Abstract
The incidence of hypersensitivity reactions to hyaluronic acid dermal fillers is between 0.3 and 4.25%, mediated by T‐lymphocytes. Flu‐like illness can trigger immunogenic reactions at the site of filler placement. Cases of SARS‐CoV‐2 are significant and pose a possible risk of inducing hypersensitivity. This case report is of a delayed‐type hypersensitivity after hyaluronic acid dermal filler treatment of the nose and subsequent infection with SARS‐CoV‐2. Risk factors for the development of such symptoms were identified as the presence of hyaluronic acid combined with flu‐like illness and repeated treatment of one area. The case resolved without intervention. Clinicians should be mindful of the risk posed by the interaction of hyaluronic acid dermal filler with SARS‐CoV‐2 in light of the pandemic.
1. INTRODUCTION
Dermal filler procedures using hyaluronic acid have become extremely popular for patients seeking temporary, non‐invasive aesthetic improvements with a relatively low‐risk profile. 2019 recorded a 15.7% increase in the number of hyaluronic acid (HA) dermal filler procedures to over 4,3 million worldwide from the preceding year 1
The properties of an ideal dermal filler are safety, to be non‐allergenic, non‐immunogenic, effective, predictable, non‐carcinogenic, non‐migratory, cost‐effective, and stable for the desired time within the target tissue. HA is often considered biologically inert, as it is found natively in mammalian tissue. 2 However, no currently available dermal filler product fulfills all requirements absolutely and while uncommon, side effects subsequent to their use have been reported. 3 , 4
Type 1 hypersensitivity reactions are immunoglobulin E (IgE)‐mediated, occur rapidly within minutes or hours, and can result in anaphylaxis or angioedema. Delayed‐type hypersensitivity to HA filler are rare reactions, occurring anywhere between 24 hours to weeks or months after placement. Presenting as a firm erythematous swelling that is often tender to touch, they are caused by a T‐lymphocyte mediated response. 3 , 5 , 6
Case reports on delayed hypersensitivity reactions to HA filler following influenza‐type illnesses have been published. 7 The incidence of delayed‐type hypersensitivity reactions is cited as between 0.3% and 4.25%. 2 , 3 , 8 , 9
The exact mechanism causing delayed‐onset reactions is not understood. However, factors contributing to their incidence include infections, filler properties, trauma, and injection technique including multiple treatment episodes and links to influenza‐like illness. 3 , 5 , 7
This case report is of a patient who presented with a delayed‐type hypersensitivity reaction to HA dermal filler after suffering infection with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), also known as COVID‐19. Transmitted via aerosol droplets, SARS‐CoV‐2 is characterized by a dry cough, fever, dyspnea, sore throat, myalgia, fatigue, and lymphopenia. 10 , 11 , 12 A possible interaction with HA dermal filler has previously not been documented.
2. CASE REPORT
A 22‐year‐old medically fit and well female patient with no known allergies except a sensitivity to raspberry underwent an uneventful non‐surgical rhinoplasty with HA dermal filler (PerfectHA SubSkin, Sinclair Pharma, London UK) in June 2020 by the author. Placement was executed using a 30G x 8 mm 0.3 ml BD Microfine to reduce injection trauma and improve precision. The syringe was backfilled with the dermal filler product using a well‐documented method under sterile conditions to avoid contamination immediately prior to use. 13 , 14 , 15 The risk of contamination of this transfer process has not yet been studied. Publications employing backfill technique have not noted any adverse events resulting from decanting, and in the author's practice this technique has been used in over 500 non‐surgical rhinoplasties without any infection or hypersensitivity events to date. 15 Placement was made to the area of the radix periosteally with the needle placement perpendicular to the bone, depositing 0.5 ml in 0.1 ml increments 5 mm apart in line with documented techniques. 16 , 17 Further placement was made at the tip, perichondrially, depositing 0.3 ml in 0.1 ml increments. The total volume injected was 0.8 ml. Due to a prominent dorsal hump, a revision treatment utilizing 0.1 ml HA dermal filler of the same product type (PerfectHA SubSkin, Sinclair Pharma, London UK) was conducted approximately three weeks later, in July 2020. The same technique was used. Again, this procedure was uneventful.
Figures 1 and 2 show the pre‐ and postoperative photographs taken in June and July, respectively.
FIGURE 1.
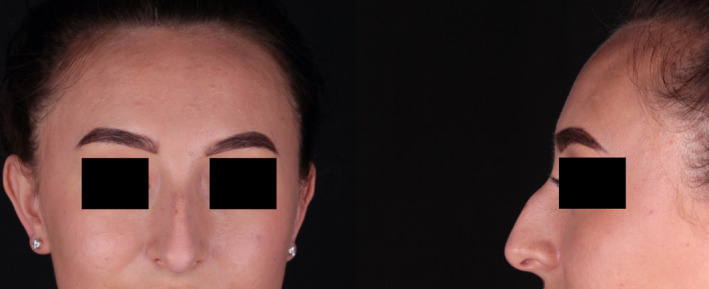
Preoperative photographs taken June 2020.
FIGURE 2.
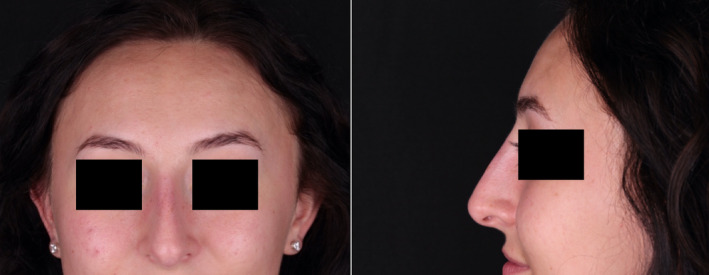
Postoperative photograph taken July 2020.
The patient developed symptoms of SARS‐CoV‐2 in October 2020 and, upon testing at a government‐approved site, was found to have a positive PCR test. Her clinical symptoms were moderate, the patient describing them as a bad cold and resolved without medical intervention.
Approximately three weeks later, in November 2020, the patient awoke with edema, induration, erythema, mild associated tenderness, and a tight feeling of the area around the radix. Figure 3 shows the patient's own photograph taken at that time, which illustrates a swelling at the radix that has significantly widened the soft tissues in this area.
FIGURE 3.
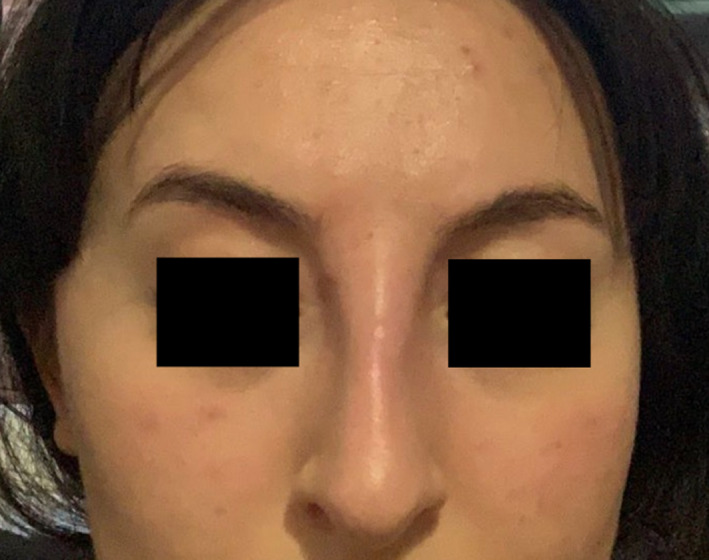
Patient's own photograph (taken on a mobile device) on the day of symptom development, showing swelling of the soft tissues at the radix.
On presentation to the author's practice one day later, a full medical history was taken of the patient. The patient had not suffered any trauma or acute skin infection to the treatment site or facial area. Further questioning revealed that she had not undergone any dental work, 18 started any medication and had not undergone any medical procedures in the time between treatment of the nose with filler in July 2020 and presentation of the swelling in November 2020. The swelling itself was localized, and there were no other extra‐site symptoms or systemic symptoms.
Much of the swelling and erythema had subsided at clinic presentation. Photographs were taken (Figure 4). The radix was no longer tender, and the tightness had subsided. Despite treatment with oral steroids being offered, the patient refused this and preferred monitoring her symptoms due to the swift resolution from the previous day. Her symptoms had entirely resolved six days later without medical intervention. At her review appointment three weeks later, no swelling or tissue changes were noted (Figure 5). However, the dorsal hump was visible again, illustrating how noticeable the swelling had been in the photographs taken some three weeks prior at presentation. At this stage, the patient declined any further investigations including biopsy and antibody testing to ascertain immunologic status after SARS‐CoV‐2.
FIGURE 4.
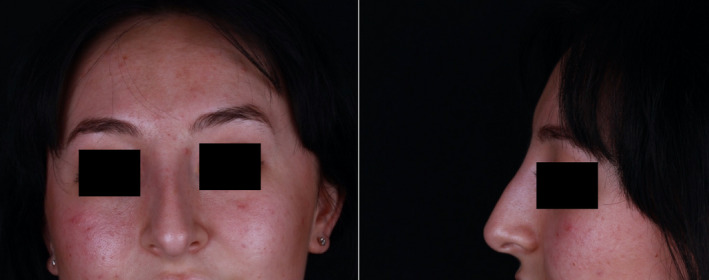
Photographs taken on the day of presentation to the author's clinic (November 2020), one day after appearance of symptoms of swelling.
FIGURE 5.
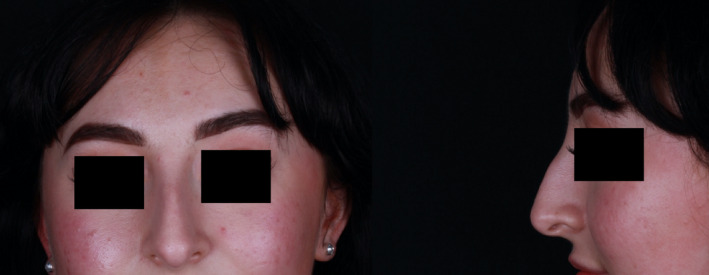
Photographs taken three weeks after the presentation of swelling, after symptoms had subsided (December 2020).
A diagnosis of delayed‐type hypersensitivity to HA dermal filler resulting from SARS‐CoV‐2 infection was made.
3. DISCUSSION
The incidence of delayed‐type hypersensitivity reactions to HA dermal filler is unpredictable and may present as a belated complication, weeks to months after treatment. 7 A number of mechanisms have been suggested, including patient factors such as systemic infections, trauma, injection technique (including filler volume, repeat treatment, and intramuscular injection), vaccines, circulating anti‐HA antibodies, and immunogenic reactions to the breakdown of filler cross‐linking agents in product degradation. 4 , 7 , 8 , 19 , 20
The mechanism for delayed swelling of HA dermal filler is thought to be due to Type IV hypersensitivity, initiated by T‐lymphocytes and mediated by CD4+ cells. The trigger to this reaction is the influenza‐like infection, in this case SARS‐CoV‐2, coupled with macrophage memory. 6 , 7
Beleznay et al noticed a seasonal correlation with an increased number of hypersensitivity reactions presenting in fall and winter, suggesting a link to cold and flu season. 39% of their cases exhibited such an immunologic stimulus prior to hypersensitivity. 2 Bhojani‐Lynch also observed a case with a flu‐like illness one week prior. 6 An immune‐mediated delayed hypersensitivity presentation after sore throat was documented by Homsy et al. 21 It is known that viral infection activates T‐lymphocytes via CD44. 21
The use of HA in cosmetic medicine is well documented, treatments improving skin support and contour due to its favorable safety profile and bio‐inert properties. HA is a high molecular weight glycosaminoglycan polysaccharide that is a component of tissue matrices and fluids, acting to stabilize intracellular components of the dermis, and regulating osmotic balance, cell proliferation, adhesion, and migration. HA has important immune functions, acting as the principal ligand of CD44, a glycoprotein expressed on mammalian cells involved in cell signaling, binding of which to HA results in activation and lymphocyte homing and recruitment. 22 , 23 , 24 , 25 , 26
HA occupies varying roles in cell signaling depending on its molecular weight. It is known that high molecular weight (HMW) HA has anti‐inflammatory effects, whereas low molecular weight (LMW) HA of less than 500 kDA is pro‐inflammatory. 24 , 27 , 28 LMW HA activates macrophages and dendritic cells and delivers co‐stimulatory signals to T cells via CD44 cell surface receptors to affect the production or degradation of HA by means of synthetases or hyaluronidases, respectively. 26 , 27
HA dermal filler may introduce two types of LMW HA: during filler product degradation and as cross‐linking agents. Beleznay et al suggested that systemic inflammatory responses, such as those to flu‐like illness, may trigger accelerated degradation of HA dermal filler due to free radical production, resulting in LMW HA fragments that result in irregular and prolonged CD44‐HA signaling and thus immunopathology as seen in this case. 2 , 22 , 29 A further consequence of the production of LMW HA is the recruitment of lymphocytes to the site of filler where the highest concentration of HA is to be found. 24 Therefore, HA dermal filler is a risk factor in the development of hypersensitivity reactions in the presence of systemic infection and this is the likely cause of the HA dermal filler swelling after SARS‐CoV‐2 infection.
Without modification, the half‐life of HA in tissues is 24–48 hours, which would make it unsuitable for use as a dermal filler. To overcome this, cross‐linking agents are used to produce a stable viscoelastic gel for cosmetic use, the exact technologies of which often remain undisclosed by dermal filler manufacturers. 29 , 30 It is thought these cross‐linking agents may precipitate an immune response. 31
PerfectHA SubSkin (Sinclair Pharma, London UK) is a biphasic non‐animal HA dermal filler cross‐linked with 1,4‐butanediol diglycidyl ether (BDDE) and various sized HA molecules. Inflammatory activity is increased with increasing modification of the HA molecule, impurities from cross‐linking procedures, and filler particle features such as size, shape, and surface. 6 , 29 With more efficient cross‐linking technologies, an increase in hypersensitivity reactions has been observed, likely stemming from the use of LMW HA in the cross‐linking processes. 2 , 23 Indeed, the same is seen in other branches of medicine: cross‐linked HA used in the treatment of osteoarthritis produces more hypersensitivity reactions than native HA. 32
SARS‐CoV‐2 is a respiratory virus causing a number of acute symptoms as previously outlined. To date, close to 120 million cases have been recorded worldwide, of which over 3.5 million in the UK. 33 98% of symptomatic infections display symptoms within 12 days of infection, and most recover spontaneously, although highest case fatality rates (CFR) have been recorded in over‐60 s and comorbid patients. 10 , 11 The patient in this report is among the 80% of cases whose disease progression is uneventful. 12
SARS‐CoV‐2 causes lymphopenia and functional exhaustion lymphocytes especially cytotoxic T cells by an unknown mechanism, possibly accounting for the delay in the presentation of symptoms. 10 , 34 , 35 HA and viral infections activate in vitro T‐lymphocytes via CD44, and a combination of both has likely resulted in this case of hypersensitivity. 7
Considering SARS‐CoV‐2, there is no evidence to suggest that there is a specific increased risk of delayed‐type hypersensitivity when compared with other influenza‐like illnesses, although due to the high virulence and infectivity and subsequent high case numbers of this virus, more delayed‐type hypersensitivity may soon present to aesthetic practitioners as a result of SARS‐CoV‐2.
Concerns have also been raised about the now‐licensed SARS‐CoV‐2 vaccines and their link to delayed‐type hypersensitivity reactions involving filler due to their ability to trigger an immune response. These vaccinations, like infection with SARS‐CoV‐2, are not thought to significantly increase the risk of soft tissue filler adverse events such as delayed swelling when compared with other vaccinations or flu‐like illnesses. 20
A further risk factor, already suggested by Bhojani‐Lynch as contributing to hypersensitivity development, was repeat treatment of the radix, the only site which developed symptoms of induration and erythema. 4 , 6 Although sub‐clinical, repeat treatment may have induced trauma, further contributing to free radical production at the site of injury and fragmentation of HA to produce inflammation 25
Although external risk factors of SARS‐CoV‐2 infection and trauma have presented in this case, we must not discount possible host susceptibility. The patient's raspberry sensitivity may seem trivial. However, 30–40% of the world's population has some form of allergy and heightened responses to interventions such as HA that may produce an immunogenic response. 27 Indeed, anything injected or implanted into the body has the propensity to cause an immune reaction.
In order to arrive at a diagnosis, it is therefore essential to take a thorough medical history and investigate the patient's treatment history for risk factors.
3.1. Management
Delayed‐type hypersensitivity reactions do not improve with the use of antihistamines. 5 , 6 Due to their often transient presentation, intervention may not be necessary. If persistent, steroids may be required to alleviate symptoms. Dose recommendations ranging between 30 and 60 mg daily and tapering down over 5 days depending on symptom severity have been advocated. 2 , 3 , 4 , 5 , 6 , 7 If the diagnosis is unclear and an infection is suspected, steroids should not be prescribed. 6 Funt and Pavicic advocated dissolution of the remaining HA dermal filler after resolution of acute symptoms to prevent recurrence. 5
The use of immune modulators such as corticosteroids has been questioned during the coronavirus pandemic. 36 It is feared they may dampen the immune response necessary to fight infection. However, short‐term or low‐dose steroids such as is indicated for delayed‐type hypersensitivity can be safely used. 37 In patients without a known positive test result for SARS‐CoV‐2, there appears to be no increased risk of contracting the virus even in the presence of comorbidities for which steroids are frequently used. 38 , 39
The patient in this report had already recovered from confirmed SARS‐CoV‐2 infection, and as such treatment with steroid would be appropriate in any case had she required its use.
Cases of dermal filler swelling resulting from SARS‐CoV‐2 vaccination with the Moderna‐type vaccine (Moderna, Cambridge MA) have been successfully treated with Lisinopril, an angiotensin‐converting enzyme inhibitor (ACE‐1) to decrease cutaneous inflammation within 72 hours. ACE‐1 treatment has previously been used in the treatment of hypertrophic scars, keloids, and other inflammatory skin disorders and can assist in downregulating CD44 by inhibiting the pro‐inflammatory Angiotensin II. 40 Lisinopril is well tolerated and a dose of 10 mg for 3–5 days suggested. 40 For cases of delayed‐type hypersensitivity, this therapy may be considered.
This case completely resolved without intervention within one week and required little more than reassurance. The patient in any case refused intervention, preferring to monitor symptoms. Although there is a time‐lag between presentation of symptoms of swelling at the filler site and SARS‐CoV‐2 infection, there are no other discernible triggers to the patient's immune system at this time that would explain her symptoms. Delayed‐type hypersensitivity secondary to SARS‐CoV‐2 was therefore the most likely diagnosis given the lack of other traumatic factors, skin infections, and absence of all other medical procedures or conditions except for the infection with SARS‐CoV‐2. Further to the positive SARS‐CoV‐2 PCR test, an antibody test was not conducted. A biopsy and histological analysis were not performed, and as such the definitive diagnosis is outstanding.
4. CONCLUSION
The exact mechanism that led to the delayed‐type hypersensitivity reaction in this case remains unclear but is likely to be an immunologic reaction between SARS‐CoV‐2, trauma, and HA dermal fillers.
Due to the activation of CD44 by LMW HA, hypersensitivity is a risk for all patients receiving HA dermal filler, as everyone will encounter flu‐like illnesses at some point. Risk may be currently heightened with increased infection rates of SARS‐CoV‐2. As the popularity of HA dermal filler treatment soars, and SARS‐CoV‐2 is widespread, patients must be consented appropriately and advice on the possible risks of hypersensitivity secondary to the risk factors outlined in this case report should be included in the preoperative education of the patient.
Further studies to exactly establish the cause and incidence of hypersensitivity reactions are required, especially those linked to SARS‐CoV‐2.
5. ETHICS STATEMENT
The patient has given consent for their anonymised images to appear in a this publication together with their case history.
6. Acknowledgements
None.
Disclosure
Dr MJ Rowland‐Warmann is a trainer and speaker for Sinclair Pharma. This publication has not received funding support.
REFERENCES
- 1. ISAPS . International Survey on Aesthetic/Cosmetic Procedures. 2019. https://www.isaps.org/wp‐content/uploads/2020/12/Global‐Survey‐2019.pdf
- 2. Beleznay K, Carruthers JDA, Carruthers A, et al. Delayed‐onset nodules secondary to a smooth cohesive 20 mg/mL hyaluronic acid filler: cause and management. Dermatol Surg. 2015;41(8):929‐939. [DOI] [PubMed] [Google Scholar]
- 3. Alijotas‐Reig J, Fernandez‐Figueras MT, Puig L. Inflammatory, immune‐mediated adverse reactions related to soft tissue dermal fillers. Semin Arthritis Rheum. 2013;43(2):241‐258. [DOI] [PubMed] [Google Scholar]
- 4. De Boulle K, Heydenrych I. Patient factors influencing dermal filler complications: prevention, assessment, and treatment. Clin Cosmet Investig Dermatol. 2015;8:205‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosmet Investig Dermatol. 2013;6:295‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bhojani‐Lynch T. Late‐onset inflammatory response to hyaluronic acid dermal fillers. Plast Reconstr Surg Glob Open. 2017;5(12):e1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Turkmani MG, De Boulle K, Philipp‐Dormston WG. Delayed hypersensitivity reaction to hyaluronic acid dermal filler following influenza‐like illness. Clin Cosmet Investig Dermatol. 2019;12:277‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Artzi O, Loizides C, Verner I, Landau M. Resistant and Recurrent Late Reaction to Hyaluronic Acid‐Based Gel. Dermatol Surg. 2016;42(1):31‐37. [DOI] [PubMed] [Google Scholar]
- 9. Alijotas‐Reig J, Fernandez‐Figueras MT, Puig L. Late‐onset inflammatory adverse reactions related to soft tissue filler injections. Clin Rev Allergy Immunol. 2013;45(1):97‐108. [DOI] [PubMed] [Google Scholar]
- 10. Emmi G, Bettiol A, Mattioli I, et al. SARS‐CoV‐2 infection among patients with systemic autoimmune diseases. Autoimmun Rev. 2020;19(7):102575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hosoki K, Chakraborty A, Sur S. Molecular mechanisms and epidemiology of COVID‐19 from an allergist's perspective. J Allergy Clin Immunol. 2020;146(2):285‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Felsenstein S, Herbert JA, McNamara PS, Hedrich CM. COVID‐19: Immunology and treatment options. Clin Immunol. 2020;215:108448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lim AC. Hyaluronic acid filler injections with a 31‐gauge insulin syringe. Australas J Dermatol. 2010;51(1):74‐75. [DOI] [PubMed] [Google Scholar]
- 14. Madsen S, Chesnut C. Use of insulin syringes for precision filler in periocular and rhinoplasty procedures. Dermatol Surg. 2020;46(4):576‐578. [DOI] [PubMed] [Google Scholar]
- 15. Kechichian E, El Khoury R, Helou J. Less pain, more gain: Lip augmentation with insulin syringes. Dermatol Surg. 2017;43(7):979‐981. [DOI] [PubMed] [Google Scholar]
- 16. Tansatit T, Moon H‐J, Rungsawang C, et al. Safe planes for injection rhinoplasty: a histological analysis of midline longitudinal sections of the asian nose. Aesthetic Plast Surg. 2016;40(2):236‐244. [DOI] [PubMed] [Google Scholar]
- 17. Mehta U, Fridirici Z. Advanced techniques in nonsurgical rhinoplasty. Facial Plast Surg Clin North Am. 2019;27(3):355‐365. [DOI] [PubMed] [Google Scholar]
- 18. Ramzi AA, Kassim M, George JV, et al. Dental procedures: is it a risk factor for injectable dermal fillers? J Maxillofac Oral Surg. 2015;14(Suppl 1):158‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Curi MM, Cardoso CL, Curra C, et al. Late‐onset adverse reactions related to hyaluronic Acid dermal filler for aesthetic soft tissue augmentation. J Craniofac Surg. 2015;26(3):782‐784. [DOI] [PubMed] [Google Scholar]
- 20. Gotkin RH, Gout U, Sattler S, et al. Global recommendations on covid‐19 vaccines and soft tissue filler reactions: a survey‐based investigation in cooperation with the international society for dermatologic and aesthetic surgery (ISDS). J Drugs Dermatol. 2021;20(4). [DOI] [PubMed] [Google Scholar]
- 21. Homsy A, Rüegg EM, Jandus P, et al. Immunological reaction after facial hyaluronic acid injection. Case Reports Plast Surg Hand Surg. 2017;4(1):68‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jordan AR, Racine RR, Hennig MJP, et al. The role of CD44 in disease pathophysiology and targeted treatment. Front Immunol. 2015;6:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sadeghpour M, Quatrano NA, Bonati LM, et al. Delayed‐onset nodules to differentially crosslinked hyaluronic acids: comparative incidence and risk assessment. Dermatol Surg. 2019;45(8):1085‐1094. [DOI] [PubMed] [Google Scholar]
- 24. Jiang D, Liang J, Noble PW. Hyaluronan as an immune regulator in human diseases. Physiol Rev. 2011;91(1):221‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Heldin P, Karousou E, Bernert B, et al. Importance of hyaluronan‐CD44 interactions in inflammation and tumorigenesis. Connect Tissue Res. 2008;49(3):215‐218. [DOI] [PubMed] [Google Scholar]
- 26. Mummert ME. Immunologic roles of hyaluronan. Immunol Res. 2005;31:189‐205. [DOI] [PubMed] [Google Scholar]
- 27. Mikkilineni R, Wipf A, Farah R, et al. New classification schemata of hypersensitivity adverse effects after hyaluronic acid injections: pathophysiology, treatment algorithm, and prevention. Dermatol Surg. 2020;46(11):1404‐1409. [DOI] [PubMed] [Google Scholar]
- 28. Scheibner KA, Lutz MA, Boodoo S, et al. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J Immunol. 2006;177(2):1272‐1281. [DOI] [PubMed] [Google Scholar]
- 29. De Boulle K, Glogau R, Kono T, et al. A review of the metabolism of 1,4‐butanediol diglycidyl ether‐crosslinked hyaluronic acid dermal fillers. Dermatol Surg. 2013;39(12):1758‐1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tuttleton Arron S, Neuhaus IM. Persistent delayed‐type hypersensitivity reaction to injectable non‐animal‐stabilised hyaluronic acid. J Cosmet Dermatol. 2007;6:167‐171. [DOI] [PubMed] [Google Scholar]
- 31. Bitterman‐Deutsch O, Kogan L, Nasser F. Delayed immune mediated adverse effects to hyaluronic Acid fillers: report of five cases and review of the literature. Dermatol Reports. 2015;7(1):5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goomer RS, Leslie K, Maris T, et al. Native hyaluronan produces less hypersensitivity than cross‐linked hyaluronan. Clin Orthop Relat Res. 2005;434:239‐245. [DOI] [PubMed] [Google Scholar]
- 33. JohnsHopkins . Coronavirus resource centre: COVID‐19 dashboard by the center for systems science and engineering. https://coronavirus.jhu.edu/map.html
- 34. Skevaki C, Fragkou PC, Cheng C, et al. Laboratory characteristics of patients infected with the novel SARS‐CoV‐2 virus. J Infect. 2020;81(2):205‐212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kumar BV, Connors TJ, Farber DL. Human T cell development, localization, and function throughout life. Immunity. 2018;48(2):202‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang C, Rademaker M, Baker C, et al. COVID‐19 and the use of immunomodulatory and biologic agents for severe cutaneous disease: An Australian/New Zealand consensus statement. Australas J Dermatol. 2020;61(3):210‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Youssef J, Novosad SA, Winthrop KL. Infection risk and safety of corticosteroid use. Rheum Dis Clin North Am. 2016;42(1):157–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McKean D, Chung SL, Fairhead R, et al. Corticosteroid injections during the COVID‐19 pandemic: experience from a UK centre. Bone Jt Open. 2020;1(9):605‐611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Roongta R, Ghosh A. Managing rheumatoid arthritis during COVID‐19. Clin Rheumatol. 2020;39(11):3237‐3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Munavalli GG, Knutsen‐Larson S, Lupo MP, Geronemus RG. Oral angiotensin converting enzyme inhibitors for the treatment of delayed inflammatory reaction of dermal hyaluronic acid fillers following COVID‐19 vaccination – a model for inhibition of angiotensin II‐induced cutaneous inflammation. JAAD Case Reports. 2021;10:63‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]


