Dear Editor,
We present the case of a 27‐year‐old female ophthalmologist, with no relevant personal history, who received the BNT162b2 vaccine in her left arm. The patient was asymptomatic until day 7 after the injection when she reported pain at the injection site and the progressive appearance of a poorly‐defined erythematous–edematous plaque, 9 × 5 cm, with an increase in local temperature (Fig. 1). She presented a fever (37.7°C) without other systemic symptoms. Real‐time reverse transcription polymerase chain reaction (RT‐PCR) test for SARS‐CoV‐2 detection from nasopharyngeal exudate was negative. Treatment was started with paracetamol, prednisone 30 mg, and dexchlorpheniramine. After 2 days, the cutaneous reaction, pain, and fever resolved.
Figure 1.
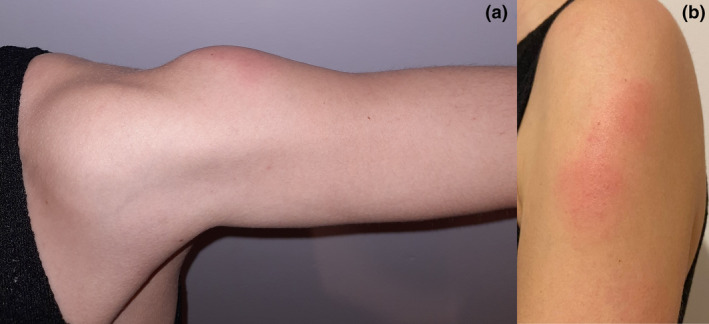
(a) Erythematous‐edematous firm plaque of several millimeters thickness over the deltoid area. (b) Surface of the same plaque, poorly limited. Erythematous millimeter papules are observed
The second dose of the vaccine was administered 21 days after the first one, in the right arm. Following 6 hours after the injection, the patient reported pain and an erythematous–edematous 3 × 2 cm plaque in the injection site. After 24 hours, fatigue was reported. The local reaction was milder than the previous, and symptoms resolved after 2 days of treatment with paracetamol.
The patient had no history of hypersensitivity reactions to previous vaccines. She had not experienced COVID‐19 symptoms. She was tested with nasopharyngeal RT‐PCR four times from April to December (because of risk contacts) with negative results. COVID‐19 serologies were performed in April, June, and December, with negative results.
In comparison with conventional vaccines, mRNA vaccines have the potential for rapid, scalable manufacturing, which makes them useful for potentially pandemic infectious diseases. 1 To date, only two mRNA vaccines for the prevention of infectious diseases are approved for commercialization: BNT162b2 and mRNA‐1273. Both are lipid‐nanoparticle‐encapsulated RNA encoding SARS‐CoV‐2 spike protein and are administered by injection into the deltoid muscle in a two‐dose regimen. 2 , 3
In the clinical trial of BNT162b2 vaccine, 2 the percentage of local reactions in patients between 16 and 55 years, 1–7 days after the first dose, was: 78% pain, 6% redness, and 6% inflammation. Most reactions were mild or moderate and resolved within 2 days. The proportion of participants reporting local reactions did not increase after the second dose. The most commonly reported systemic events were fatigue and headache. Systemic events appeared with more frequency and severity after the second dose. Fever was reported by 0.2% and 0.8% of participants after the first and second dose, respectively.
Compared to the clinical trial of the mRNA‐1273 vaccine, 3 and to a phase I trial of another lipid‐nanoparticle‐mRNA vaccine, 4 local and systemic reactions were similar among all mRNA and conventional vaccines. The only difference was that the mRNA‐1273 vaccine produced delayed injection‐site reactions characterized by erythema, induration, and tenderness, with onset on or after day 8, and resolving over the following 4–5 days. Those appeared in 0.8% of participants after the first dose and in 0.2% after the second dose.
Local hypersensitivity reactions to conventional vaccines are frequent. Mild pain, redness, and/or swelling at injection site within 72 hours after vaccination are attributed to nonspecific inflammation. Arthus reaction (type III hypersensitivity) might be considered in larger reactions, but it is described mostly with toxoid vaccines because of preexisting IgG antibodies from earlier immunizations. Delayed local eczematous reactions usually represent immuno‐mediated type IV reactions. 5
Delayed injection‐site reactions described in mRNA1273’s trial are similar to our case, but the suspected mechanism of this reaction is unspecified in the trial. An immune‐mediated hypersensitivity mechanism seems unlikely because of the lack of previous sensitization to lipid‐nanoparticle‐mRNA and the low potential for hypersensitivity of the excipients. We have not found any published report about delayed‐local reactions after injection of lipid‐nanoparticles. We hypothesize that this delayed injection‐site reaction could be mediated by nonspecific inflammation in the spectrum of normal immune response. More studies are needed to classify hypersensitivity reactions to vaccines.
Conflict of interest: None.
Funding source: None.
References
- 1. Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines – a new era in vaccinology. Nat Rev Drug Discov 2018; 17: 261–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med 2020; 383: 2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med 2021; 384: 403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bahl K, Senn JJ, Yuzhakov O, et al. Preclinical and clinical demonstration of immunogenicity by mRNA vaccines against H10N8 and H7N9 influenza viruses. Mol Ther 2017; 25: 1316–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stone CA, Rukasin CRF, Beachkofsky TM, Phillips EJ. Immune‐mediated adverse reactions to vaccines. Br J Clin Pharmacol 2019; 85: 2694–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]


