Abstract
For the treatment of Covid‐19 patients with remdesivir, poor renal and liver function were both exclusion criteria in randomized clinical trials and contraindication for treatment. Also, nephrotoxicity and hepatotoxicity are reported as adverse events. We retrospectively reviewed renal and liver functions of Covid‐19 103 patients who received remdesivir in the 15 days after treatment initiation. Approximately 20% of the patient population met randomized clinical trial exclusion criteria. In total, 11% of the patients had a decrease in estimated glomerular filtration rate >10 mL/min/1.73m2. Also, 25 and 35% had increased alanine transaminase and aspartate transaminase levels, respectively. However, serious adverse events were limited. Therefore, based on these preliminary results, contraindications based on kidney and liver function should not be absolute for remdesivir treatment in patients with Covid‐19 if these functions are monitored regularly. A larger patient cohort is warranted to confirm our results.
Keywords: adverse events, Covid‐19, liver function, remdesivir, renal function
What is already known about this subject
Nephrotoxicity and hepatotoxicity are reported as adverse events during randomized controlled trials of remdesivir treatment in patients with Covid‐19.
Patients with an impaired kidney or liver function were excluded from the trials.
Impaired kidney or liver function are stated as contraindication for treatment with remdesivir.
What this study adds
This real‐world population of patients with Covid‐19 includes 20% of patients potentially excluded from clinical trials based on their renal or liver function.
Hepatoxicity and nephrotoxicity of remdesivir are mostly mild, and severe adverse events are limited.
Contraindications based on kidney and liver function should not be absolute if functions are monitored regularly.
1. INTRODUCTION
Nephrotoxicity and hepatoxicity were reported as adverse drug events in patients with coronavirus disease 2019 (Covid‐19) treated with remdesivir, a viral RNA‐dependent, RNA polymerase inhibitor. 1 , 2 Renal toxicity due to remdesivir is attributed to 2 different mechanisms. First, remdesivir triphosphate itself has low potential for mitochondrial toxicity as it weakly inhibits the mammalian DNA and RNA polymerases, and therefore may cause mitochondrial injury in renal tubular epithelial cells. 2 , 3 However, after treatment with similar type of antiviral drugs (e.g. tenofovir), this adverse effect only occurred after long‐term treatment. 4 Second, the intravenous preparation of remdesivir contains solfobutylether‐β‐cyclodextrin as vehicle, since remdesivir itself has poor water solubility. Accumulation of this vehicle was found in patients with a creatinine clearance <50 mL/min, and associated with liver necrosis and obstruction of renal tubules in animal studies with high doses. 2 , 3 , 5 However, a clinical trial investigating remdesivir as treatment for Ebola did not report any severe hepatotoxicity or nephrotoxicity. 6
Nonetheless, Covid‐19 patients with impaired kidney (estimated glomerular filtration rate [eGFR] <30 or <50 mL/min/1.73m2) or liver function (alanine transaminase [ALT] or aspartate transaminase [AST] 5 times upper limit of normal [ULN]; ALT male: 45 U/L, female: 35 U/L; AST male: 35 U/L, female: 30 U/L]) were excluded from randomised clinical trials (RCTs) with remdesivir. 1 , 7 , 8 These impaired functions were included as contraindications for treatment in the summary of product characteristics. 1 , 9
We report changes in renal and liver functions during treatment with remdesivir in a Covid‐19 patient population, regardless of the initial laboratory values. Hereby, additional information was obtained about the incidence and severity of nephro‐ and hepatotoxicity due to remdesivir treatment in clinical practice.
2. PATIENTS AND METHODS
We included hospitalized (Leiden University Medical Center, The Netherlands) adult patients on a regular ward with oxygen supplementation who started 5‐day remdesivir treatment (intention to treat) between 17 August and 4 November 2020. We received a waiver for the need of informed consent from the Medical Ethics Review Committee of LUMC, Leiden. All patients had a severe acute respiratory syndrome coronavirus‐2 infection confirmed by polymerase chain reaction. Per patient, age, sex, remdesivir prescription data, and laboratory parameters (eGFR, AST, ALT) were extracted from the electronic health record using CTcue text mining software (CTcue B.V., Amsterdam, The Netherlands). We determined renal and liver function at the start of treatment and change of parameters in a follow‐up period of 15 days after remdesivir treatment initiation, both per patient and as mean change per day. Adverse event classification was according to the Common Terminology Criteria for Adverse Events version 5.0. Patients were excluded if no laboratory measurements were available.
3. RESULTS
In total, 103 patients were included in this study, of whom at least 1 of the laboratory measurements was available, the majority of them being male (68%), and the median (interquartile range, IQR) age was 64 (56–75) years (Table 1).
TABLE 1.
Patient characteristics
| Total population | |
|---|---|
| N = 103 | |
| Sex, male, n (%) | 70 (68) |
| Age, median years (1st and 3rd qu.) | 64 (56–75) |
| Baseline eGFR | N = 95 |
| eGFR, median mL/min/1.73 m2 (1st and 3rd qu.) | 74.0 (54.5–87.0) |
| >50 mL/min/1.73m2, n (%) | 74 (78) |
| 30–50 mL/min/1.73m2, n (%) | 15 |
| <30 mL/min/1.73m2, n (%) | 6 |
| Baseline ALT | N = 95 |
| ALT, median U/L (1st and 3rd qu.) | 32.0 (22.0–55.8) |
| <ULN, n (%) | 54 |
| ULN ‐ 5x ULN, n (%) | 36 |
| >5x ULN, n (%) | 5 |
| Baseline AST | N = 81 |
| AST, median, U/L (1st and 3rd qu.) | 40.0 (27.0–59.0) |
| <ULN, n (%) | 33 |
| ULN–5× ULN, n (%) | 44 |
| >5× ULN, n (%) | 5 |
ALT, alanine transaminase; AST, aspartate transaminase; eGFR, estimated glomerular filtration rate; IQR, interquartile range; ULN, upper limit of normal
Figure 1 shows the change in eGFR, AST and ALT compared to the baseline measurements per individual patient and mean change per day after remdesivir initiation.
FIGURE 1.
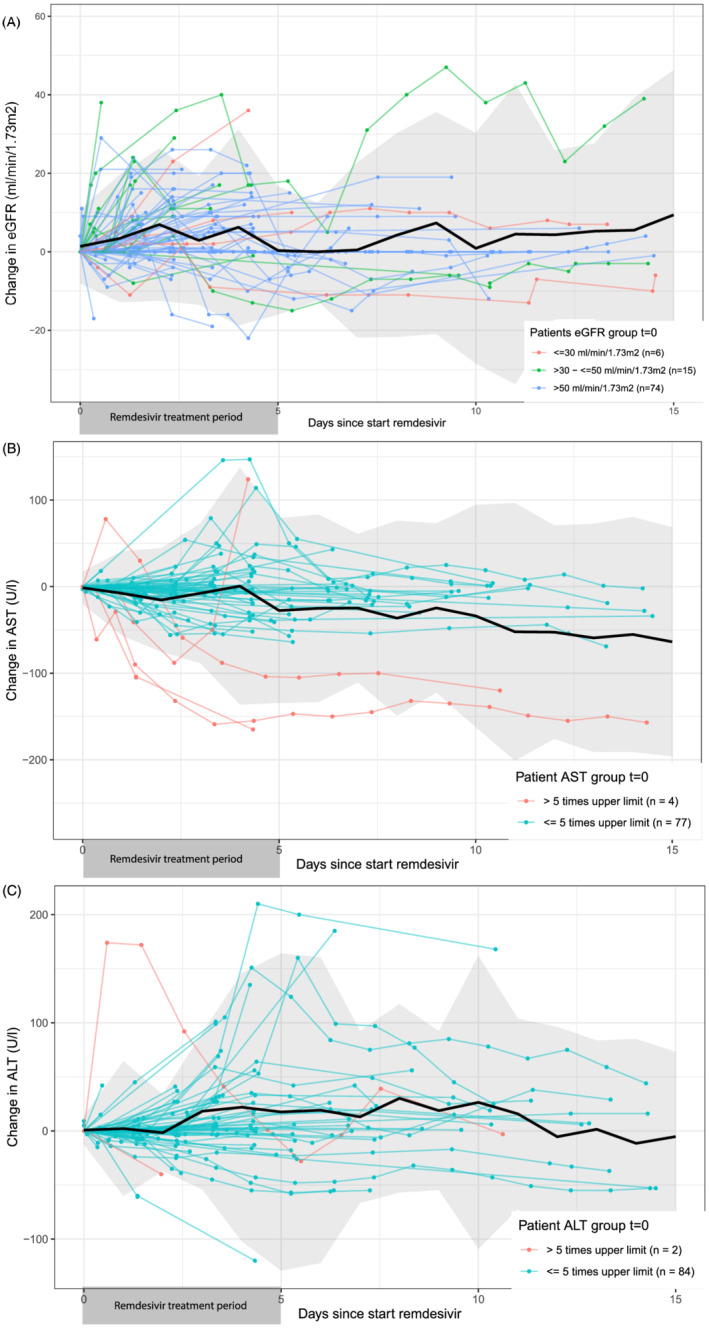
Changes in renal and liver function parameters after start of remdesivir treatment in patients with Covid‐19. The data originate from the electronic health record of 1 hospital (Leiden University Medical Center, The Netherlands). In this figure changes in estimated glomerular filtration rate (eGFR; A), aspartate transaminase (AST; B) and alanine transaminase (ALT; C) laboratory measurements compared to baseline are shown. Baseline measurement was the most recent before start (max. 30 days), or if not available, the first measurement within 24 hours after the remdesivir prescription in the electronic health record. All coloured data represent individual patient measurements. Patients in red and green would have been excluded from clinical trials, based on their baseline measurements (eGFR: <30 [red] and 30–50 [green] mL/min/1.73m2, AST: >5× upper limit of normal [ULN], AST: >5× ULN). In black, the mean change per day is shown including a 95% confidence interval in grey
At start of treatment, the median baseline eGFR in 95 patients was 74.0 (IQR 54.5–87.0) mL/min/1.73m2. In total, 10 (10.5%) patients had a decrease in eGFR of >10 mL/min 1.73m2 and the maximum decrease in eGFR was 21 mL/min/1.73m2. Of all patients, 21 (22.1%) started treatment with an eGFR below the trial exclusion limit of 50 mL/min/1.73m2 and 2 (9.5%) had a decrease in eGFR of >10 mL/min/1.73m2 (Figure 1A).
The baseline median AST was 40.0 (IQR 27.0–58.5) U/L in 81 patients, of which 33 patients started with a normal AST and 48 patients with AST above ULN, including 4 patients out of the trial exclusion limit of 5× ULN. After start with remdesivir, 13 patients (39%) with baseline AST in normal range had an increase in AST >ULN (grade 1 adverse event), 1 patient (3%) had an increase in AST above 3× ULN (grade 2 adverse event) and 1 patient AST >5× ULN (grade 3 adverse event). Of the 48 patients where baseline was initially above ULN, 2 met the exclusion limit and 5 (10.4%) had an increase of 1.5× baseline (grade 1 adverse event). The maximum increase in AST was 147 U/L (Figure 1B).
The baseline median ALT was 32.0 (IQR 22.0–55.8) U/L in 95 patients, 54 started remdesivir treatment with a normal ALT and 41 with ALT above ULN, of whom 2 had ALT >5× ULN. In total 19 (35%) of patients starting with a normal ALT had an increase of ALT >ULN (grade 1 adverse event), 3 patients (6%) had ALT >3× ULN (grade 2) and 1 patient had AST >5× ULN (grade 3). Nine patients (22%) who started remdesivir treatment with ALT above ULN had an increase in ALT >1.5× baseline (grade 1); however, in none of them was >3.0× baseline (grade 2) reported. The maximum increase in ALT was 210 U/L (Figure 1C).
We included 11 patients with at least 1 contraindication based on kidney or liver function. Of these, 9 patients received the complete full dose 5‐day treatment. In 1 patient the remdesivir treatment was ended when dismissed from the hospital and in 1 patient treatment was ended due to hepatitis, which was suspected to be viral or ciprofloxacin induced. However, in none of these patients, remdesivir‐induced toxicity was a reason to end treatment.
4. DISCUSSION AND CONCLUSION
Overall, in 103 hospitalized Covid‐19 patients who received a maximum of 5 days remdesivir treatment, no severe nephrotoxicity and in 2 patients grade 3 hepatotoxicity was found in the first 15 days after treatment initiation.
The incidence of decreased glomerular filtration rate was comparable with data reported in the RCTs last year. 7 , 8 A quarter of our population had AST elevation and a third ALT elevation, which is more than the adverse events reported in RCTs (3.4–5% and 2.3–6%, respectively). However, Beigel et al. only reported adverse events of grade 2 or higher; Wang et al. and Goldman et al. did not specify the grade of adverse events included in the study. 1 , 7 , 8 Most of the liver transaminase elevation in patients in our study was mild (grade 1). Only 1% showed grade 3 elevation, whereas Goldman et al. reported grade 3 and 4 adverse events in 2–6% of the patients. 8 None of the patients that started with transaminases above ULN, including patients meeting exclusion criteria, had grade 2 or higher transaminase elevation.
It is important to emphasize that remdesivir is not the only independent factor that could cause nephrotoxicity or hepatotoxicity in these patients. Both Covid‐related and non‐Covid related risk factors are known, of which age of 65 years and older is 1. 10 , 11 However, in our patients, age was not identified as an independent risk factor for nephro‐ or hepatotoxicity. Remdesivir is extensively metabolized; for the prodrug and its metabolites, respectively, no and modest (<2‐fold) accumulation was observed after multiple dosing of 150 mg for 14 days. 12 Also, comparable clearance was shown between a patient with an impaired and a patient with normal kidney function. 13 Therefore, although remdesivir metabolites are mostly renally excreted, we do not expect that poor renal function will cause clinically relevant blood level elevation within 5 days of treatment resulting in increased toxicity or efficacy.
In a real‐world population, we found that the incidence of decreased eGFR during treatment with remdesivir was comparable to the RCTs and was at most moderate. Furthermore, we reported a higher incidence of patients with non‐severe transaminase elevation. However, with only 2 patients with a reversible severe adverse event, this was not more than in RCTs. Patients meeting contraindications seem not to develop more nephro‐ or hepatotoxicity on remdesivir treatment. Therefore, there may be no reason for these contraindications to be absolute. However, monitoring remains necessary. In case of a further decline of organ functions during treatment with remdesivir, we believe it is the role of the clinician to assess the origin of the impairment.
Our observations indicate that kidney and liver dysfunction should not be an absolute contraindication for the use of remdesivir in Covid‐19 patients, and that by regularly monitoring kidney and liver function, treatment with remdesivir can be justified in these patients. Verification of these findings in a larger cohort is needed.
ACKNOWLEDGEMENT
This research received no specific grand from any funding agency in public, commercial or not‐for‐profit sectors.
COMPETING INTERESTS
There are no competing interests to declare.
CONTRIBUTORS
S.L., M. B, K. G, H. G, and J.Z. wrote the manuscript; S.L., J.Z. and K.G. designed the research; S.L. performed the research and S.L. analysed the data.
5.
van Laar SA, de Boer MGJ, Gombert‐Handoko KB, Guchelaar H‐J, Zwaveling J, LUMC‐Covid‐19 research group. Liver and kidney function in patients with Covid‐19 treated with remdesivir. Br J Clin Pharmacol. 2021;87(11):4450-4454. 10.1111/bcp.14831
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the Treatment of Covid‐19 — Final Report. N Engl J Med. 2020;383(19):1813–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adamsick ML, Gandhi RG, Bidell MR, et al. Remdesivir in Patients with Acute or Chronic Kidney Disease and COVID‐19. J Am Soc Nephrol. 2020;31(7):1384–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ackley TW, McManus D, Topal JE, Cicali B, Shah S. A Valid Warning or Clinical Lore: An Evaluation of Safety Outcomes of Remdesivir in Patients with Impaired Renal Function from a Multicenter Matched Cohort. Antimicrob Agents Chemother. 2020;65(2):e02290–20. 10.1128/aac.02290-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Herlitz LC, Mohan S, Stokes MB, Radhakrishnan J, D'Agati VD, Markowitz GS. Tenofovir nephrotoxicity: acute tubular necrosis with distinctive clinical, pathological, and mitochondrial abnormalities. Kidney Int. 2010;78(11):1171–1177. [DOI] [PubMed] [Google Scholar]
- 5. Kiser TH, Fish DN, Aquilante CL, et al. Evaluation of sulfobutylether‐beta‐cyclodextrin (SBECD) accumulation and voriconazole pharmacokinetics in critically ill patients undergoing continuous renal replacement therapy. Crit Care. 2015;19(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mulangu S, Dodd LE, Davey RT Jr, et al. A Randomized, Controlled Trial of Ebola Virus Disease Therapeutics. N Engl J Med. 2019;381(24):2293–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID‐19: a randomised, double‐blind, placebo‐controlled, multicentre trial. The Lancet. 2020;395(10236):1569–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goldman JD, Lye DCB, Hui DS, et al. Remdesivir for 5 or 10 Days in Patients with Severe Covid‐19. N Engl J Med. 2020;383(19):1827–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gilead Sciences . 2020. Summary of Product Characteristics: Veklury (remdesivir).
- 10. Nadim MK, Forni LG, Mehta RL, et al. COVID‐19‐associated acute kidney injury: consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup. Nat Rev Nephrol. 2020;16(12):747–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mercado MG, Smith DK, Guard EL. Acute Kidney Injury: Diagnosis and Management. Am Fam Physician. 2019;100(11):687–694. [PubMed] [Google Scholar]
- 12. Humeniuk R, Mathias A, Cao H, et al. Safety, Tolerability, and Pharmacokinetics of Remdesivir, An Antiviral for Treatment of COVID‐19, in Healthy Subjects. Clin Transl Sci. 2020;13(5):896–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yan VC, Muller FL. Captisol and GS‐704277, but Not GS‐441524, Are Credible Mediators of Remdesivir's Nephrotoxicity. Antimicrob Agents Chemother. 2020;64:e01920. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


