Abstract
Background
With the recent approval of COVID‐19 vaccines, recovered COVID‐19 subjects who are vaccinated may be ideal candidates to donate COVID‐19 convalescent plasma (CCP).
Case Series
Eleven recovered COVID‐19 patients were screened to donate CCP. All had molecularly confirmed COVID‐19, and all but one were antibody positive by chemiluminescence immunoassay (DiaSorin) prior to vaccination. All were tested again for antibodies 11–21 days after they were vaccinated (Pfizer/Moderna). All showed dramatic increases (~50‐fold) in spike‐specific antibody levels and had at least a 20‐fold increase in the IC50 neutralizing antibody titer based on plaque reduction neutralization testing (PRNT). The spike‐specific antibody levels following vaccination were significantly higher than those seen in any non‐vaccinated COVID‐19 subjects tested to date at our facility.
Conclusion
Spike‐specific and neutralizing antibodies demonstrated dramatic increases following a single vaccination after COVID‐19 infection, which significantly exceeded values seen with COVID‐19 infection alone. Recovered COVID‐19 subjects who are vaccinated may make ideal candidates for CCP donation.
Keywords: immunology (other than RBC serology), transfusion Practices (Adult)
1. INTRODUCTION
The emergence of SARS‐CoV‐2, the cause of COVID‐19, has resulted in intense efforts to identify new and effective treatments. The lack of proven effective antiviral therapies against coronaviruses has led to the broad utilization of COVID‐19 convalescent plasma (CCP) obtained from survivors of COVID‐19 to treat patients with active disease. 1 , 2 , 3 While the mechanism of action of CCP is uncertain, the most prevalent hypothesis is that CCP contains neutralizing antibodies that limit viral spread and replication. 4 Multiple reports describe the rationale for this therapy and provide some evidence of efficacy. 5 , 6 , 7 , 8 , 9 , 10 One recent study demonstrated that CCP reduced severe disease by nearly 50% when used within 72 h of disease onset. 10 However, other studies have failed to show clear evidence of efficacy. 8 , 11 One study linked high titer CCP to better outcomes than low titer CCP. 12 While there are many possible explanations for the variable results with CCP to date, one potential explanation is that studies that employ just a single unit of CCP fail to provide enough dose to consistently improve outcomes. Transfusion of only one or two units would be expected to only modestly increase antibody levels in COVID‐19 patients. Approaches to identify CCP donors with very high titer of antibodies may allow for future studies that would allow investigators to address these important and unanswered questions. With COVID‐19 vaccine use increasing, there will likely be numerous vaccinated subjects who have recovered from COVID‐19 and are able to donate CCP.
A CCP donor program was established in our academic hospital with an in‐house donor center. This program was established under an IRB‐approved protocol that obtained informed consent from all subjects, allowing for COVID‐19 related research on blood samples obtained from these subjects. 13 A biorepository of serum samples from these subjects was established, which included serum samples from screening or CCP donation. Of these subjects, several were health care providers and per hospital policy were offered COVID‐19 vaccine if they were 90 or more days out from the infection. Given the uncertainty about the durability of the immune response in recovered COVID‐19 patients, several of the subjects in our study elected to get the vaccine when offered, and blood samples were obtained from them as part of this study. The eligibility of vaccinated subjects to donate convalescent plasma has been hotly debated by the convalescent plasma community with some believing that recovered COVID‐19 subjects who are vaccinated may be ideal candidates for donation. The results described here examine antibody levels in these subjects and compare these levels to those detected following infection.
2. MATERIALS AND METHODS
2.1. CCP donor screening and testing
Potential CCP donors were screened following FDA guidance instructions under an IRB‐approved protocol (#202003554). Subjects with molecularly confirmed (PCR) COVID‐19 at the time of their symptoms or subjects with symptoms consistent with COVID‐19 whose diagnosis was confirmed by antibody testing were eligible to donate CCP. The consent signed by all subjects allowed the use of blood samples for research purposes. After vaccine administration was initiated at our facility, subjects were contacted to obtain additional blood samples for research as allowed under this protocol. The date of COVID‐19 diagnosis was recorded for all subjects based on positive molecular (PCR) or antibody testing of the subject. Since the date of disease onset was not systematically recorded for these subjects, the date of diagnosis is defined as day 0 for the purposes of this study. In subjects with molecular testing, this was generally within a week of symptom onset. Serum from all subjects was stored at ‐30 C or colder prior to SARS‐CoV‐2 antibody testing.
2.2. SARS‐CoV‐2 immunoassays
Samples for this study were tested using the DiaSorin LIAISON® SARS‐CoV‐2 S1/S2 IgG chemiluminescence immunoassay (Saluggia, Italy), which has a positive cutoff of 15 arbitrary units per ml (AU/ml) and an upper range limit of 400 AU/mL. 14 Samples above 400 AU/mL were diluted with antibody‐negative serum until the value was within the assay range. For these samples, the value reported represents the measured value multiplied by the fold dilution. For example, if a sample was originally >400 AU/mL and a dilution of 100 ul subject serum into 400 ul of negative serum resulted in a value at 300 AU/mL the reported antibody level for that subject would be 1500 AU/mL (5 × 300). Some samples were also tested with the Roche Diagnostics Elecys Anti‐SARS‐CoV‐2 electrochemiluminescence immunoassay, which target total antibodies (IgG, IgM, and IgA) to the nucleocapsid protein. This assay uses a cutoff index (COI) of 1.0 or higher to indicate a positive result. Both the Roche and the DiaSorin assays were granted emergency use authorization by the FDA and demonstrate similar performance. 14 Both the Roche and the DiaSorin assays were granted emergency use authorization by the FDA and demonstrate similar performance. 14 After vaccination samples were tested with the Beckman Coulter Access SARS‐CoV‐2 IgG assay, which the FDA has recently (February 4, 2021) approved to label CCP as high titer. This assay uses a cutoff index (COI) of 1.0 or higher to indicate a positive result and a cutoff of ≥3.3 to qualify as high titer.
2.3. SARS‐CoV‐2 neutralization assay
Vero E6 cells (ATCC) were maintained in 10% FBS‐DMEM in a humidified incubator at 37°C in 5% CO2. The SARS‐CoV‐2 isolate 2019n‐COV/USA‐WA1/2019 was used for these studies in a BSL‐3 facility (accession #MT123290). Serum samples were diluted 1:10 in DMEM and then serially diluted (eight total dilutions) in DMEM before mixing with 100 PFU SARS‐CoV‐2 in an equal volume (1:20 initial dilution). Under these conditions, the highest dilution tested was 43,740 so samples that still showed more than a 50% reduction in plaque formation at that dilution were given a titer of >43740. Following 1‐hour incubation at 24°C, aliquots were added in duplicates of each dilution to Vero E6 cells in 12 well plates and incubated at 37°C in 5% CO2 for 45 minutes, with gentle rocking performed every 15 minutes to ensure even distribution. Wells were then overlaid with 1.2% agarose/DMEM/2% fetal calf serum. After further incubation for 3 days, cells were fixed with 10% formalin, then agarose plugs were removed with a small spatula, and plaques were visualized by staining with 0.1% crystal violet and subsequently counted. PRNT‐50 was determined as the serum dilution that resulted in a 50% reduction in plaques relative to healthy control serum, estimated by linear interpolation of log‐transformed dilutions. 15 , 16
2.4. Case series
Case 1
A 61‐year‐old Caucasian male was diagnosed and recovered from COVID‐19 in September 2020. His diagnosis was molecularly confirmed via a nasal swab and PCR testing on day 0, 2 days after he developed symptoms consistent with COVID‐19. He recovered without hospitalization or treatment by day 12 and consented to participate in our CCP study on day 70. Antibody screening using the DiaSorin immunoassay was positive (109 AU/mL) and above our cutoff of 100 AU/mL so he successfully donated CCP on day 76. At that time, his SARS‐CoV2 IgG antibody level was below our cutoff so he was not asked to donate CCP again. He received the Pfizer vaccine on day 103 and experienced arm pain, body aches, and a headache, all symptoms commonly observed with vaccination. He returned to donate platelets on day 120 when additional research samples were obtained for SARS‐CoV‐2 specific (immunoassay and neutralizing) antibody measurements. These were compared to samples obtained prior to his vaccination (Table 1). His antibody level by immunoassay was estimated to be 3940 AU/mL, nearly 40‐fold higher than it was prior to vaccination. The PRNT assay demonstrated that his IC50 neutralizing antibody titer on day 76 was 486 while on day 120, 17 days after his first vaccination, the IC50 neutralizing antibody titer was 9683. This represents a 20‐fold increase in his neutralizing antibody titer. The spike immunoassay was repeated 14 days after his second vaccination and the level was 4137 AU/mL, roughly the same as after his first vaccination. The timeline for the disease, screening, donations, and vaccinations for all three subjects are shown in Figure 1.
Case 2
A 60‐year‐old Caucasian female was diagnosed and recovered from COVID‐19 in September 2020. Her diagnosis was molecularly confirmed via a nasal swab and PCR testing, the same day she developed symptoms consistent with COVID‐19. She recovered without hospitalization or treatment by day 10 and consented to participate in our CCP study on day 70. Antibody screening using the DiaSorin immunoassay was positive (24.1 AU/mL), but below our cutoff for donation, so she did not donate CCP. She received the Pfizer vaccine on day 105 and returned when additional research samples were obtained on day 126 for SARS‐CoV‐2 specific antibody testing (Table 1). Her antibody level by immunoassay was estimated to be 1515 AU/mL, about a 60‐fold increase relative to her antibody level 35 days prior to vaccination. The PRNT assay demonstrated that her IC50 neutralizing antibody titer on day 70 was <20 while on day 126, 21 days after her first vaccination, the IC50 neutralizing antibody titer was 3533. This represents a > 170‐fold increase in her neutralizing antibody titer. The spike immunoassay was repeated 14 days after her second vaccination and the level was 1452 AU/mL, roughly the same as after the first vaccination.
Case 3
A 57‐year‐old Caucasian male was diagnosed and recovered from COVID‐19 in April 2020. His diagnosis was molecularly confirmed via a nasal swab and PCR testing on day 0, 11 days after he developed symptoms consistent with COVID‐19. He recovered without hospitalization or treatment by day 7 and consented to participate in our CCP study on day 14. Antibody screening using the DiaSorin immunoassay was positive (55.2 AU/mL) and he successfully donated CCP on days 18, 119, and 147. The cutoff for CCP donation was increased to 100 AU/mL after that and he was no longer eligible to donate CCP. He received the Pfizer vaccine on day 247 and returned when additional research samples were obtained on day 269, 22 days after he received the first vaccine. These were compared to samples obtained prior to his vaccination on day 147 (Table 1). His antibody level by immunoassay was estimated to be 2960 AU/mL, more than 40‐fold higher than it was prior to vaccination. The PRNT assay demonstrated that his IC50 neutralizing antibody titer on day 144 was 184 while on day 269, 22 days after his first vaccination, the IC50 neutralizing antibody titer was 31,094. This represents a 169‐fold increase in his neutralizing antibody titer. The spike immunoassay was repeated 14 days after his second vaccination and the level was 3410 AU/mL, just slightly higher than after the first vaccination.
TABLE 1.
Vaccinated subject antibody levels
| Pre | Post | Fold change | ||
|---|---|---|---|---|
| Case 1 61‐M‐P | Spike IgG (AU/ml) | 98.7 | 3940 | 40 |
| Nucleocapsid Total Ig (COI) | 165 | 180 | 1.1 | |
| PRNT IC50 titer | 476 | 9683 | 20 | |
| Case 2 60‐F‐P | Spike IgG (AU/ml) | 24.1 | 1515 | 63 |
| Nucleocapsid Total Ig (COI) | 40.8 | 25.6 | 0.6 | |
| PRNT IC50 titer | <20 | 3533 | >177 | |
| Case 3 57‐M‐P | Spike IgG (AU/ml) | 63.6 | 2960 | 47 |
| Nucleocapsid Total Ig (COI) | 3.42 | 1.82 | 0.5 | |
| PRNT IC50 titer | 184 | 31,094 | 169 | |
FIGURE 1.
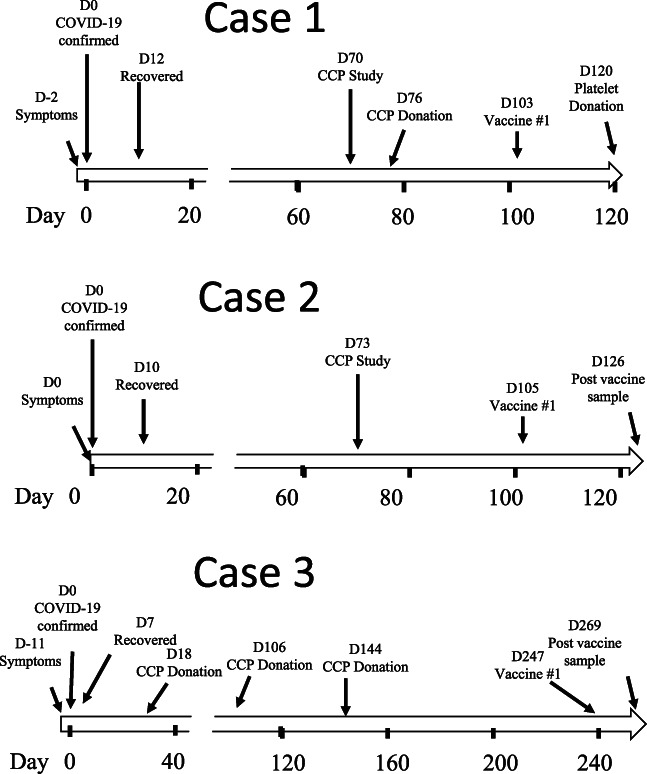
Subject timelines. The timeline for the three initial CCP subjects described in this report. Day 0 is the day that COVID‐19 was confirmed by nasal swab molecular testing
2.5. ELISA results on COVID‐19 infected subjects
During this study, 126 subjects with molecularly confirmed COVID‐19 have been tested using the DiaSorin immunoassay. The initial testing results on these subjects are shown in Figure 2(A). As previously reported for other populations, 14 a relatively high (21%) fraction of COVID‐19 patients were found to be antibody negative (<15 AU/mL). A majority (57%) were positive but had values under 100 AU/mL. Only 22% had values over 100 AU/mL, which is the antibody level currently in use for CCP donation eligibility at our site. The mean of the initial positive results was 81.7 AU/mL and the range was 15–324 AU/mL. Looking at all test results from this study, the DiaSorin immunoassay has been positive on 205 samples from 145 subjects in this study. When we look at these positive results relative to when subjects were confirmed to have COVID‐19 (initial molecular or serological testing), all but one was under 500 AU/mL. This contrasts sharply with the three cases detailed in this report and shown as solid triangles in Figure 2(B).
FIGURE 2.

Spike specific IgG levels from CCP subjects. (A) the initial/screening immunoassay results (DiaSorin) on 126 subjects with molecularly confirmed COVID‐19 are shown. Of the positive results, the mean was 81 (range 15–324). (B) the spike‐specific immunoassay results for all positive (>15) samples are shown relative to the time the subject was confirmed to have COVID‐19. The open triangles are non‐vaccinated subjects and the filled diamonds are from the 11 vaccinated subjects outlined in this case series, three of who were tested twice as described in the results
2.6. SPIKE and PRNT levels on additional subjects
Given the dramatic increases seen in the three subjects described here, eight additional CCP subjects were recruited following vaccination. All eight show significant increases in spike IgG levels as measured by immunoassay (Table 2). In addition, seven of the eight subjects had PRNT values higher than 1000 (Table 2). Two of these subjects received the Moderna (M) vaccine and both showed a significant increase in antibody levels, suggesting that dramatic increases in antibody levels are not unique to the Pfizer vaccine. Of note, only one subject in this cohort had spike antibody levels less than 1000 AU/mL and PRNT titer less than 1000 and this subject was the only subject tested to date who was antibody negative (<3.8 AU/mL) at the time they were screened. This suggests that this subject did not mount an antibody response following COVID‐19 infection and thus may have responded to the vaccine like an uninfected subject. Excluding this “antibody‐negative” subject, the other 10 subjects had an average spike‐specific IgG level of 4166 AU/mL (range 1235–7854). This compares to an average of 81.7 AU/mL in the non‐vaccinated antibody positive subjects screened for this study (N = 109). Neutralizing antibodies by PRNT were similarly increased in these 10 subjects with a median IC50 value of 32,657 (Range 3533 to >43,740).
TABLE 2.
DiaSorin antibody levels on additional subjects who received either the Pfizer (P) or Moderna (M) vaccine
| Case age‐sex‐vaccine | Pre (days before vaccine) | First vaccine days from diagnosis | Post (days after vaccine(s) | Fold change | PRNT |
|---|---|---|---|---|---|
| Case4 61‐F‐P | 31.9 (23) | 185 | 1235 (22) | 39 | 6171 |
| Case 5 56‐F‐P | <3.8 (55) | 113 | 245 (36/14) | 66 | 887 |
| Case 6 41‐F‐M | 39.4 (254) | 276 | 6132 (25) | 156 | 30,848 |
| Case 7 46‐F‐P | 39.1 (118) | 281 | 6027 (20) | 154 | 34,221 |
| Case 8 39‐F‐P | 39.2 (247) | 273 | 4851 (20) | 124 | >43,740 |
| Case 9 27‐F‐P | 379 (20) | 250 | 3810 (16) | 10 | 41,045 |
| Case 10 40‐F‐M | 84 (123) | 292 | 7854 (17) | 93 | >43,740 |
| Case 11 61‐F‐P | 37.6 (146) | 195 | 3333 (21) | 89 | 37,538 |
2.7. Vaccinated CCP donors antibody results with FDA high titer assay
The FDA has recently expanded the tests available to qualify CCP as high titer and has put an emphasis on the collection and use of high titer plasma. The Beckman Access IgG assay is one of the tests approved, so after vaccination, samples from all 11 cases were tested using this assay. All 11 samples tested met the cutoff for high titer with an average value of 32.5 and range from 10.9 to 41.65 (Table 3).
TABLE 3.
Beckman antibody results on these subjects
3. DISCUSSION
The results described here in a total of 11 subjects suggest that antibody positive recovered COVID‐19 patients mount a strong amnestic response following just a single vaccination with either the Pfizer or the Moderna vaccine. These subjects all had spike‐specific antibody levels at least 10‐fold higher than what was detected prior to vaccination and all subjects who were antibody positive values prior to vaccination had antibody levels greater than 1200 AU/mL using a spike‐specific IgG immunoassay. This contrasts with non‐vaccinated subjects who had an average of 81.7 AU/mL in this study. These dramatic results suggest that recovered and vaccinated COVID‐19 patients who were antibody positive following the initial infection would be strong candidates for CCP donation. Whether CCP collected from these donors would be more efficacious than CCP from non‐vaccinated subjects is not known but the recent demonstration that patients receiving high titer plasma may do better than other CCP recipients certainly supports this possibility. 12 The nearly 10‐fold increase in antibody levels raises the possibility that one unit of plasma from these donors would be enough to increase antibody levels in recipients to levels seen following infection alone. Given the FDA has recently emphasized the collection of high titer plasma, it is also important to note that the vaccinated subjects in this study all met the criteria for high titer plasma using the Beckman assay with a S/Co of ≥3.3 for high titer. In fact, the average value from these subjects using this test was nearly 10‐fold above this cutoff. Recent reports suggest other groups have demonstrated similar results with vaccinated recovered COVID‐19 patients. 17 , 18
This study also provides data regarding the durability of an amnestic response in subjects who have recovered from COVID‐19. The 10 subjects in this study who were antibody positive when initially screened for CCP donation were vaccinated from 103 to 292 days after they were diagnosed. Six subjects were vaccinated 8 months or more after they were diagnosed, and all six had antibody levels greater than 2900 AU/mL after just a single vaccine. Previous studies demonstrated that a single dose of the Pfizer vaccine had lower antibody levels than observed in recovered COVID‐19 patients. 19 , 20 The results here suggest that these subjects had a strong amnestic response up to 8 months after they had been infected. The FDA has recently (Jan 15, 2021) issued a guidance for industry document on the collection of CCP that includes information about the use of CCP from vaccinated subjects. These guidelines state CCP can be collected from vaccinated subjects if the subject previously had confirmed COVID‐19 and the CCP is collected within 6 months of the end of symptoms. It is unfortunate that under these guidelines, 8 of the 11 subjects in this study would not be eligible to donate CCP as they had recovered from COVID‐19 more than 6 months before they were tested. Each of these subjects had antibody spike specific IgG levels higher than any of 100+ previous donors we have screened for this study, and each of the three tested using the PRNT assay had extremely high levels of neutralizing antibody as well.
The COVID‐19 vaccine trials (NCT04368728/Pfizer;NCT04470427/Moderna) excluded subjects who had recovered from COVID‐19, so data regarding the use of these vaccines in these patients are lacking. 21 Given that limitation, healthcare facilities have been left to develop their own policies regarding whether and/or when to vaccinate recovered COVID‐19 patients. As mentioned earlier, our facility selected 90 days after infection before recovered COVID‐19 patients are offered the vaccine. Other sites have shorter time periods as some studies have shown that antibody responses may wane following infection. 22 This small case series provides evidence that some, if not most, recovered patients have evidence of an amnestic antibody response 8–10 months following infection. In this case series, all (10 of 10) subjects who had been antibody positive prior to CCP donation had robust antibody response to just a single vaccination. Given that vaccine demand currently greatly exceeds supply, these results raise the potential that antibody testing could be used to determine vaccine eligibility. One possible scenario is that antibody‐negative patients could be eligible for the vaccine at any point after infection while antibody positives be deferred for longer periods of time. Additionally, the strong antibody responses in these subjects following the first vaccination suggest that the second vaccine dose may not be necessary. The modest changes in antibody levels in our first three subjects following the second vaccine seem to support the fact that the second dose may provide little benefit in these subjects.
In conclusion, this small case series provides evidence to support a strong amnestic antibody response in recovered COVID‐19 subjects who had previously been antibody positive. These subjects may be preferentially selected for CCP donation and all qualified as high titer donors in this study. This raises the possibility that CCP collected from these vaccinated “super donors” could be more efficacious to infected patients than the CCP that has been used to date.
CONFLICT OF INTEREST
The authors declare no competing financial interests.
ACKNOWLEDGMENTS
We would like to thank the staff at the state hygienic lab who assisted in testing CCP donors with the Beckman assay including Michelle Sexton, Haley Peden, and Michael Pentella. We would also like to thank Julie Kurt and Gail Drey for assistance with our CCP program.
Vickers MA, Sariol A, Leon J, et al. Exponential increase in neutralizing and spike specific antibodies following vaccination of COVID‐19 convalescent plasma donors. Transfusion. 2021;61:2099–2106. 10.1111/trf.16401
Reprints will not be available from the authors.
REFERENCES
- 1. Cao H, Shi Y. Convalescent plasma: Possible therapy for novel coronavirus disease 2019. Transfusion. 2020;60:1078–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lung T, Kazatchkine MD, Risch L, Risch M, Nydegger UE. A consideration of convalescent plasma and plasma derivatives in the care of severely‐ill patients with COVID‐19. Transfus Apher Sci. 2020;59:102936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Richard SA, Kampo S, Esquijarosa HM. Elucidating the pivotal role of convalescent plasma therapy in critically ill COVID‐19 patients: A review. Hematol Rep. 2020;12:8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rojas M, Rodríguez Y, Monsalve DM, Acosta‐Ampudia Y, Camacho B, Gallo JE, et al. Convalescent plasma in Covid‐19: Possible mechanisms of action. Autoimmun Rev. 2020;19:102554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang M, Wu T, Zuo Z, You Y, Yang X, Pan L, et al. Evaluation of current medical approaches for COVID‐19: A systematic review and meta‐analysis. BMJ Support Palliat Care. 2021;11(1):45–52. [DOI] [PubMed] [Google Scholar]
- 6. Al‐Riyami AZ, Schäfer R, van der Berg K, Bloch EM, Escourt LJ, Goel R, et al. Clinical use of convalescent plasma in the COVID‐19 pandemic; a transfusion‐focussed gap analysis with recommendations for future research priorities. Vox Sang. 2021;116(1):88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ruiz‐Argüelles GJ. Infusion of convalescent plasma is associated with clinical improvement in critically ill patients with Covid‐19: A comment. Rev Invest Clin. 2020;72(5). https://clinicalandtranslationalinvestigation.com/frame_esp.php?id=338. [DOI] [PubMed] [Google Scholar]
- 8. Li L, Zhang W, Hu Y, Tong X, Zheng S, Yang J, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life‐threatening COVID‐19: A randomized clinical trial. JAMA. 2020;324:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu STH, Lin H‐M, Baine I, Wajnberg A, Gumprecht JP, Rahman F, et al. Convalescent plasma treatment of severe COVID‐19: A propensity score–matched control study. Nat Med. 2020;26:1708–13. [DOI] [PubMed] [Google Scholar]
- 10. Libster R, Pérez Marc G, Wappner D, Coviello S, Bianchi A, Braem V, et al. Early high‐titer plasma therapy to prevent severe Covid‐19 in older adults. N Engl J Med. 2021;384(7):610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Simonovich VA, Burgos Pratx LD, Scibona P, Beruto MV, Vallone MG, Vázquez C, et al. A randomized trial of convalescent plasma in Covid‐19 severe pneumonia. N Engl J Med. 2021;384(7):619–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Joyner MJ, Carter RE, Senefeld JW, Klassen SA, Mills JR, Johnson PW, et al. Convalescent plasma antibody levels and the risk of death from Covid‐19. N Engl J Med. 2021;384(11):1015–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Knudson CM, Jackson JB. COVID‐19 convalescent plasma: Phase 2. Transfusion. 2020;60:1332–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Merrill AE, Jackson JB, Ehlers A, Voss D, Krasowski MD. Head‐to‐head comparison of two SARS‐CoV‐2 serology assays. J Appl Lab Med. 2020;5:1351–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zheng J, Wang Y, Li K, Meyerholz DK, Allamargot C, Perlman S. SARS‐CoV‐2‐induced immune activation and death of monocyte‐derived human macrophages and dendritic cells. J Infect Dis. 2021;223(5):785–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wong LR, Li K, Sun J, Zhuang Z, Zhao J, McCray PB Jr, et al. Sensitization of non‐permissive laboratory mice to SARS‐CoV‐2 with a replication‐deficient adenovirus expressing human ACE2. STAR Protoc. 2020;1:100169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abu Jabal K, Ben‐Amram H, Beiruti K, Batheesh Y, Sussan C, Zarka S, et al. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID‐19 vaccine: Real‐world evidence from healthcare workers, Israel, December 2020 to January 2021. Euro Surveill. 2021;26(6):2100096. https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2021.26.6.2100096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bradley T, Grundberg E, Selvarangan R. Antibody responses boosted in seropositive healthcare workers after single dose of SARS‐CoV‐2 mRNA vaccine. medRxiv 2021. 10.1101/2021.02.03.21251078. [DOI] [PMC free article] [PubMed]
- 19. Mulligan MJ, Lyke KE, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Phase I/II study of COVID‐19 RNA vaccine BNT162b1 in adults. Nature. 2020;586:589–93. [DOI] [PubMed] [Google Scholar]
- 20. Sahin U, Muik A, Derhovanessian E, Vogler I, Kranz LM, Vormehr M, et al. Türeci Ö. COVID‐19 vaccine BNT162b1 elicits human antibody and T(H)1 T cell responses. Nature. 2020;586:594–9. [DOI] [PubMed] [Google Scholar]
- 21. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med. 2020;383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Girardin RC, Dupuis AP, Payne AF, Sullivan TJ, Strauss D, Parker MM, et al. Temporal analysis of serial donations reveals decrease in neutralizing capacity and justifies revised qualifying criteria for COVID‐19 convalescent plasma. J Infect Dis. 2021;223:743–51. [DOI] [PMC free article] [PubMed] [Google Scholar]


