Dear Editor,
1.
The coronavirus disease 2019 (COVID‐19) disease has become a global pandemic and caused over a 120 million cases and 2.7 million deaths so far. There are not yet efficient antiviral agents available, and the only current approach which has proven effective in reducing mortality in patients with the severe disease has been the use of corticosteroids to control the hyperinflammatory response 1 , 2 Among immunomodulatory and antiviral agents, interferons (IFNs) are essential in the regulation of the activation and function of various immune cell populations and in restraining viral replication. 3 , 5 Antiviral research during the last three coronavirus outbreaks resulted in the identification of IFNs as agents that may potentially target coronavirus replication directly, can modulate the immune response to coronavirus infection, and improve respiratory distress. 6 , 7 IFN dysregulation represents a key determinant of COVID‐19 pathogenesis 3 and highlights its potential for therapeutic intervention.
The present study evaluates the effect of interferon β‐1b (IFNβ‐1b), administered in the hyperinflammatory phase, on mortality and intensive care unit (ICU) admission among moderate to severe hospitalized patients with COVID‐19.
The present work was a multicenter, controlled, retrospective cohort study, conducted at the Arnau de Vilanova–Llíria Health Department in Valencia, Spain. We reviewed all patients who were diagnosed with suspected COVID‐19 between March 3, 2020, and April 30, 2020. Patients were eligible for study inclusion if they were aged ≥ 18 years, had oxygen saturation while breathing room air (SatO2) < 92%, and had laboratory confirmation of COVID‐19 infection by reverse‐transcription polymerase chain reaction (RT‐PCR; SARS‐CoV‐2 Real Time PCR Detection Kit, Certest Biotec S.L.). Patients with any terminal disease were excluded. They were followed until death or discharge from the hospital. Our study was conducted according to the Declaration of Helsinki. It was approved by the Arnau de Vilanova‐Llíria Hospitals’ Ethics Committee and registered with the AEMPS (Spanish Agency of Drugs and Health Products). Register: CTA‐ARA‐2020‐04.
In our Health Department, the interim guidelines for management of COVID‐19 were developed by a committee of experts following the guidelines issued by the Spanish Ministry of Health. These guidelines included the use of IFNβ‐1b (Betaferon®, Bayer AG) 250 μg subcutaneously every 48 h for a maximum of 14 days. Patients could also receive tocilizumab and methylprednisolone or dexamethasone. As antiviral therapy, patients received hydroxychloroquine, with or without azithromycin, and/or lopinavir/ritonavir. Data were recorded from patients’ electronic medical records, including demographics, comorbidities, chest radiography, SatO2, laboratory values, other therapies received for COVID‐19, and outcome. Patients were assessed to fit in one of the categories of the WHO's Eight‐category scale for clinical improvement, based on oxygen support requirement, 8 at admission and at days 7 and 21 (see Table legend). If a given patient was discharged before reaching the 21st day of hospitalization, and there were not electronic register of a new readmission or medical visit, the patient was considered to have at day 21 the same category as that at discharge. The primary composite endpoint of the study was admission to ICU or in‐hospital death. The secondary endpoint was time to clinical improvement. Improvement was defined as two points decrease in the WHO's Eight‐category scale or live discharge from the hospital, whichever occurred first. Descriptive analysis was performed to compare baseline characteristics of patients who received IFNβ‐1b and those who did not. We used Kaplan‐Meier analysis and log‐rank test for survival analysis between both groups. The hazard ratio (HR) and 95% confidence interval (CI) for clinical improvement and the composite endpoint death or ICU admission were estimated by the Cox proportional hazards model and were adjusted for propensity score index and confounding variables. P‐values were two‐sided and values < 0.05 were considered statistically significant. Statistical analysis was performed using the Statistical Package for Social Science (SPSS 19.0 Inc.).
Of 287 eligible patients evaluated for study inclusion, 46 were excluded because laboratory confirmation of SARS‐CoV‐2 infection by RT‐PCR was negative, 126 were excluded because SatO2 was not lower than 92%, and 10 were excluded because of a terminal disease. From the remaining 105 patients included in our analysis, 28 of them received INFβ‐1b. In the IFNβ‐1b group, Table 1 summarizes patients’ demographic, baseline clinical data, and outcome.
Table 1.
Demographic clinical baseline characteristics of patients and outcome
| Control group (n = 77) | IFNβ‐1b group (n = 28) | p value | |
|---|---|---|---|
| Male, % (n) | 53 (41) | 79.0 (22) | .02 |
| Age, years (IQR) | 77 (64–86) | 68.5 (49–75) | .01 |
| Hypertension, % (n) | 58 (45) | 64 (18) | .60 |
| Diabetes mellitus, % (n) | 29 (22) | 29 (8) | .99 |
| Coronary artery disease, % (n) | 13 (14) | 14 (4) | .86 |
| Chronic heart failure, % (n) | 1 (1) | 4 (1) | .50 |
| COPD, % (n) | 8 (6) | 7 (2) | .91 |
| CKD (GFR < 60), % (n) | 5 (4) | 0 | .57 |
| Liver cirrhosis, % (n) | 0 | 0 | |
| Tobacco smoker, % (n) | .51 | ||
| Active | 5 (4) | 0 (0) | |
| Never | 38 (29) | 39 (11) | |
| Former | 30 (23) | 39 (11) | |
| Unknown | 27 (21) | 21 (6) | |
| Use of ARBs or ACE inhibitors, % (n) | 39 (30) | 43 (12) | .72 |
| Obesity | 19.5 (15) | 11 (3) | .38 |
| CURB‐65 score at admission, % (n) | .51 | ||
| 0 | 16 (12) | 18 (5) | |
| 1 | 37 (28) | 50 (14) | |
| 2 | 33 (25) | 29 (8) | |
| 3 | 12 (9) | 4 (1) | |
| 4 | 3 (2) | 0 (0) | |
| Chest radiography, % (n) | .44 | ||
| Bilateral infiltrates | 79 (59) | 89 (24) | |
| Unilateral infiltrates | 19 (14) | 11 (3) | |
| Missing | 3 (2) | 0 (0) | |
| SatO2/FiO2, median (IQR) | 423 (310–435) | 421 (295–433) | .76 |
| SatO2, % (IQR) | 90 (85–92) | 89 (85–91) | .95 |
| ANC, cells/mm3, (IQR) | 4530 (3145 –6550) | 6165 (4742–7475) | .02 |
| LC, cells/mm3, (IQR) | 995 (600–1375) | 800 (8600–1032) | .01 |
| Platelets count, 109/L, (IQR) | 217 (171−266) | 201 (160–260) | .5 |
| d‐dimer, ng/ml (IQR) | 911 (487−1328) | 737 (475–1146) | .05 |
| LDH, mU/ml (IQR)a | 256 (205–319) | 328 (288–409) | .08 |
| Ferritin, ng/ml (IQR)a | 513 (278−1053) | 872 (427−1486) | .5 |
| C‐reactive protein, mg/L (IQR) | 73 (45−150) | 135 (103–216) | <.01 |
| Other treatment % (n) | |||
| Hydroxychloroquine | 95 (73) | 100 (28) | .57 |
| Azithromycin | 61 (54) | 36 (10) | .02 |
| Lopinavir/ritonavir | 70 (61) | 97 (27) | <.01 |
| Tocilizumab | 8 (6) | 11 (3) | .7 |
| Corticosteroids | 27 (21) | 39 (11) | .23 |
| Eight‐category scale at admission, % (n) | .70 | ||
| 1. Ambulatory, no limitation of activities | 0 | 0 | |
| 2. Ambulatory, limitation of activities | 0 | 0 | |
| 3. Hospitalized, no oxygen therapy | 0 | 0 | |
| 4. Hospitalized, oxygen therapy (FiO2 < 0.40) | 71 (55) | 75 (21) | |
| 5. Severe disease, NIMV (FiO2 > 0.40) | 29 (22) | 25 (7) | |
| 6. ICU and ventilation | 0 | 0 | |
| 7. Ventilation and additional organ support | 0 | 0 | |
| 8. Death | 0 | 0 | |
| Eight‐category scale at day 7, % (n) | .07 | ||
| 1. Ambulatory, no limitation of activities | 10 (8) | 7 (2) | |
| 2. Ambulatory, limitation of activities | 3 (2) | 0 | |
| 3. Hospitalized, no oxygen therapy | 9 (7) | 7 (2) | |
| 4. Hospitalized, oxygen therapy (FiO2 < 0.40) | 39 (30) | 47 (13) | |
| 5. Severe disease, NIMV (FiO2 > 0.40) | 10 (8) | 32 (9) | |
| 6. ICU and ventilation | 8 (6) | 7 (2) | |
| 7. Ventilation and additional organ support | 9 (7) | 0 | |
| 8. Death | 12 (9) | 0 | |
| Eight‐category scale at day 21, % (n) | .03 | ||
| 1. Ambulatory, no limitation of activities | 51 (39) | 71 (20) | |
| 2. Ambulatory, limitation of activities | 12 (9) | 18 (5) | |
| 3. Hospitalized, no oxygen therapy | 5 (4) | 0 (0) | |
| 4. Hospitalized, oxygen therapy (FiO2 < 0.40) | 5 (4) | 11 (3) | |
| 5. Severe disease, NIMV (FiO2 > 0.40) | 0 | 0 | |
| 6. ICU and ventilation | 0 | 0 | |
| 7. Ventilation and additional organ support | 1 (1) | 0 (0) | |
| 8. Death | 26 (20) | 0 (0) | |
| Time to IFN initiation from symptoms onset, days (IQR) | ‐ | 10 (9−13) | |
| Time on treatment with IFNβ‐1b, days (IQR) | ‐ | 8 (6–9) | |
| Clinical improvementb, % (n) | 73 (56) | 100 (28) | <.01 |
| Intensive care unit admission, % (n) | 19 (15) | 7 (2) | .3 |
| Deathc, % (n) | 27 (21) | 0 (0) | <.01 |
| Time to discharge, days (IQR) | 12 (9–16) | 17 (13–21) | .02 |
| Time to clinical improvement, days (IQR) | 12 (9−16) | 15 (12–21) | .02 |
Note: Continuous data are median IQR. p values were calculated using the χ2 or the Fisher's exact test for categorical variables and the t test for continuous variables.
Abbreviations: ACE, angiotensin‐converting enzyme; ANC, absolute neutrophils count; ARB, angiotensin receptor blocker; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; FiO2, fraction of inspired oxygen; GRF, glomerular filtration rate (mL min−1 per 1.73 m2 body surface area); ICU, intensive care unit; IFN, interferon; IQR, interquartile range; LC, lymphocytes count; LDH, lactate dehydrogenase; NIMV, noninvasive mechanical ventilation; SatO2, oxygen saturation in room air.
LDH was calculated in a sample of 74 patients; ferritin was calculated in a sample of 58 patients.
Clinical improvement was defined as two points decreased in the WHO eight‐category scale or live discharge from the hospital whichever occurs first.
Patients who were admitted to the ICU and died are counted as both UCI admission and death.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
At admission, categorization by WHO's Eight‐category scale was similar in both groups (Table 1). Median time from symptoms onset to IFNβ‐1b start was 10 days, while patients received a median of 8 days of interferon treatment. The primary composite endpoint (ICU admission or death) occurred in 7% of patients in the INFβ‐1b group and in 35% in the control group (Table 1).
Among all patients, cumulative survival incidence in IFNβ‐1b versus control group was 92% (95% CI, 100–82) and 51% (95% CI, 68–34), respectively, p = .002 (Figure 1A). HR for death or ICU admission was 0.06 (95% CI, 0.01–0.34), p = .001. Median time to clinical improvement was not significantly different between groups: 15 days in the interferon‐treated group, and 12 days in the control group (Figure 1B). Log‐rank test: p = .53, HR, 0.80 (95% CI, 0.42–1.36). No IFNβ‐1b treatment had to be stopped due to side effects.
Figure 1.
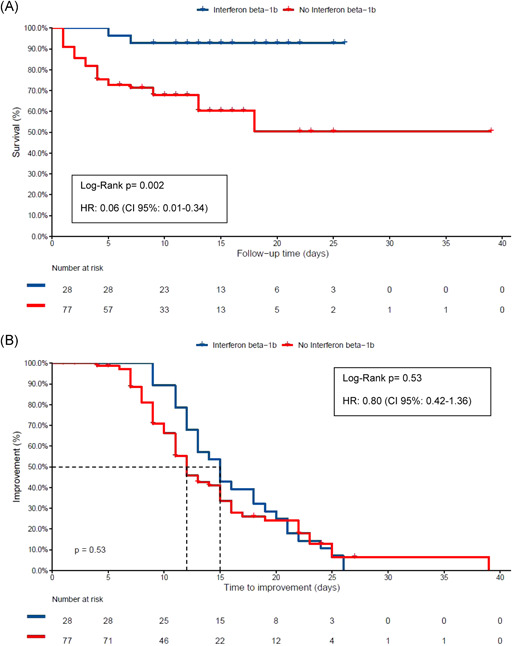
(A) Cumulative survival and hazard ratio (HR) in the control group and interferon β‐1b (INFβ‐1b) treated group. Patients not discharged were exitus letalis or hospitalized in the intensive care unit. Kaplan‐Meier and Cox hazards analyses were used. (b) Kaplan‐Maier plot for time to clinical improvement. Clinical improvement was defined as two points decrease in the WHO's Eight‐category scale or live discharge from the hospital, whichever occurs first. Not discharged patients (censored patients) died. Cox regression was adjusted for propensity score index for interferon use, oxygen requirements (patients requiring noninvasive mechanical ventilation [NIMV], high flow nasal cannula or FiO2 > 0.40, or patients requiring supplemental oxygen with FiO2 < 0.40) and corticosteroids. To calculate the propensity score index, sex, age, diabetes, hypertension, obesity, CURB‐65 at admission, and C‐reactive protein were included
The results of this study showed that IFNβ‐1b, when given in the inflammatory phase of the disease, is associated with reduction in mortality and ICU admission in COVID‐19 hospitalized patients that require oxygen support, while time to reach clinical response did not change.
Along the same lines, some real‐world setting studies have been recently published. 9 , 10 , 11 IFNα‐2b significantly reduced elevated blood levels of inflammatory markers. 5 IFNβ−1a combined with the standard antiviral treatment have shown efficacy in reducing mortality. 9 IFNβ‐1b, in severe patients, was effective in shortening the time to clinical improvement and in decreasing admission to ICU, although it could not offer an estimation of the survival benefit. 11 Recent data from the WHO SOLIDARITY trial 12 which repurposes IFN as an antiviral drug, exclude a mortality reduction among these patients. However, a caveat of the Solidarity trial is that the time from symptom onset to IFN initiation is not available.
Severe COVID‐19 has two phases, an initial viral replication phase followed by an inflammatory phase. It has been proposed that the window of opportunity for antiviral treatment should be no longer than 1 week from symptom onset. 1 After that, immunomodulatory therapy should be the main course of action. Since the patients’ median time from symptoms onset to treatment was 10 days, our results showed the therapeutical potential of IFNβ‐1b immunomodulatory effects on the treatment of the hyperinflammatory phase of the disease.
Our work had some limitations. Baseline characteristics differed between both groups. We tried to overcome this issue by using Cox regression adjusted for confounding variables. Moreover, our study was limited to one Health Department, and the groups are small, which could affect its external validation and its statistical power. Therefore, our results should be interpreted cautiously.
Recent studies suggest that type I IFNs might be a safe and efficient treatment against SARS‐CoV‐2. In this line of research, our study shows that IFNβ‐1b, when administered in the inflammatory phase, is associated with reduced mortality and ICU admission in patients with moderate to severe COVID‐19.
REFERENCES
- 1. Horby P, Lim WS, Emberson JR, RECOVERY Collaborative Group , et al. Dexamethasone in Hospitalized Patients with COVID‐19—Preliminary Report. 2020. N Engl J Med. Jul 17: NEJMoa2021436. 10.1056/NEJMoa2021436 [DOI]
- 2. Tortajada C, Colomer E, Andreu‐Ballester JC, Esparcia A, Oltra C, Flores J. Corticosteroids for COVID‐19 patients requiring oxygen support? Yes, but not for everyone: effect of corticosteroids on mortality and intensive care unit admission in patients with COVID‐19 according to patients' oxygen requirements. J Med Virol. 2020. Oct 27 10.1002/jmv.26635 [DOI] [PubMed] [Google Scholar]
- 3. Acharya D, Liu G, Gack MU. Dysregulation of type I interferon responses in COVID‐19. Nat Rev Immunol. 2020;20(7):397‐398. 10.1038/s41577-020-0346-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sallard E, Lescure FX, Yazdanpanah Y, Mentre F, Peiffer‐Smadja N. Type 1 interferons as a potential treatment against COVID‐19. Antiviral Res. 2020;178:104791. 10.1016/j.antiviral.2020.104791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou Q, Chen V, Shannon CP, et al. Interferon‐α2b treatment for COVID‐19. Front Immunol. 2020;May 11:1061. 10.3389/fimmu.2020.01061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Loutfy MR, Blatt LM, Siminovitch KA, et al. Interferon alfacon‐1 plus corticosteroids in severe acute respiratory syndrome: a preliminary study. JAMA. 2003;290:3222‐3228. 10.1001/jama.290.24.3222 [DOI] [PubMed] [Google Scholar]
- 7. Omrani AS, Saad MM, Baig K, et al. Ribavirin and interferon alfa‐2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect Dis. 2014;14(11):1090‐1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. WHO . COVID‐19 Therapeutic Trial Synopsis [Internet]. Geneva, Switzerland: WHO; February 18, 2020. https://www.who.int/blueprint/priority-diseases/key-action/COVID-19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020.pdf
- 9. Davoudi‐Monfared E, Rahmani H, Khalili H, et al. A randomized clinical trial of the efficacy and safety of interferon β‐1a in treatment of severe COVID‐19. Antimicrob Agents Chemother. 2020;64(9):e01061‐20. 10.1128/AAC.01061-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang YQ, Tang SQ, Xu XL, et al. No Statistically apparent difference in antiviral effectiveness observed among ribavirin plus interferon‐alpha, lopinavir/ritonavir plus interferon‐alpha, and ribavirin plus lopinavir/ritonavir plus interferon‐alpha in patients with mild to moderate coronavirus disease 2019. Results of a randomized, open‐labeled prospective study. Front Pharmacol. 2020; Jul 11:1071. 10.3389/fphar.2020.01071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rahmani H, Davoudi‐Monfared E, Nourian A, et al. Interferon β‐1b in treatment of severe COVID‐19: a randomized clinical trial. Int Immunopharmacol. 2020;Nov 88:106903. 10.1016/j.intimp.2020.106903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Repurposed antiviral drugs for COVID‐19‐interim. WHO Solidarity trial results (version posted October 15, 2020; 10.1101/2020.10.15.20209817. medRxiv preprint [DOI] [PMC free article] [PubMed]


