Abstract
Background
Diabetes is a risk factor for poor COVID‐19 outcomes, but pediatric patients with type 1 diabetes are poorly represented in current studies.
Methods
T1D Exchange coordinated a US type 1 diabetes COVID‐19 registry. Forty‐six diabetes centers submitted pediatric cases for patients with laboratory confirmed COVID‐19. Associations between clinical factors and hospitalization were tested with Fisher's Exact Test. Logistic regression was used to calculate odds ratios for hospitalization.
Results
Data from 266 patients with previously established type 1 diabetes aged <19 years with COVID‐19 were reported. Diabetic ketoacidosis (DKA) was the most common adverse outcome (n = 44, 72% of hospitalized patients). There were four hospitalizations for severe hypoglycemia, three hospitalizations requiring respiratory support (one of whom was intubated and mechanically ventilated), one case of multisystem inflammatory syndrome in children, and 10 patients who were hospitalized for reasons unrelated to COVID‐19 or diabetes. Hospitalized patients (n = 61) were more likely than nonhospitalized patients (n = 205) to have minority race/ethnicity (67% vs 39%, P < 0.001), public insurance (64% vs 41%, P < 0.001), higher A1c (11% [97 mmol/mol] vs 8.2% [66 mmol/mol], P < 0.001), and lower insulin pump and lower continuous glucose monitoring use (26% vs 54%, P < 0.001; 39% vs 75%, P < 0.001). Age and gender were not associated with risk of hospitalization. Higher A1c was significantly associated with hospitalization, with an odds ratio of 1.56 (1.34‐1.84) after adjusting for age, gender, insurance, and race/ethnicity.
Conclusions
Higher A1c remained the only predictor for hospitalization with COVID‐19. Diabetic ketoacidosis is the primary concern among this group.
Keywords: COVID‐19, DKA, pediatric, type 1 diabetes
Highlights Outcomes for children with type 1 diabetes who experience COVID‐19 are currently unknown. We report 266 cases from US diabetes clinics of children with type 1 diabetes who had COVID‐19. Diabetic ketoacidosis was the major adverse outcome, and it was associated with higher A1c.
摘要
背景
糖尿病是新型冠状病毒肺炎预后差的危险因素, 但在目前的研究中, 1型糖尿病儿童患者的情况鲜有报道。
方法
T1D交流处协调了一个美国新型冠状病毒肺炎1型糖尿病的临床注册研究。46个糖尿病中心提交了实验室确认的新型冠状病毒肺炎患者的儿童病例。采用Fisher‘s 确切检验分析临床因素与住院时间的相关性。用Logistic回归计算住院的优势比。
结果
报道了266例既往确诊的<19岁的1型糖尿病患者患有新型冠状病毒肺炎的资料。糖尿病酮症酸中毒(DKA)是最常见的不良结局(n=44, 72%的住院患者)。因严重低血糖住院4例, 需要呼吸支持住院3例(其中1例经气管插管机械通气), 1例儿童多系统炎症综合征, 10例因与新型冠状病毒肺炎或糖尿病无关的原因住院。住院患者(n=61)与非住院患者(n=205)相比:少数族裔占比更高(67%比39%, P<0.001)、公共保险覆盖率更高(64%比41%, P<0.001)、糖化血红蛋白更高(11%[97 mmol/mol]比8.2%[66 mmol/mol], P<0.001)、胰岛素泵以及连续血糖监测系统使用率更低(26%比54%, P<0.001, 39%比75%, P<0.001)。年龄和性别与住院风险无关。较高的糖化血红蛋白与住院显著相关, 在校正了年龄、性别、保险和种族后, 优势比为1.56(1.34‐1.84)。
结论
较高的糖化血红蛋白仍然是新型冠状病毒肺炎住院的唯一预测因素。糖尿病酮症酸中毒是这一群体的首要问题。
Keywords: 新型冠状病毒肺炎, DKA, 儿科, 1型糖尿病
1. INTRODUCTION
In people with coronavirus disease 2019 (COVID‐19), adults with type 2 diabetes mellitus are at increased risk for severe illness and increased mortality. 1 , 2 , 3 , 4 , 5 However, there are fewer reports from populations of people with type 1 diabetes, and very little is known about these risks among children with type 1 diabetes. 6 , 7 , 8 , 9 , 10 Severe illness and death from COVID‐19 appear to be very rare among people with type 1 diabetes under age 55.
Adults with type 1 diabetes are more likely than those without diabetes to have infections of the urinary tract, skin and mucous membranes, and lower respiratory tract, as well as serious bacterial infections requiring hospitalization. 11 , 12 However, children and adolescents with type 1 diabetes whose A1c is in target range do not appear to have increased risk for contracting viral infections. 13 Healthy children are susceptible to COVID‐19, but usually have a milder course than adults. 14 , 15 , 16 Several underlying conditions are associated with poorer outcomes in children with COVID‐19, but little evidence suggests diabetes as a unique risk factor for COVID‐19 severity or mortality in this population.
Infectious diseases in people with type 1 diabetes often trigger diabetic ketoacidosis (DKA), and COVID‐19 may therefore lead to poorer outcomes in children and adolescents with type 1 diabetes than in their peers without diabetes. Higher A1c and minority race and ethnicity are independently associated with increased rates of DKA in pediatric patients with type 1 diabetes, indicating that these subgroups may be particularly vulnerable during the current pandemic. 17 , 18 Moreover, the relatively high rates of COVID‐19 in racial and ethnic minority communities may further increase their risk of poor outcomes. 19
To improve understanding of the impact of COVID‐19 on people with type 1 diabetes, the T1D Exchange Quality Improvement Collaborative, comprising 30 pediatric and adult clinical sites in the United States, partnered with additional endocrinology clinics in the United States to form a registry to collect data on the symptoms and outcomes of people with type 1 diabetes and COVID‐19 across the lifespan. In this report, we describe characteristics of and outcomes of COVID‐19 in children and adolescents with type 1 diabetes and whether higher A1c, minority race/ethnicity, older age, and male gender increase the risk of severe disease.
2. METHODS
In April 2020, T1D Exchange invited 81 pediatric and adult diabetes centers from the T1D Exchange Quality Improvement Collaborative, 20 the T1D Exchange Clinic Registry, 21 and throughout the US to join a COVID‐19 clinical registry for patients with type 1 diabetes. Fifty‐six diabetes centers submitted cases, 52 of whom submitted cases for at least one patient <19 years of age. Investigators were asked to identify all known COVID‐19 cases among their clinic population occurring on or after 1 March 2020. Using online survey software (Qualtrics.com), diabetes centers submitted de‐identified data for patients who have type 1 diabetes and had tested positive for SARS‐CoV‐2 infection. Patients with newly diagnosed type 1 diabetes were excluded from this analysis and reported separately. 22
The 33‐question survey captures patient demographics, duration of type 1 diabetes, presenting symptoms, diabetes management and device use, comorbidities, relevant behaviors, highest level of care, duration of COVID‐19 symptoms, and clinical outcomes in patients with positive SARS‐CoV‐2 PCR tests. Data presented here were collected between 9 April 2020 and 15 January 2021. The registry remains open.
Data fields were coded and categorized. Insurance types were classified as public, private, uninsured, or unknown. Patients who were hospitalized on inpatient floors or admitted to intensive care were grouped as hospitalized.
Quantitative data were represented as mean (SD) or median [interquartile range]. Categorical data were represented as the percentage of patients. Summary statistics, including frequency and percentage for categorical variables, were calculated for all patient‐related and clinical characteristics.
We used the Fisher's exact test to assess associations between hospitalization and demographics, A1c, diabetes duration, symptoms, and adverse outcomes. Univariate and multivariate logistic regression were used to calculate odds ratios (OR) for hospitalization associated with A1c, age, insurance, continuous glucose monitor (CGM) use, and duration of diabetes. All tests were two‐sided with type 1 error set at 5%. Fisher's exact test and logistic regression were performed using statistical software R version 3.6.2 (R: A Language and Environment for Statistical Computing, R Core Team, R Foundation for Statistical computing, Vienna, Austria, 2020, https://www.R-project.org).
This non‐interventional, cross‐sectional study was sponsored and coordinated by T1D Exchange. T1D Exchange did not collect data belonging to any of the 18 categories defined as protected health information. 23 This study was performed in accordance with the ethical standards laid down by the Declaration of Helsinki. It was reviewed and approved as non‐human subjects’ research by the Western Institutional Review Board (Approval #1‐1290115‐1), and therefore consent or waiver of consent was not required. All participating centers also obtained local institutional review board approvals as appropriate.
3. RESULTS
There were 266 patients <19 years of age with type 1 diabetes who had confirmed COVID‐19 reported to the registry. Patient characteristics and a comparison of those who were hospitalized to those who were not are shown in Table 1. Race/ethnicity was different between the groups (P < 0.001), with fewer non‐Hispanic White (33% vs 61%) and more non‐Hispanic Black (34% vs 13%) patients among those hospitalized. Hospitalized patients were more likely than non‐hospitalized patients to have public insurance (64% vs 41%, P < 0.001), higher A1c (11% [97 mmol/mol] vs 8.2% [66 mmol/mol], P < 0.001), lower insulin pump use (26% vs 54%, P < 0.001), and lower continuous glucose monitor use (39% vs 75%, P < 0.001). Age, gender, and diabetes duration were similar between patients who were hospitalized compared to those who were not.
TABLE 1.
Characteristics of hospitalized vs non‐hospitalized among patients with laboratory confirmed COVID‐19 with previously established type 1 diabetes
| Hospitalized N = 61 | Non‐Hospitalized N = 205 | P value | |
|---|---|---|---|
| Age | 0.91 | ||
| 0 to 5 | 3 (5) | 7 (3) | |
| 6 to 10 | 6 (10) | 19 (9) | |
| 11 to 15 | 29 (48) | 97 (47) | |
| 16 to 18 | 23 (38) | 82 (40) | |
| Female | 33 (54) | 100 (49) | 0.55 |
| Race/Ethnicity | <0.001 | ||
| Non‐Hispanic White | 20 (33) | 126 (61) | |
| Non‐Hispanic Black | 21 (34) | 26 (13) | |
| Hispanic | 17 (28) | 46 (22) | |
| Other | 3 (5) | 7 (3) | |
| Insurance Type | <0.001 | ||
| Public | 39 (64) | 84 (41) | |
| Private | 19 (31) | 119 (58) | |
| Uninsured | 3 (5) | 2 (1) | |
| A1c % | 11 [3.6] | 8.2 [2.3] | <0.001 |
| A1c mmol/mol | 97 [16] | 66 [1.6] | <0.001 |
| Last A1c Group | <0.001 | ||
| <7% (<53 mmol/mol) | 0 (0) | 35 (17) | |
| 7%‐9% (53‐65 mmol/mol) | 11 (18) | 106 (52) | |
| >9% (>65 mmol/mol) | 50 (82) | 64 (31) | |
| Duration of T1D | 1 | ||
| Less than 1 year | 7 (11) | 23 (11) | |
| 1‐5 years | 32 (52) | 107 (52) | |
| More than 5 years | 22 (36) | 75 (37) | |
| Insulin Pump Use | 16 (26) | 110 (54) | <0.001 |
| CGM Use | 24 (39) | 153 (75) | <0.001 |
| Most prevalent symptoms | |||
| High Blood Sugar | 29 (48) | 58 (28) | 0.007 |
| Elevated Temperature | 17 (28) | 61 (30) | 0.87 |
| Dry Cough | 16 (26) | 46 (22) | 0.6 |
| Nausea | 20 (33) | 12 (6) | <0.001 |
| Excess Fatigue | 12 (20) | 30 (15) | 0.42 |
| Body aches/Headaches | 14 (23) | 58 (28) | 0.51 |
| Vomiting | 30 (49) | 6 (3) | <0.001 |
| Shortness of breath | 10 (16) | 10 (5) | 0.005 |
| Adverse Acute T1D Outcome | <0.001 | ||
| Death | 0 (0) | 0 (0) | |
| DKA | 44 (72) | 0 (0) | |
| Severe Hypoglycemia | 4 (7) | 0 (0) | |
| Respiratory support a | 3 (5) | 0 (0) | |
| None b | 10 (16) | 205 (100) | |
Note: N (%) or median [IQR].
Abbreviations: CGM, continuous glucose monitoring; DKA, diabetic ketoacidosis; IQR, interquartile range; T1D, type 1 diabetes. Other Race include 3 Asians, 2 people with more than one race.
Two patients were managed with nasal cannula for respiratory support while one of the patients was mechanically intubated.
The 10 hospitalized patients with no adverse acute T1D outcome were hospitalized for other non‐COVID19 or diabetes reasons for example prescheduled urologic procedures, salmonella enteritis, suicidal ideation etc.
Hyperglycemia, shortness of breath, nausea, and vomiting were more common among hospitalized children compared to those who were not hospitalized (Table 1). The frequencies of other symptoms did not differ between these two groups. The most common adverse outcome was DKA, which occurred in 72% of hospitalized patients and 16.5% of all patients. Most recent HbA1c was >9% (>65 mmol/mol; median 11%, 97 mmol/mol) in 38 of the 44 patients (86%) with DKA. Four patients were hospitalized with severe hypoglycemia, and three received respiratory support, two of whom received oxygen by nasal cannula and one who underwent intubation with mechanical ventilation. There were no deaths.
The patient who underwent intubation and ventilation was a 15 year‐old, non‐Hispanic White male with most recent A1c of 8.9% (74 mmol/mol). He presented to the emergency department with tachypnea. An echocardiogram showed moderate to severely depressed function. He progressed to severe hypoxic respiratory failure, cardiogenic shock, and wide complex arrhythmia requiring intubation and extracorporeal membrane oxygenation, which was weaned after 6 days. As he continued to improve, he was transferred to a rehabilitation facility and has recovered sufficiently to be discharged home.
One patient was diagnosed with the COVID‐19 associated multi‐system inflammatory syndrome of childhood (MIS‐C). 24 This 13 year‐old non‐Hispanic White male with a recent A1c of 11.1% (98 mmol/mol), presented to the emergency department with fever, nausea, vomiting, abdominal pain, and difficulty breathing. He was admitted to the intensive care unit and received oxygen via nasal cannula. He also recovered and was discharged home.
Ten of the 61 hospitalized patients were diagnosed with COVID‐19 during admissions that were unrelated to COVID‐19 or type 1 diabetes. These included admissions for such reasons as scheduled urologic procedures, salmonella enteritis, and suicidal ideation.
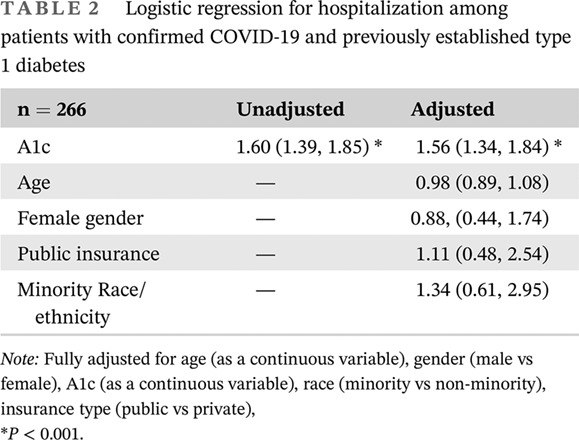
Table 2 shows the logistic regression analysis for hospitalization. In the multivariable model, the odds of hospitalization significantly increased with higher A1c, but not with age, gender, insurance, or race and ethnicity.
TABLE 2.
Logistic regression for hospitalization among patients with confirmed COVID‐19 and previously established type 1 diabetes
| n = 266 | Unadjusted | Adjusted |
|---|---|---|
| A1c | 1.60 (1.39, 1.85) * | 1.56 (1.34, 1.84) * |
| Age | — | 0.98 (0.89, 1.08) |
| Female gender | — | 0.88, (0.44, 1.74) |
| Public insurance | — | 1.11 (0.48, 2.54) |
| Minority Race/ethnicity | — | 1.34 (0.61, 2.95) |
Note: Fully adjusted for age (as a continuous variable), gender (male vs female), A1c (as a continuous variable), race (minority vs non‐minority), insurance type (public vs private),
P < 0.001.
4. DISCUSSION
This national cohort is the first report of the outcomes of pediatric patients with type 1 diabetes and COVID‐19 from the United States, representing 266 known cases from 46 diabetes centers across the US, a country with a disproportionately high number of COVID‐19 cases per capita compared with other high‐income nations. This novel registry was developed quickly within existing T1D Exchange infrastructure and incorporated participation from new partners.
Diabetic ketoacidosis was the most common adverse outcome, accounting for 72% of patients with type 1 diabetes and concurrent COVID‐19.
Thirty‐eight of the 44 patients (86%) who were hospitalized with DKA had an A1c level greater than 9% (75 mmol/mol). This underscores the strong association between elevated A1c and DKA, which is consistent with studies before the pandemic showing a strong positive correlation between progressively higher A1c levels and a steep increase in the frequency of DKA. 17 , 18 Over 30% of children with type 1 diabetes in the United States have A1c levels above 9%, 17 which may make the COVID‐19 pandemic particularly dangerous for this sub‐population. This is an especially important time for healthcare teams to emphasize diabetes self‐management and sick day practices and for them to target high risk children and families for additional support to improve glycemic outcomes and reduce the risk of DKA.
Hyperglycemia, reported in a third of cases, can increase the risk for hypercoagulability. Acknowledging the link between COVID‐19 and hypercoagulability, 25 it is vital for clinicians caring for children with type 1 diabetes and apparent COVID‐19 to combat osmotic diuresis through oral hydration, to minimize glycemic excursions, and to have a low threshold for increasing acuity of care. The symptoms reported among hospitalized patients were consistent with the high rate of DKA among that group.
There were more than twice as many Non‐Hispanic Black patients who were hospitalized vs not hospitalized. This is consistent with our earlier report which includes adults. 26 This finding is also consistent with other studies showing that COVID‐19 has been more common and more severe among racial/ethnic minority populations. 27 , 28 Further, racial/ethnic minority patients with type 1 diabetes have been reported to have higher A1c levels, 18 , 29 which was a risk factor for hospitalization in our population. Racial and ethnic minority youth with type 1 diabetes are clearly at higher risk than their Non‐Hispanic White counterparts for severe outcomes with COVID‐19. These findings underscore the need for novel clinical approaches to help patients improve baseline glycemic management, especially for patients who identify as a member of a minority group.
Children who were hospitalized were also more likely to have public insurance and less likely to use insulin pumps and continuous glucose monitors, though these variables did not predict hospitalization in the regression models. Large registry studies show improved metabolic control and lower frequency of DKA in pediatric patients with type 1 diabetes who use CGM. 30 Among our patients with COVID‐19, only 65 of 123 (53%) of those with public insurance were using CGM, compared to 113 of 138 (82%) of those with private insurance. This is also consistent with registry studies that show that racial/ethnic minority patients use CGM at about half the rate that non‐minority patients do, highlighting the need to improve access for this important technology, including universal coverage of CGM by state insurance programs. 31
Strengths of this study include the geographic distribution of a diverse group of children with type 1 diabetes and participation from a large number of diabetes centers. To our knowledge, it is the largest report of pediatric patients with type 1 diabetes and confirmed COVID‐19. We also demonstrate how an existing network can quickly be leveraged to provide insight about new problems. Even as the pandemic evolved and data collection continued, this quality improvement network has kept contributing centers informed through serial videoconference meetings.
Our study has several limitations. It is cross‐sectional study and cannot demonstrate causality. We did not collect information on the timing of the COVID‐19 presentation and confirmation of SARS‐CoV‐2. Importantly, case ascertainment relies on several factors. Testing availability was poor in the US before summer, and many cases may have gone undiscovered. Although investigators actively sought out COVID‐19 cases in their local patient populations, we acknowledge that not all SARS‐CoV‐2 infections were discovered: around 20% of patients may be asymptomatic, 32 those with mild or no symptoms may not have been tested, and case reporting depended on the diabetes providers receiving notification of a positive test. Therefore, this report overestimates the proportion of all COVID‐19 cases in children with type 1 diabetes who were hospitalized. Nonetheless, comparison of the hospitalized to the nonhospitalized known COVID‐19 cases reveals DKA as the most common poor outcome and elevated A1c as the clearest risk factor. Severe outcomes were relatively rare and very unlikely to have gone unreported.
We did not collect diabetes antibody data, so it is possible that some patients had other forms of diabetes. We also did not collect the background demographics from each clinic, so the racial/ethnic proportions may not be generalizable. In addition to the data presented, we also attempted to collect smoking status and whether the patient had received an influenza vaccine, but these were reported as unknown in nearly half of patients. Improved capture of these items in medical records could improve clinical care and registry insights.
5. CONCLUSIONS
As early reports identified diabetes as a risk factor for increased morbidity and mortality with COVID‐19, the findings from this surveillance study should provide measured reassurance for families of children with type 1 diabetes as well as pediatric endocrinologists and their care teams. Most children with type 1 diabetes and COVID‐19 were cared for at home without adverse outcomes, less than 2% needed respiratory support, and there were no deaths reported. Hospitalization in children with type 1 diabetes and COVID‐19 was usually in the setting of DKA. The major risk factor remained the same in our population as has been demonstrated in registry studies before the pandemic: elevated A1c levels.
Our data reveal a disproportionate rate of hospitalization and DKA among racial and ethnic minority groups, children who were publicly insured, and those with higher A1c. It is essential to find pathways for the most vulnerable patients to have adequate, equitable access to medical care via in person and telehealth services, to obtain and successfully use diabetes technology, and to optimize sick day management. Quality improvement networks are viable platforms for generating this type of knowledge.
DISCLOSURE
Robert Rapaport is senior editor for Journal of Diabetes. The remaining authors have no disclosures.
ACKNOWLEDGEMENTS
The Leona M. and Harry B. Helmsley Charitable Trust funds the T1D Exchange QI Collaborative. The T1D Exchange received partial financial support for this study from Abbott Diabetes, Dexcom, Medtronic, Insulet Corporation, JDRF, Eli Lilly, and Tandem Diabetes Care. None of the sponsors were involved in initiating, designing, or preparing the manuscript for this study.
Alonso GT, Ebekozien O, Gallagher MP, et al. Diabetic ketoacidosis drives COVID‐19 related hospitalizations in children with type 1 diabetes. Journal of Diabetes. 2021;13:681–687. 10.1111/1753-0407.13184
Funding information Abbott Diabetes; Dexcom; Eli Lilly and Company; Insulet Corporation; JDRF; Leona M. and Harry B. Helmsley Charitable Trust; Medtronic; Tandem Diabetes Care
REFERENCES
- 1. Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID‐19 pneumonia ‐ a systematic review, meta‐analysis, and meta‐regression. Diabetes Metab Syndr Clin Res Rev. 2020;14(4):395‐403. 10.1016/j.dsx.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kumar A, Arora A, Sharma P, et al. Is diabetes mellitus associated with mortality and severity of COVID‐19? A meta‐analysis. Diabetes Metab Syndr Clin Res Rev. 2020;14(4):535‐545. 10.1016/j.dsx.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tadic M, Cuspidi C, Sala C. COVID‐19 and diabetes: is there enough evidence? J Clin Hypertens. 2020;22:943‐948. 10.1111/jch.13912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guan W‐J, Ni Z‐Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fadini GP, Morieri ML, Longato E, Avogaro A. Prevalence and impact of diabetes among people infected with SARS‐CoV‐2. J Endocrinol Invest. 2020;43(6):867‐869. 10.1007/s40618-020-01236-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barron E, Bakhai C, Kar P, et al. Type 1 and type 2 diabetes and COVID‐19 related mortality in England: a whole population study. 2020. [DOI] [PMC free article] [PubMed]
- 7. Cariou B, Wargny M, Pichelin M, et al. Phenotypic characteristics and prognosis of inpatients with COVID‐19 and diabetes: the CORONADO study. Diabetologia. 2020;63:1500‐1515. 10.1007/s00125-020-05180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wargny M, Gourdy P, Ludwig L, et al. Type 1 diabetes in people hospitalized for COVID‐19: new insights from the CORONADO study. Diabetes Care. 2020;43(11):e174‐e177. 10.2337/dc20-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gregory JM, Slaughter JC, Duffus SH, et al. COVID‐19 severity is tripled in the diabetes community: a prospective analysis of the Pandemic's impact in type 1 and type 2 diabetes. Diabetes Care. 2020;44(2):526‐532. 10.2337/dc20-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cardona‐Hernandez R, Cherubini V, Iafusco D, Schiaffini R, Luo X, Maahs DM. Children and youth with diabetes are not at increased risk for hospitalization due to COVID‐19. Pediatr Diabetes. 2020;22(2):202‐206. 10.1111/pedi.13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Muller LMAJ, Gorter KJ, Hak E, et al. Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin Infect Dis. 2005;41(3):281‐288. 10.1086/431587. [DOI] [PubMed] [Google Scholar]
- 12. Shah BR, Hux JE. Quantifying the risk of infectious diseases for people with diabetes. Diabetes Care. 2003;26(2):510‐513. 10.2337/diacare.26.2.510. [DOI] [PubMed] [Google Scholar]
- 13. Laffel LM, Limbert C, Phelan H, Virmani A, Wood J, Hofer SE. ISPAD clinical practice consensus guidelines 2018: sick day management in children and adolescents with diabetes. Pediatr Diabetes. 2018;19:193‐204. 10.1111/pedi.12741. [DOI] [PubMed] [Google Scholar]
- 14. CDCMMWR . Coronavirus disease 2019 in children—United States, February 12–April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:422‐426. 10.15585/mmwr.mm6914e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lu X, Zhang L, Du H, et al. SARS‐CoV‐2 infection in children. N Engl J Med. 2020;382(17):1663‐1665. 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID‐19 among children in China. Pediatrics. 2020;145(6):e20200702. 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 17. Cengiz E, Xing D, Wong JC, et al. Severe hypoglycemia and diabetic ketoacidosis among youth with type 1 diabetes in the T1D exchange clinic registry. Pediatr Diabetes. 2013;14(6):447‐454. 10.1111/pedi.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kahkoska AR, Shay CM, Crandell J, et al. Association of race and ethnicity with glycemic control and hemoglobin A1c levels in youth with type 1 diabetes. JAMA Netw Open. 2018;1(5):e181851. 10.1001/jamanetworkopen.2018.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tai DBG, Shah A, Doubeni CA, Sia IG, Wieland ML. The disproportionate impact of COVID‐19 on racial and ethnic minorities in the United States. Clin Infect Dis. 2020;72(4):703‐706. 10.1093/cid/ciaa815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alonso GT, Corathers S, Shah A, et al. Establishment of the T1D exchange quality improvement collaborative (T1DX‐QI). Clin Diabetes. 2020;38(2):141‐151. 10.2337/cd19-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Beck RW, Tamborlane WV, Bergenstal RM, et al. The T1D exchange clinic registry. J Clin Endocrinol Metab. 2012;97(12):4383‐4389. 10.1210/jc.2012-1561. [DOI] [PubMed] [Google Scholar]
- 22. Beliard K, Ebekozien O, Demeterco‐Berggren C, et al. Increased DKA at presentation among newly diagnosed type 1 diabetes patients with or without COVID‐19: data from a multi‐site surveillance registry. J Diabetes. 2020;13(3):270‐272. 10.1111/1753-0407.13141. [DOI] [PubMed] [Google Scholar]
- 23. Office for Civil Rights . Summary of the HIPAA privacy rule. HHS.Gov. 2008. https://www.hhs.gov/hipaa/for-professionals/privacy/laws-regulations/index.html. Accessed 19 June, 2020.
- 24. Capone CA, Subramony A, Sweberg T, et al. Characteristics, cardiac involvement, and outcomes of multisystem inflammatory disease of childhood (MIS‐C) associated with SARS‐CoV‐2 infection. J Pediatr. 2020;224:141‐145. 10.1016/j.jpeds.2020.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fei Y, Tang N, Liu H, Cao W. Coagulation dysfunction: a hallmark in COVID‐19. Arch Pathol Lab Med. 2020;44(10):1223‐1229. 10.5858/arpa.2020-0324-SA. [DOI] [PubMed] [Google Scholar]
- 26. Ebekozien O, Agarwal S, Noor N, et al. Inequities in diabetic ketoacidosis among patients with type 1 diabetes and COVID‐19: data from 52 US clinical centers. J Clin Endocrinol Metab. 2021;106(4):e1755‐e1762. 10.1210/clinem/dgaa920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mahajan UV, Larkins‐Pettigrew M. Racial demographics and COVID‐19 confirmed cases and deaths: a correlational analysis of 2886 US counties. J Public Health. 2020;42(3):445‐447. 10.1093/pubmed/fdaa070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Price‐Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among black patients and white patients with Covid‐19. N Engl J Med. 2020;382(26):2534‐2543. 10.1056/NEJMsa2011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Miller KM, Foster NC, Beck RW, et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D exchange clinic registry. Diabetes Care. 2015;38(6):971‐978. 10.2337/dc15-0078. [DOI] [PubMed] [Google Scholar]
- 30. Tauschmann M, Hermann JM, Freiberg C, et al. Reduction in diabetic ketoacidosis and severe hypoglycemia in pediatric type 1 diabetes during the first year of continuous glucose monitoring: a multicenter analysis of 3,553 subjects from the DPV registry. Diabetes Care. 2020;43(3):e40‐e42. 10.2337/dc19-1358. [DOI] [PubMed] [Google Scholar]
- 31. DeSalvo DJ, Miller KM, Hermann JM, et al. Continuous glucose monitoring and glycemic control among youth with type 1 diabetes: international comparison from the T1D exchange and DPV initiative. Pediatr Diabetes. 2018;19(7):1271‐1275. 10.1111/pedi.12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Buitrago‐Garcia D, Egli‐Gany D, Counotte MJ, et al. Occurrence and transmission potential of asymptomatic and presymptomatic SARS‐CoV‐2 infections: a living systematic review and meta‐analysis. PLoS Med. 2020;17(9):e1003346. 10.1371/journal.pmed.1003346. [DOI] [PMC free article] [PubMed] [Google Scholar]


