform future intervention strategies aimed at prevention. Ongoing education of health care professionals about the early symptoms of DM1 in children is important in preventing DKA at diagnosis. Public health campaigns on DKA prevention during the pandemic would likely be challenging due to the constant barrage of health information already being presented to the public.
This current study was limited by the fact that it was a retrospective data collection. Some elements, such as, symptoms and duration of symptoms relied on documentation of self‐reported information which could be subject to recall bias. While the majority of cases of new onset DM1 in Alberta would be seen in the two major tertiary care centers of Calgary and Edmonton, some cases of new onset DM1 are seen in peripheral hospitals. Our case identification was limited to our databases in Calgary and Edmonton and did not include rural hospitals.
During the COVID‐19 pandemic, initial presentations of DM1 were more severe, likely due to the pandemic's impact on the healthcare system and health delivery. There was no increase in incidence of DM1 in children during the pandemic; however, there was a significant increase in rates of DKA. Early recognition of clinical signs and symptoms of DM1 by healthcare providers and families can lead to rapid initiation of effective outpatient stabilization and treatment. We stress the importance of timely access to healthcare and immediate referral to pediatric diabetes services or an emergency department upon recognition of the symptoms of DM1. When the diagnosis of DM1 is delayed, as we have seen during the COVID‐19 pandemic, individuals will continue to have rapid metabolic decompensation, resulting in DKA. DKA is associated with morbidity and mortality, and our data supports the need for targeted awareness campaigns aimed at preventing DKA at DM1 diagnosis through early symptom recognition and early treatment.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/pedi.13205.
ACKNOWLEDGEMENT
No funding was received in association with this manuscript
Ho J, Rosolowsky E, Pacaud D, et al. Diabetic ketoacidosis at type 1 diabetes diagnosis in children during the COVID‐19 pandemic. Pediatr Diabetes. 2021;22:552–557. 10.1111/pedi.13205
DATA AVAILABILITY STATEMENT
The authors are unable to share the data repository as it would compromise the ethical standards and legal requirements.
REFERENCES
- 1. World Health Organization. WHO Director‐General's opening remarks at the media briefing on COVID‐19‐ 11 March. Accessed October 20. 2020.
- 2. Government of Alberta. COVID‐19 orders and legislation Mar 16. Accessed October 20, 2020.
- 3. Isba R, Edge R, Jenner R, Broughton E, Francis N, Butler J. Where have all the children gone? Decreases in paediatric emergency department attendances at the start of the COVID‐19 pandemic of 2020. Arch Dis Child. 2020;105(7):704. [DOI] [PubMed] [Google Scholar]
- 4. Chanchlani N, Buchanan F, Gill PJ. Addressing the indirect effects of COVID‐19 on the health of children and young people. CMAJ. 2020;192(32):E921‐E927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dabelea D, Rewers A, Stafford JM, et al. Trends in the prevalence of ketoacidosis at diabetes diagnosis: the SEARCH for diabetes in youth study. Pediatrics. 2014;133(4):e938‐e945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alonso GT, Coakley A, Pyle L, Manseau K, Thomas S, Rewers A. Diabetic ketoacidosis at diagnosis of type 1 diabetes in Colorado children, 2010‐2017. Diabetes Care. 2020;43(1):117‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Robinson ME, Li P, Rahme E, Simard M, Larocque I, Nakhla MM. Increasing prevalence of diabetic ketoacidosis at diabetes diagnosis among children in Quebec: a population‐based retrospective cohort study. CMAJ Open. 2019;7(2):E300‐E305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bui H, Stein R, Fung K, Daneman D. Is diabetic ketoacidosis at disease onset a result of missed diagnosis? J Pediatr. 2010;156(3):472‐477. [DOI] [PubMed] [Google Scholar]
- 9. Elbarbary NS, Dos Santos TJ, de Beaufort C, Agwu JC, Calliari LE, Scaramuzza AE. COVID‐19 outbreak and pediatric diabetes: perceptions of health care professionals worldwide. Pediatr Diabetes. 2020;21:1083‐1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kamrath C, Monkemoller K, Biester T, et al. Ketoacidosis in children and adolescents with newly diagnosed type 1 diabetes during the COVID‐19 pandemic in Germany. JAMA. 2020;324(8):801‐804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rabbone I, Schiaffini R, Cherubini V, Maffeis C, Scaramuzza A. Diabetes study Group of the Italian Society for pediatric E, et al. has COVID‐19 delayed the diagnosis and worsened the presentation of type 1 diabetes in children? Diabetes Care. 2020;43(11):2870‐2872. [DOI] [PubMed] [Google Scholar]
- 12. Lawrence C, Seckold R, Smart C, et al. Increased paediatric presentations of severe diabetic ketoacidosis in an Australian tertiary Centre during the COVID‐19 pandemic. Diabet Med. 2020;38:e14417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Diabetes Canada Clinical Practice Guidelines Expert C , Punthakee Z, Goldenberg R, Katz P. Definition, classification and diagnosis of diabetes, prediabetes and metabolic syndrome. Can J Diabetes. 2018;42(Suppl 1):S10‐S15. [DOI] [PubMed] [Google Scholar]
- 14. Diabetes Canada Clinical Practice Guidelines Expert C , Goguen J, Gilbert J. Hyperglycemic emergencies in adults. Can J Diabetes. 2018;42(Suppl 1):S109‐S114. [DOI] [PubMed] [Google Scholar]
- 15. Wolfsdorf JI, Glaser N, Agus M, et al. ISPAD Clinical Practice Consensus Guidelines 2018: diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Pediatr Diabetes. 2018;19(Suppl 27):155‐177. [DOI] [PubMed] [Google Scholar]
- 16. Government of Alberta Treasury Board and Finance. Annual Population Report, Alberta 2019. Accessed October 26, 2020.
- 17. Tittel SR, Rosenbauer J, Kamrath C, et al. Did the COVID‐19 lockdown affect the incidence of pediatric type 1 diabetes in Germany? Diabetes Care. 2020;43(11):e172‐e173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Unsworth R, Wallace S, Oliver NS, et al. New‐onset type 1 diabetes in children during COVID‐19: multicenter regional findings in the U.K. Diabetes Care. 2020;43(11):e170‐e171. [DOI] [PubMed] [Google Scholar]
- 19. Lazzerini M, Barbi E, Apicella A, Marchetti F, Cardinale F, Trobia G. Delayed access or provision of care in Italy resulting from fear of COVID‐19. Lancet Child Adolesc Health. 2020;4(5):e10‐e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jefferies CA, Nakhla M, Derraik JG, Gunn AJ, Daneman D, Cutfield WS. Preventing diabetic ketoacidosis. Pediatr Clin North Am. 2015;62(4):857‐871. [DOI] [PubMed] [Google Scholar]
- 21. Duca LM, Wang B, Rewers M, Rewers A. Diabetic ketoacidosis at diagnosis of type 1 diabetes predicts poor long‐term glycemic control. Diabetes Care. 2017;40(9):1249‐1255. [DOI] [PubMed] [Google Scholar]
- 22. Fox DA, Islam N, Sutherland J, Reimer K, Amed S. Type 1 diabetes incidence and prevalence trends in a cohort of Canadian children and youth. Pediatr Diabetes. 2018;19(3):501‐505. [DOI] [PubMed] [Google Scholar]
- 23. Vanelli M, Chiari G, Ghizzoni L, Costi G, Giacalone T, Chiarelli F. Effectiveness of a prevention program for diabetic ketoacidosis in children. An 8‐year study in schools and private practices. Diabetes Care. 1999;22(1):7‐9. [DOI] [PubMed] [Google Scholar]
- 24. King BR, Howard NJ, Verge CF, et al. A diabetes awareness campaign prevents diabetic ketoacidosis in children at their initial presentation with type 1 diabetes. Pediatr Diabetes. 2012;13(8):647‐651. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors are unable to share the data repository as it would compromise the ethical standards and legal requirements.


