Dear Editor,
Coronavirus disease (COVID‐19) has spread across the globe from December 2019 determining the current pandemic. It is caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), an RNA virus of the betacoronavirus family. Besides COVID‐19 respiratory symptoms, skin manifestations have been largely reported, such as chilblain‐like lesions, urticaria, maculopapular lesions, and also hair loss. 1
We report a case of alopecia areata(AA) which occurred after SARS‐CoV‐2 infection.
In October 2020, a female patient aged 29 was admitted to our post‐COVID Dermatology Office, complaining progressive hair loss starting 6 months before our observation. The patient had been diagnosed with SARS‐CoV‐2 infection in March 2020, following positive naso‐oropharyngeal swab test. Reported symptoms were fever and dry cough, treated with paracetamol and azithromycin and requiring no hospitalization.
The patient recalled that hair loss started 1 month after the onset of COVID‐19, firstly on vertex and parietal regions, with patchy distribution, then involving the total scalp surface.
A trichological evaluation showed a generalized hair loss of the scalp, with regrowing hairs of different lengths (Figure 1A,B) and the loss of the outer third of the eyebrows. Trichoscopy showed black dots, yellow dots, exclamation mark hairs, and regrowing hairs (Figure 1C–E). The clinical and trichoscopic aspects were compatible with the diagnosis of alopecia areata totalis. No other skin manifestations of COVID‐19 were present.
FIGURE 1.
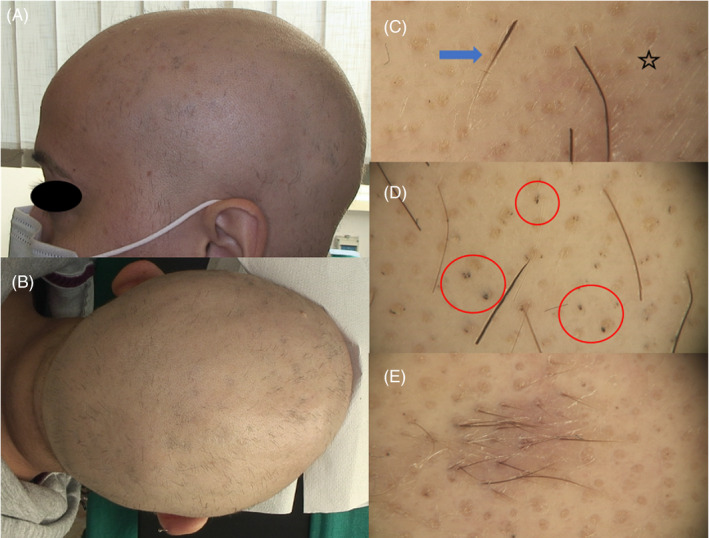
(A, B) Macroscopic images of AA totalis, with complete scalp hair loss and diffuse regrowing hairs; (C, D) trichoscopic images showing multiple yellow dots (star), black dots (red circles), exclamation mark hairs (blue arrow); and (E) trichoscopic image of regrowing hairs
Personal history was negative for autoimmune and trichological disorders; family history was negative for AA, but her brother was affected by Bruton syndrome (agammaglobulinemia). Laboratory findings including thyroid function tests, celiac disease tests, antinuclear antibody (ANA) test, and Extractable Nuclear Antigen Antibodies (ENA) panel were normal.
Triamcinolone acetonide injections, oral vitamin D and probiotics, and topical steroids and bimatoprost for the eyebrows were prescribed with good response.
In literature, only few reports describe a possible association between AA and COVID‐19.
Some works reported an increase of AA as a consequence of the pandemic, as a stress‐related factor causing hair loss in non‐infected individuals.
Only two previous cases of new‐onset AA after SARS‐CoV‐2 infection have been described, 2 , 3 and our report is the third published case of new‐onset AA in a COVID‐19 patient, and the second one with total distribution.
AA is a complex autoimmune disease, in which inflammation and loss of hair follicle immune privilege(HFIP) lead to T‐cells activation and non‐scarring hair loss. Several environmental factors have been suggested as possible triggers in genetically predisposed individuals, such as stress, viruses, vaccination, and hormonal alterations. Stress is one of the most common trigger exacerbating or inducing AA; thus, cases of AA in individuals with COVID‐19 could be a consequence of the psychological distress related to the infection. Although is not possible to directly link the SARS‐CoV‐2 infection and AA onset in our patient, there seems to be a close association in timing between them, suggesting a possible correlation.
Therefore, the connection between AA and other viruses has been suggested by many authors, particularly cytomegalovirus (CMV), Epstein‐Barr virus (EBV), and hepatitis B vaccination. 4 Viral infections could cause AA via the interferon (IFN)‐mediated antiviral response, which induces major histocompatibility complex (MHC) class I expression in the proximal outer root sheath with consequent loss of HFIP. Other possible mechanisms include molecular mimicry, superantigens, and epitope spreading. 4 SARS‐CoV‐2 could activate one of these processes activating the cytokine cascade involving IFN and other pro‐inflammatory cytokines such as IL‐6, which is usually elevated in both COVID‐19 and AA. 5
In particular, IL‐6 seems to dose‐dependently inhibit the proliferation of follicular keratinocytes and the HF stem cells, functionally blocking the transition from telogen to anagen. 6
In conclusion, our report provides a contribution to the current knowledge about the mechanisms undergoing the dermatological manifestations of COVID‐19, and to the possible association between SARS‐CoV‐2 and AA.
CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
Alfredo Rossi and Teresa Grieco revised the work and the conceptual ideas and supervised the work. Camilla Chello, Gemma Caro, and Marco di Fraia contributed to the enrollment, collected the data, and helped writing the work. Francesca Magri, Alvise Sernicola, and Simone Michelini planned the work and wrote the paper. Marta Muscianese and Maria Caterina Fortuna critically revised the manuscript. All authors discussed the results and contributed to the final manuscript.
INFORMED CONSENT
The patients in this manuscript have given written informed consent to publication of their case details.
REFERENCES
- 1. Moreno‐Arrones OM, Lobato‐Berezo A, Gomez‐Zubiaur A, et al. SARS‐CoV‐2‐induced telogen effluvium: a multicentric study. J Eur Acad Dermatol Venereol. 2020;5(3):e181‐e183. 10.1111/jdv.17045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. FIvenson David. COVID‐19: association with rapidly progressive forms of alopecia areata. Int J Dermatol. 2020;60:127. 10.1111/ijd.15317. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sgubbi P, Savoia F, Calderoni O, Longo R, Stinchi C, Tabanelli M. Alopecia areata in a patient with SARS‐Cov‐2 infection. Dermatol Ther. 2020;10:e14295. [DOI] [PubMed] [Google Scholar]
- 4. Richardson CT, Hayden MS, Gilmore ES, Poligone B. Evaluation of the relationship between alopecia areata and viral antigen exposure. Am J Clin Dermatol. 2018;19(1):119‐126. [DOI] [PubMed] [Google Scholar]
- 5. Rossi A, Cantisani C, Carlesimo M, et al. Serum concentrations of IL‐2, IL‐6, IL‐12 and TNF‐α in patients with alopecia areata. Int J Immunopathol Pharmacol. 2012;25(3):781‐788. [DOI] [PubMed] [Google Scholar]
- 6. Huang WY, Huang YC, Huang KS, et al. Stress‐induced premature senescence of dermal papilla cells compromises hair follicle epithelial‐mesenchymal interaction. J Dermatol Sci. 2017;86(2):114‐122. [DOI] [PubMed] [Google Scholar]


