Abstract
Emerging data are linking coronavirus disease 2019 (COVID‐19) with an increased risk of developing new‐onset diabetes. The gut has been so far out of the frame of the discussion on the pathophysiology of COVID‐19‐induced diabetes, with the pancreas, liver, and adipose tissue being under the spotlight of medical research. Sodium‐glucose co‐transporters (SGLT) 1 represent important regulators of glucose absorption, expressed in the small intestine where they mediate almost all sodium‐dependent glucose uptake. Similar to what happens in diabetes and other viral infections, SGLT1 upregulation could result in increased intestinal glucose absorption and subsequently promote the development of hyperglycaemia in COVID‐19. Considering the above, the question whether dual SGLT (1 and 2) inhibition could contribute to improved outcomes in such cases sounds challenging, deserving further evaluation. Future studies need to clarify whether putative benefits of dual SGLT inhibition in COVID‐19 outweigh potential risks, particularly with respect to drug‐induced euglycaemic diabetic ketoacidosis, gastrointestinal side effects, and compromised host response to pathogens.
Keywords: COVID‐19, diabetes, SGLT1, SGLT2
1. BACKGROUND
Emerging data are linking coronavirus disease 2019 (COVID‐19) with an increased risk of developing new‐onset diabetes and severe diabetic complications, including diabetic ketoacidosis (DKA). Whether this type of diabetes resembles type 1 (T1D) type 2 (T2D) or comprises a new form of the disorder remains vague. In a recently published observational study from Scotland, McGurnaghan et al. 1 demonstrated substantially increased risk of fatal or critical care unit‐treated COVID‐19 in people with both type of diabetes compared with the background population. Among patients with diabetes and after adjustment for age, sex, diabetes type, and duration, those with poor glycaemic control, presence of complications (retinopathy and renal disease), previous history of hospitalization due to hypoglycaemia or DKA, and being on treatment with multiple antidiabetes agents and other medication were found to be at greater risk for severe COVID‐19 than individuals who didn't have the above‐mentioned characteristics. Obesity, a major risk factor for the development of T2D, has been also linked to poor outcomes, including severe pneumonia, intensive care unit admission, and mortality during COVID‐19, probably related to increased viral entry into cells and defects in immune response. 2 COVID‐19‐related DKA seems to manifest peculiar—still interesting—characteristics, including the occurrence in people without a previous diagnosis of diabetes, or in those with non‐insulin‐dependent diabetes and adequate metabolic control prior to infection. In addition, hyperglycaemia without diabetes and new‐onset diabetes have been both linked to a poorer prognosis in the context of COVID‐19. 3
The development of hyperglycaemia in COVID‐19, either being genuinely new or the unmasking of a previously existing occult diabetes, is the result of a pathophysiological interplay between impaired insulin secretion and action. Up to now, putative explanations for the underlying pathophysiological mechanisms through which the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) can induce severe hyperglycaemia and subsequent DKA have mainly focused on two directions: first, a direct viral attack against beta‐cells and, second, an exacerbation of insulin resistance (IR), as a result of systemic inflammation. 4
In this paper, we discuss a novel hypothesis according to which, intestinal sodium‐glucose transport proteins might be implicated in the pathogenesis of COVID‐19‐induced diabetes and could represent a therapeutic target for the disorder in the future.
2. HYPOTHESIS
The angiotensin‐converting enzyme 2 (ACE2), which is the entry point for the virus into the cells, has been shown to be expressed in the pancreatic tissue. In line with what is already known from studies conducted with other coronaviruses, it has been demonstrated that SARS‐CoV‐2 exerts cytotoxic effects that cause islet cell injury and impair insulin production. 5 Moreover, downregulation of ACE2 following the endocytosis of the virus complex results in increased angiotensin II concentrations, which are known to impede insulin secretion. 6 , 7 Similar to what happens with other viral infections, an immune‐mediated beta‐cell destruction triggered by the SARS‐CoV‐2 infection has been also suggested. 4 , 7 In a parallel way, COVID‐19‐related cytokine storm leading to overproduction of proinflammatory molecules, predominantly interleukin (IL)‐6 and tumour necrosis factor‐α (TNF‐α), further aggravates beta‐cell dysfunction and induces IR.
Over the past few years, the importance of the gut in the regulation of glucose homeostasis has been greatly recognized by identifying its multiple roles in modulating nutrient absorption, satiety, systemic and adipose tissue inflammation and insulin secretion and action. Although patients with COVID‐19 mainly present with respiratory symptoms, a recent meta‐analysis proved that approximately 12% of SARS‐CoV‐2‐infected individuals will manifest gastrointestinal (GI) symptoms, with diarrhoea, nausea, or vomiting being the most commonly reported among them. 8 While the exact mechanisms through which the virus attacks the GI tract are still under investigation, it is believed that following the infection of the lung tissue by SARS‐CoV‐2, effector CD4+ T‐cells move to the small intestine through the gut‐lung axis and cause intestinal immune damage. However, the gut has been so far out of the frame of the discussion on the pathophysiology of COVID‐19‐induced diabetes, with the pancreas, liver, and adipose tissue being under the spotlight of medical research. Only recently, alterations in the gut microbiome have been proposed to contribute to the pathogenesis, severity, and disease course of COVID‐19, potentially leading to an upregulated systemic inflammatory state, negatively affecting this way insulin production and sensitivity. 7
Sodium‐glucose co‐transporters (SGLT) 1 and 2 represent important regulators of glucose uptake across apical cell membranes. SGLT1 are particularly expressed in the small intestine where they mediate almost all sodium‐dependent glucose uptake, whereas they are also located in other tissues, including heart and kidneys. High expression of ACE2 in mucosal cells of the intestine renders the organ vulnerable to SARS‐CoV‐2 invasion and replication. Previous research has highlighted that ACE2‐mediated downregulation of SGLT1 in the intestinal epithelium has the potential to improve glycaemic status in an experimental model of T1D. 9 Inversely, ACE2‐mediated SGLT1 upregulation occurs in diabetes and diabetes‐related diseases, which results in increased intestinal glucose absorption and subsequently to the development of hyperglycemia. 6 In support of the perspective that SGLT1 might be implicated in the metabolic disarrangement observed in some patients with COVID‐19, Dai et al. 10 have demonstrated that infection with transmissible gastroenteritis virus—another coronavirus—results in augmented glucose uptake through enhanced SGLT1 expression in porcine intestinal columnar epithelial cells.
On the other hand, recent studies have suggested an important role of SGLT1 activation in the host response against specific pathogens. Sharma et al. 11 have showed that genetic knockout of SGLT1 in a murine model of Listeria infection increased bacterial load in liver, spleen, kidney, and lung and significantly upregulated the hepatic expression of IL‐12 a and TNF‐α, resulting in the death of SGLT1‐deficient mice, in contrast with the wild‐type animals which survived the infection. In a similar way, SGLT1 inhibition with phlorizin was correlated with a decreased survival rate in a rat model of bronchial inflammation and sepsis. 12
SGLT activation has recently been a key therapeutic target in T2D, where excessive glucose reabsorption by the kidneys is observed. 13 Available drugs mainly inhibit SGLT2, located in the early segment of the proximal renal tube, thus leading to reduced renal glucose reabsorption. 14 However, specific agents within the class, namely, sotagliflozin and canagliflozin, present only 20‐ and 250‐fold higher inhibitory activity for SGLT2 compared with SGLT1, respectively, and are considered “dual inhibitors,” manifesting their glucose‐lowering actions by reducing—apart from renal—intestinal glucose absorption, as well 15 (Table 1). Interestingly, dual inhibition has been questionably linked to a greater risk of adverse events, including lower extremity amputations and euglycaemic DKA, compared with the more selective SGLT2 inhibitors. 14 , 15 Although the exact mechanisms that could explain these differences are still debatable, they may be related to the volume depletion‐mediated increased ketone body reabsorption in the distal tubules, which, in turn, could amplify the magnitude of ketonemia already observed with SGLT2 inhibition. 16 GI adverse events, such as osmotic diarrhoea, are also more common with dual inhibitors, attributable to their effects on the brush border of the small intestine. 14 , 15 , 16
TABLE 1.
Pharmacological characteristics of different SGLT inhibitors
| Drug | SGLT2 over SGLT1 selectivity | Site of action | Glucose‐lowering action by | HbA1c reduction (%) a | Anti‐inflammatory potency | Effects on heart failure and kidney disease outcomes | |
|---|---|---|---|---|---|---|---|
| SGLT2 inhibitors | Empagliflozin | 2500‐fold | Almost exclusively kidney | Reducing renal glucose reabsorption | 0.7–0.9 | Similar | Beneficial |
| Ertugliflozin | 2000‐fold | 0.5 | |||||
| Dapagliflozin | 1200‐fold | 0.5–0.7 | |||||
| Dual inhibitors | Canagliflozin | 250‐fold | Mostly in kidney and small intestine | Reducing renal glucose reabsorption and dietary absorption of glucose and galactose | 0.91–1.16 | ||
| Sotagliflozin | 20‐fold | 0.52–0.92 |
Abbreviations: HbA1c, glycated haemoglobin; SGLT, sodium‐glucose co‐transporters.
Data refer to individual and not to head‐to‐head trials.
Relevant to their remarkable cardiorenal protective properties in people with or without diabetes, previous research has highlighted the potential of SGLT2 inhibitors to decrease the concentrations of proinflammatory cytokines, reduce oxidative stress, downregulate sympathetic activity and improve cell autophagy, leading to amelioration of both systemic and adipose‐tissue inflammation. 17 These seem to be class effects and not related either to SGLT1 or SGLT2 inhibition alone. In this context, dapagliflozin, a molecule with a 1200‐fold higher potency for SGLT2 compared with SGLT1, is currently being tested in clinical trials as a potential treatment for acute respiratory COVID‐19 disease, 17 and their results are anticipated in order to clarify whether the class could represent an additional weapon in the therapeutic arsenal against SARS‐CoV‐2. Considering the hypothesis that ACE2‐mediated intestinal SGLT1 dysregulation is implicated in the development of COVID‐19‐related hyperglycaemia, the question whether dual SGLT (1 and 2) inhibition could contribute to improved outcomes in such cases sounds challenging, deserving further evaluation in clinical studies (Figure 1).
FIGURE 1.
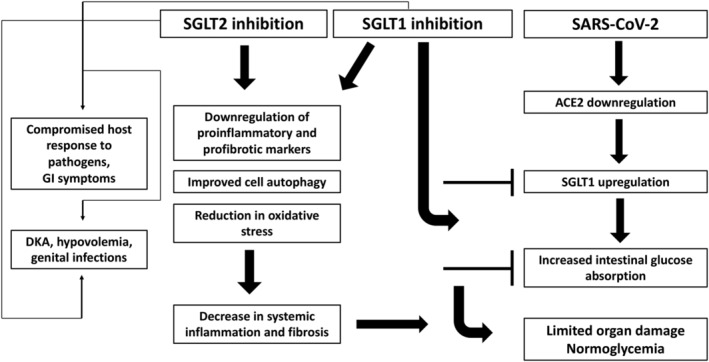
Potential harms and benefits of dual SGLT (1 and 2) inhibition in COVID‐19‐induced diabetes. Abbreviations: ACE2, angiotensin‐converting enzyme 2; COVID‐19, coronavirus disease 2019; DKA, diabetic ketoacidosis; GI, gastrointestinal; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; SGLT, sodium‐glucose co‐transporters
3. CONCLUSION
In conclusion, there is indirect evidence linking ACE2‐mediated SGLT1 upregulation to the development of hyperglycaemia and diabetes‐related adverse outcomes in COVID‐19. Thus, the excessive activation of SGLT1 could be a potential therapeutic target for COVID‐19‐induced diabetes. The testing of the hypothesis seems to be relatively feasible, considering that dual SGLT inhibitors are already widely used in daily clinical practice for the management of T2D.
On the other hand, it should be noted that available data are still very limited. Therefore, the development of animal models for the mechanistic study of the key molecular mechanisms implicated in the SARS‐CoV‐2 binding receptor ACE2 mediated dysregulation of intestinal nutrient transporters is needed. 6 Moreover, the great heterogeneity of the clinical presentation of COVID‐19 implies complex underlying interactions between genetic and environmental components that determine inter‐individual variations in disease severity and possibly in response to various treatments. Clinical studies are hence required to investigate the potential of a tailored approach in the therapeutic management of COVID‐19, but also to clarify whether putative benefits of dual SGLT inhibition outweigh potential risks, particularly with respect to drug‐induced euglycaemic DKA, GI side effects, and compromised host response to pathogens.
COMPETING INTERESTS
T.K. has received honoraria as a speaker from AstraZeneca, Boehringer Ingelheim, and Novo Nordisk and has participated in sponsored studies by Eli‐Lilly. K.K. has received honoraria for lectures/advisory boards and research support from Astra Zeneca, Boehringer Ingelheim, Pharmaserve Lilly, Sanofi‐Aventis, ELPEN, MSD, and Novo Nordisk. Other authors report no conflict of interest.
CONTRIBUTORS
T.K. reviewed the literature and drafted the first version of the manuscript. S.M., P.Z., and K.K. reviewed the literature and edited the manuscript. All authors have read and approved the final version of the manuscript.
ACKNOWLEDGEMENT
The authors received no financial support for the research, authorship, and/or publication of this article.
Koufakis T, Metallidis S, Zebekakis P, Kotsa K. Intestinal SGLT1 as a therapeutic target in COVID‐19‐related diabetes: A “two‐edged sword” hypothesis. Brit Jnl Clinical Pharma. 2021;87(10):3643–3646. 10.1111/bcp.14800
REFERENCES
- 1. McGurnaghan SJ, Weir A, Bishop J, et al. Risks of and risk factors for COVID‐19 disease in people with diabetes: a cohort study of the total population of Scotland. Lancet Diabetes Endocrinol. 2021;9(2):82‐93. 10.1016/S2213-8587(20)30405-8 Epub 2020 Dec 23. PMID: 33357491; PMCID: PMC7832778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hussain A, Mahawar K, Xia Z, Yang W, El‐Hasani S. Obesity and mortality of COVID‐19. Meta‐analysis. Obes Res Clin Pract. 2020;14(4):295‐300. 10.1016/j.orcp.2020.07.002 Epub 2020 Jul 9. Erratum in: Obes Res Clin Pract. 2021 Jan‐Feb;15(1):100. PMID: 32660813; PMCID: PMC7346803. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3. Singh AK, Singh R. Hyperglycemia without diabetes and new‐onset diabetes are both associated with poorer outcomes in COVID‐19. Diabetes Res Clin Pract. 2020;167:108382. 10.1016/j.diabres.2020.108382 Epub 2020 Aug 25. PMID: 32853686; PMCID: PMC7445123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hussain A, Bhowmik B, do Vale Moreira NC. COVID‐19 and diabetes: knowledge in progress. Diabetes Res Clin Pract. 2020;162:108142. 10.1016/j.diabres.2020.108142 Epub 2020 Apr 9. PMID: 32278764; PMCID: PMC7144611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Müller JA, Groß R, Conzelmann C, et al. SARS‐CoV‐2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat Metab. 2021;1‐17. 10.1038/s42255-021-00347-1 Epub ahead of print. PMID: 33536639. [DOI] [PubMed] [Google Scholar]
- 6. Kumar A, Faiq MA, Pareek V, et al. Relevance of SARS‐CoV‐2 related factors ACE2 and TMPRSS2 expressions in gastrointestinal tissue with pathogenesis of digestive symptoms, diabetes‐associated mortality, and disease recurrence in COVID‐19 patients. Med Hypotheses. 2020;144:110271. 10.1016/j.mehy.2020.110271 Epub 2020 Sep 13. PMID: 33254575; PMCID: PMC7487155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tadic M, Cuspidi C, Sala C. COVID‐19 and diabetes: is there enough evidence? J Clin Hypertens (Greenwich). 2020;22(6):943‐948. 10.1111/jch.13912 Epub 2020 May 29. PMID: 32472662; PMCID: PMC7300807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Parasa S, Desai M, Thoguluva Chandrasekar V, et al. Prevalence of gastrointestinal symptoms and fecal viral shedding in patients with coronavirus disease 2019: A systematic review and meta‐analysis. JAMA Netw Open. 2020;3(6):e2011335. 10.1001/jamanetworkopen.2020.11335 PMID: 32525549; PMCID: PMC7290409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wong TP, Ho KY, Ng EK, Debnam ES, Leung PS. Upregulation of ACE2‐ANG‐(1‐7)‐Mas axis in jejunal enterocytes of type 1 diabetic rats: implications for glucose transport. Am J Physiol Endocrinol Metab. 2012;303(5):E669‐E681. 10.1152/ajpendo.00562.2011 Epub 2012 Jul 17. PMID: 22811473. [DOI] [PubMed] [Google Scholar]
- 10. Dai L, Hu WW, Xia L, Xia M, Yang Q. Transmissible gastroenteritis virus infection enhances SGLT1 and GLUT2 expression to increase glucose uptake. PLoS One. 2016;11(11):e0165585. 10.1371/journal.pone.0165585 PMID: 27851758; PMCID: PMC5112927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sharma P, Khairnar V, Madunić IV, et al. SGLT1 deficiency turns listeria infection into a lethal disease in mice. Cell Physiol Biochem. 2017;42(4):1358‐1365. 10.1159/000479197 Epub 2017 Jul 14. PMID: 28704812. [DOI] [PubMed] [Google Scholar]
- 12. Cardoso‐Sousa L, Aguiar EMG, Caixeta DC, et al. Effects of salbutamol and phlorizin on acute pulmonary inflammation and disease severity in experimental sepsis. PLoS One. 2019;14(9):e0222575. 10.1371/journal.pone.0222575 PMID: 31536570; PMCID: PMC6752759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. DeFronzo RA, Davidson JA, Del Prato S. The role of the kidneys in glucose homeostasis: a new path towards normalizing glycaemia. Diabetes Obes Metab. 2012;14(1):5‐14. 10.1111/j.1463-1326.2011.01511.x PMID: 21955459. [DOI] [PubMed] [Google Scholar]
- 14. Washburn WN, Poucher SM. Differentiating sodium‐glucose co‐transporter‐2 inhibitors in development for the treatment of type 2 diabetes mellitus. Expert Opin Investig Drugs. 2013;22(4):463‐486. 10.1517/13543784.2013.774372 Epub 2013 Mar 1. PMID: 23452053. [DOI] [PubMed] [Google Scholar]
- 15. Dominguez Rieg JA, Rieg T. What does sodium‐glucose co‐transporter 1 inhibition add: prospects for dual inhibition. Diabetes Obes Metab. 2019;21(Suppl 2):43‐52. 10.1111/dom.13630 PMID: 31081587; PMCID: PMC6516085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tsimihodimos V, Filippas‐Ntekouan S, Elisaf M. SGLT1 inhibition: pros and cons. Eur J Pharmacol. 2018;838:153‐156. 10.1016/j.ejphar.2018.09.019 Epub 2018 Sep 18. PMID: 30240793. [DOI] [PubMed] [Google Scholar]
- 17. Kosiborod M, Berwanger O, Koch GG, et al. Effects of dapagliflozin on prevention of major clinical events and recovery in patients with respiratory failure due to COVID‐19: the design and rationale for the DARE‐19 study. Diabetes Obes Metab. 2020. 10.1111/dom.14296 Epub ahead of print. PMID: 33319454. [DOI] [PMC free article] [PubMed] [Google Scholar]


