Key Points.
Although COVID‐19 affects adults more, children can develop severe forms of this disease.
It is known that COVID‐19 causes very different clinical findings, endothelial damage with different mechanisms and arterial and venous thrombosis.
COVID‐related pro‐thrombotic events such as stroke, as seen in adult patients, can also occur in children.
Cerebrovascular events associated with COVID‐19 may be due to systemic inflammation and hypercoagulable state resulting from cytokine storm, post‐infectious immune‐mediated response and direct viral‐induced endotheliitis or endotheliopathy. 1 , 2 The reported incidence of stroke in COVID‐19‐hospitalised patients is 1.3–2.4%, 3 , 4 and young adult patients in their 30s are also reported to have COVID‐19‐related stroke. 5 , 6
Recently published case series and case reports revealed thromboembolic events in adult patients with COVID‐19. 4 , 7 To our knowledge, there are no reported cases of paediatric patients suffering stroke and resultant brain death due to cerebral arterial thrombosis associated with COVID‐19. Here, we present the case of a 7‐year‐old girl who suffered from a catastrophic intracranial arterial thromboembolic event.
Case Report
A previously healthy 7‐year 10‐month‐old girl was brought to the hospital due to a generalised tonic–clonic seizure, which lasted for 3–4 min. She was admitted to the paediatric ward, had two further vomits and ceftriaxone and acyclovir treatment was initiated. She awoke 4 h later, but was transferred to the paediatric intensive care unit due to listlessness and aphasia.
No pathological finding was found on the initial cranial computed tomography (CT) taken in the emergency room immediately after the seizure (Fig. 1a). At admission, prothrombin time and international normalised ratio values, fibrinogen, thrombocyte count, liver and kidney functions were normal, but complete blood count showed marked lymphopenia (0.75 × 109/L). The child had no other medical history. There was no family history of thrombophilia or stroke.
Fig 1.
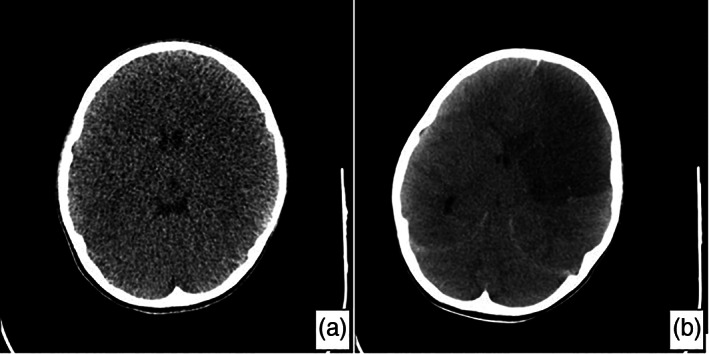
(a) Cranial cranial computed tomography (CT) taken on the first day in emergency room: normal. (b) Cranial CT 2 h after acute neurological decompensation that developed on the second day of hospitalisation: a large area of infarct involving the left frontal lobe and anterior parietal lobe, causing compression on the ventricle.
Six hours after the transfer to paediatric intensive care unit, the patient remained listless and had aphasia. Her vital signs showed high blood pressure and bradycardia. The patient was assumed to have cerebral oedema and empiric treatment with 3% sodium chloride and elevated head position were prescribed. Throat and nasal swabs were taken for SARS‐CoV‐2 polymerase chain reaction (PCR) in the context of the COVID‐19 pandemic and the patient's lymphopenia. Over the subsequent 7 h, the patient's pulse remained at about 60 beats per minute and her neurological findings were unchanged. She then rapidly developed tachycardia, her respiration became superficial and anisocoria developed. She was intubated using the rapid sequence intubation method and connected to mechanical ventilation. Shortly after (about 15 min), her pupils became fixed and dilated, and no respiratory effort nor reflex response was observed. Cranial CT was performed 2 h after neurological deterioration and revealed a large infarcted area within the brain parenchyma (Fig. 1b). Of the tests performed simultaneously, d‐dimer and C‐reactive protein were 3669 ng/L (normal range: 0–243 ng/L) and 37.5 mg/L (normal range: 0–5 mg/L), respectively. Her prothrombin time, international normalised ratio, ferritin level and homocysteine level were found to be in the normal range. Antiphospholipid antibodies IgG and IgM were negative, and both protein C (29%, normal range: 70–130%) and protein S (30%, normal range: 50–134%) were found to be below normal. Although the first test result was negative, the COVID‐19 SARS‐CoV‐2 PCR test result was positive in the sample taken from the tracheal aspirate 24 h later.
Brain magnetic resonance imaging revealed a large infarcted area affecting most of the left frontal lobe and the anterior part of the parietal lobe, creating a compression effect on the left lateral ventricle. Signal void in the left middle cerebral artery could not be clearly observed (Fig. 2).
Fig 2.
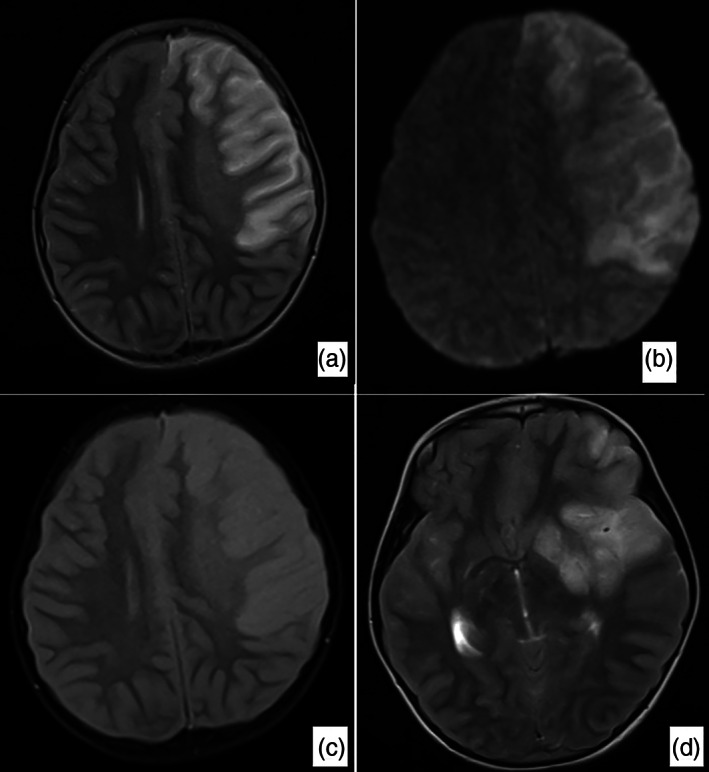
Brain magnetic resonance imaging, a large infarct area affecting the majority of the left frontal lobe and the anterior part of the parietal lobe and creating a compression effect on the left lateral ventricle was detected. Widespread signal increase was present in T2‐weighted, Fluid‐Attenuated Inversion Recovery (FLAIR) and Diffusion‐Weighted Imaging (DWI) sequences (a–c). In the T2‐weighted sequence, the signal void in the right middle and anterior cerebral artery was observed in the cross section passing through the Willis polygon level, while the signal void in the middle cerebral.
Cranial magnetic resonance angiography and CT angiography were also performed in our patient, but as these examinations were performed on the second day, total cerebral circulatory arrest was detected and local vascular occlusion could not be demonstrated angiographically.
Discussion
COVID‐19 is linked to a prothrombotic state causing venous and arterial thromboembolism and elevated d‐dimer levels. 5 Besides, hypercoagulation state caused by systemic inflammation and cytokine storm, post‐infectious immune‐mediated responses 1 and direct virally induced endothelitis or endotheliopathy can potentially lead to angiopathic thrombosis. 2
The rate of thromboembolic events in the course of viral infections is not low. In a study involving young adults, the stroke rate was found to be 7.6 times higher in COVID‐19 compared to that seen in influenza infection. 8 Due to the novelty of the disease and the insufficiency of data, thromboembolic complications and incidence estimates of COVID‐19 are not clearly defined. The incidence of the composite outcome of arterial and venous thromboembolic disease has been reported as 17.8% (range: 9.9–27.4) and the incidence of ischemic stroke is 1.8% (range: 1.3–2.4). 3
However, these rates reflect adult statistics and the prevalence in paediatric COVID‐19 patients is unknown. It has been reported that arterial and venous thromboembolism due to COVID‐19 is encountered in adults. 9 In another study in which five major vascular stroke cases were reported, the cases of stroke in young adults were evaluated, but the youngest patient in this patient group was reported to be 33 years. 5
In our case, there was no previous contact history or symptoms associated with COVID‐19. There was no finding in initial cranial CT when the child presented with an acute convulsion. The patient died of acute cerebral arterial infarction while she was awake but aphasic and listless in the postictal period after the seizure. SARS‐CoV‐2 PCR positivity was detected in the sample taken from the patient's tracheal aspirate. Thrombosis‐related laboratory parameters were determined. Our patient had no symptoms of COVID‐19 or a history of contact, so anticoagulation treatment was not given initially; however, early anticoagulation therapy should be considered in patients who are thought to have COVID‐19 to prevent thromboembolic events. 6 , 10
The low protein C and S values of our case were attributed to the fact that they were measured in the blood taken after a very rapidly developing thrombosis event and the consumption of these factors.
To our knowledge, our case is the first paediatric patient younger than 10 years who presented with generalised convulsion without any other symptoms associated with COVID‐19 and died due to acute cerebral arterial thrombosis associated with COVID‐19.
Acknowledgements
The authors thank all the intensive care staff and all health‐care professionals fighting COVID‐19.
Conflict of interest: None declared.
References
- 1. Ellul MA, Benjamin L, Singh B et al. Neurological associations of COVID‐19. Lancet Neurol. 2020; 19: 767–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Varga Z, Flammer AJ, Steiger P et al. Endothelial cell infection and endotheliitis in COVID‐19. Lancet 2020; 395: 1417–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kunutsor SK, Laukkanen JA. Incidence of venous and arterial thromboembolic complications in COVID‐19: A systematic review and meta‐analysis. Thromb. Res. 2020; 196: 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yaghi S, Ishida K, Torres J et al. SARS‐CoV‐2 and stroke in a New York healthcare system. (Published correction appears in Stroke 2020; 51: e179.). Stroke 2020; 51: 2002–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oxley TJ, Mocco J, Majidi S et al. Large‐vessel stroke as a presenting feature of Covid‐19 in the young. N. Engl. J. Med. 2020; 382: e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fifi JT, Mocco J. COVID‐19 related stroke in young individuals. Lancet Neurol. 2020; 19: 713–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mao L, Jin H, Wang M et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020; 77: 683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Merkler AE, Parikh NS, Mir S et al. Risk of ischemic stroke in patients with coronavirus disease 2019 (COVID‐19) vs patients with influenza. JAMA Neurol. 2020; 77: 1366–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guan WJ, Ni ZY, Hu Y et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020; 382: 1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moores LK, Tritschler T, Brosnahan S et al. Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019: CHEST guideline and expert panel report. Chest 2020; 158: 1143–63. [DOI] [PMC free article] [PubMed] [Google Scholar]


