Abstract
Introduction
During this long COVID‐19 pandemic outbreak, continuous positive airway pressure (CPAP) and noninvasive ventilation (NIV) are being widely used to treat patients with moderate to severe acute respiratory failure (ARF). As for now, data on the efficacy of NIV in COVID‐19 acute respiratory distress syndrome (ARDS) are lacking, and for this reason it is extremely important to accurately determine the outcomes of this strategy. This study aimed to evaluate clinical data and outcomes of NIV in patients with COVID‐19 ARDS.
Matherials and methods
Seventy‐nine consecutive patients with sudden worsening of respiratory failure were evaluated. All patients (71% male) had a confirmed SARS‐CoV‐2 infection and signs, symptoms and radiological findings compatible with COVID‐19 pneumonia and all of them underwent a trial of NIV. Primary outcomes were NIV success and failure defined by intubation and mortality rate. Secondary outcome was the duration of NIV.
Results
NIV was successful in 38 (48.1%) patients (Table 1). EOT was necessary in 21 patients (26.6%). Death occurred in 20 patients (25.3%). In the group of patients having failed a trial with NIV and then being intubated, compared to those who continued NIV, there was no higher mortality rate. By evaluating the ICU survival outcome of the subgroup of patients intubated after NIV, 57% of the patients were discharged and 43% died.
Conclusion
Previous studies conducted on patients undergoing invasive mechanical ventilation showed higher mortality rate than the present study. Our data showed that NIV can avoid intubation in almost half of the patients. Therefore, this data could reassure clinicians who would consider using NIV in COVID‐19 ARDS‐related treatment.
Keywords: acute respiratory failure, ARDS, COVID‐19, endotracheal intubation, noninvasive ventilation
1. INTRODUCTION
During the COVID‐19 pandemic outbreak, continuous positive airway pressure (CPAP) and noninvasive ventilation (NIV) are being widely used to treat patients with moderate to severe acute respiratory failure (ARF). 1 These ventilation methods allowed clinicians to treat a larger number of patients, mainly because they can be applied outside of intensive care units (ICU). Indeed, in this pandemic, a rapid increase in numbers of critically ill patients requiring invasive ventilation and NIV has occurred, resulting in a dramatic ICU beds saturation. 2 As for now, data on the efficacy of NIV in COVID‐19 acute respiratory distress syndrome (ARDS) are lacking, and for this reason it is extremely important to accurately determine the outcomes of this strategy.
2. MATERIALS AND METHODS
We retrospectively analysed clinical data on characteristics, ventilatory and pharmacological treatment and outcomes of patients with COVID‐19 ARDS admitted to the Pulmonology Unit of Azienda USL di Reggio Emilia‐IRCCS.
Primary outcomes were NIV success and failure defined by intubation and mortality rate. The secondary outcome was the duration of NIV.
NIV has been applied to patients admitted to our ward, who had a pO2/FiO2 ratio > 100 and ≤ 200 mmHg despite oxygen delivered through a Venturi mask (FiO2 60%) (Figure 1). NIV was provided using Philips V680 ™ (Respironics INC®, Pennsylvania, USA) or Hamilton G‐5 (Hamilton Medical AG, Bonaduz, Switzerland) mechanical ventilators through a full‐face mask. For NIV settings see Table 1.
FIGURE 1.
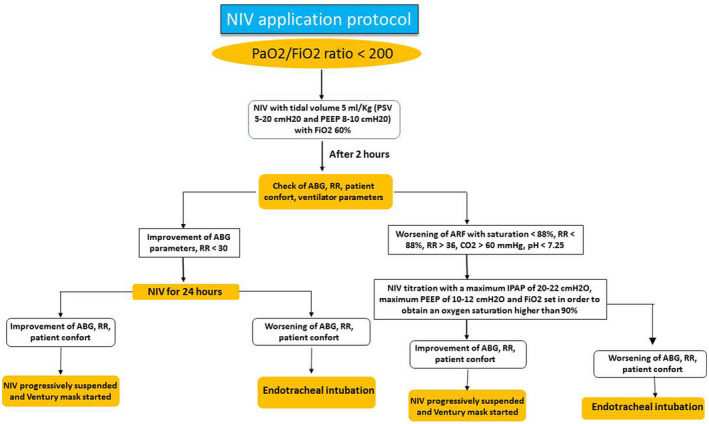
NIV application protocol
TABLE 1.
Demographics and clinical features
| P value | ||||
| No. of patients | 79 | |||
| Age (y), mean ± SD | 66.5 ± 11.4 | |||
| Male, n (%) | 56 (71) | |||
| Ex smokers, n (%) | 22 (28) | |||
| Former smokers, n (%) | 54 (68) | |||
| BMI, mean ± SD | 29.7 ± 5.2 | |||
| Number of comorbidities, mean ± SD | 2.9 ± 2.1 | |||
| Charlson Comorbidity index, mean ± SD | 3.4 ± 2.2 | |||
| SOFA index at admission, mean ± SD | 4.3 ± 1.3 | |||
| Symptoms | ||||
| Fever, n (%) | 78 (98) | |||
| Dyspnea, n (%) | 42 (53) | |||
| Cough, n (%) | 46 (58) | |||
| Fatigue, n (%) | 7 (9) | |||
| Days from symptoms onset to NIV application, mean ± SD | 10.2 ± 5.3 | |||
| CT features | ||||
| % extent, mean ± SD | 44.1 ± 17 | |||
| Presence of consolidations, n (%) | 49 (64) | |||
| Concomitant medication | ||||
| ACE‐i/ARB, n (%) | 41 (52) | |||
| Antiaggregants, n (%) | 19 (24) | |||
| β blockers, n (%) | 18 (23) | |||
| NIV settings | ||||
| Average duration (days), mean ± SD | 6.6 ± 4.5 | |||
| EPAP (cm H2O), mean ± SD | 9.46 ± 2.37 | |||
| IPAP (cm H2O), mean ± SD | 17.7 ± 2.2 | |||
| FiO2 (mmHg), mean ± SD | 63.1 ± 10.9 | |||
| ABG | Admission | 72 hour | 7 days | |
| pH | 7.45 ± 0.05 | 7.44 ± 0.07 | 7.44 ± 0.03 | 0.2 |
| pCO2 (mmHg), mean ± SD | 36.5 ± 6.2 | 40.7 ± 11.1 | 39.9 ± 5.8 | 0.006 |
| pO2 (mmHg), mean ± SD | 67.3 ± 20.2 | 85.7 ± 29.9 | 100.8 ± 42.7 | <0.0001 |
| pO2/FiO2 ratio mean ± SD | 120.1 ± 41.6 | 155.6 ± 78.6 | 191 ± 86.8 | <0.0001 |
| Respiratory rate | Admission | 72 h | 7 days | |
| Breaths/min. mean ± SD | 24.6 ± 4.9 | 25.6 ± 7.3 | 21.2 ± 5.7 | 0.04 |
| Outcomes | ||||
| NIV success, n (%) | 38 (48.1) | |||
| NIV failure, n (%) | 41 (51.9) | |||
| Intubations, n (%) | 21 (26.6) | |||
| Deaths, n (%) | 20 (25.3) | |||
| Not eligible for ICU, n (%) | 18 (22.7) | |||
| NIV duration, overall (days), mean ± SD | 6.6 ± 4.5 | |||
| In NIV success (days), mean ± SD | 8.7 ± 3.9 | |||
| In intubated (days), mean ± SD | 2.9 ± 3.2 | |||
| In deaths (days), mean ± SD | 6.3 ± 4.2 | |||
| Treatment | ||||
| Tocilizumab | ||||
| IV, n (%) | 28 (35.4) | |||
| SC, n (%) | 13 (16.5) | |||
| Steroids (at least methylprednisolone 0.75‐1 mg/kg/die), n (%) | 55 (70) |
Abbreviations: ACEi, angiotensin‐converting‐enzyme inhibitors; ARB, angiotensin II receptor blockers; BMI, body mass index; DC, subcutaneous; EPAP, expiratory positive airway pressure; FiO2, fraction of inspired oxygen; IPAP, inspiratory positive airway pressure; IV, intravenous; NIV, noninvasive ventilation; paCO2, Partial Pressure of Carbon Dioxide; pO2, partial pressure of oxygen; SD, standard deviation; SOFA, sequential organ failure assessment.
When FiO2 was < 50%, respiratory rate (RR) < 30 breaths per minute, expiratory tidal volume > 5 mL/kg body weight expected with a pressure support < 10 cmH2O and PEEP < 8 cmH2O; NIV was progressively suspended, and a Venturi oxygen mask with variable FiO2 was started on the basis of ABG data (Figure 1). Early (<48 h) and late (>48 h) NIV failure was defined according to ERS/ATS guidelines. 3 NIV failure and consequent need for intubation was defined as the persistence of low levels of oxygen saturation and high respiratory rate despite NIV; in addition, the persistency of a low pO2/FiO2 ratio (less than 100 mmHg despite optimal NIV settings) was indicative of NIV failure. (1) Before intubation and in order to avoid this procedure, NIV was titrated with a maximum IPAP of 20–22 cmH2O, maximum PEEP of 10–12 cmH2O and FiO2 set in order to obtain an oxygen saturation higher than 90%. On the other hand, these settings were checked in the single patient in order to avoid barotrauma and intolerance. We add these clarifications in the text.
In detail, the decision to intubate a patient was made on the basis of the following criteria: persistent or worsening of ARF (oxygen saturation <88%, respiratory rate >36/min) despite NIV, development of conditions requiring endotracheal intubation (EOT) in order to protect airways (coma or convulsive disorder) or to manage abundant tracheal and/or bronchial secretions; hemodynamic or electrocardiographic instability.
Prism 8.0 statistical software package (GraphPad Software, Inc., La Jolla, CA, USA) and SPSS software version 25 (IBM Corp., Armonk, NY, USA) were used for statistical analysis. Descriptive statistics for continuous variables were presented as mean ± standard deviation (SD). The nonparametric Wilcoxon test (Mann‐Whitney) and Student’s t test were used for comparison of two continuous variables, whilst Brown–Forsythe with Welch ANOVA test and Kruskal–Wallis test were used for comparison of more than two continuous variables, where appropriate. A Cox regression model was built to evaluate the factors associated with NIV failure. A P‐value less than 0.05 was considered significant.
3. RESULTS
Seventy‐nine consecutive patients with sudden worsening of respiratory failure were admitted to the Pulmonology Unit of the Santa Maria Nuova Hospital, between 10 March 2020 and 14 April 2020. All patients (71% male) had a confirmed SARS‐CoV‐2 infection and signs, symptoms and radiological findings compatible with COVID‐19 pneumonia, and all of them underwent a trial of NIV. Demographic and clinical features are shown in Table 1.
Of the 79 patients evaluated, mean arterial oxygen partial pressure (pO2 in mmHg) to fractional inspired oxygen (FiO2) ratio at baseline was 120.1 (SD = ±41.6), after 72 h of ventilation 155.6 (SD = ±78.6) and after 7 days was 191.0 (SD = ±86.8) (Figure 2).
FIGURE 2.
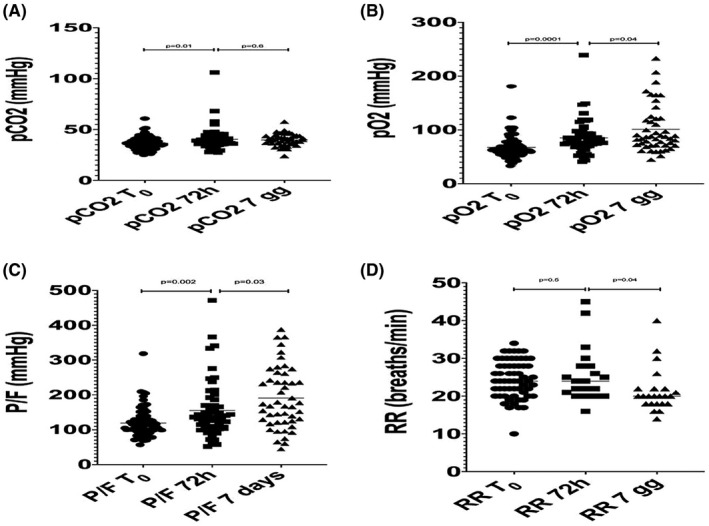
A, pCO2 variation; B, pO2 variation; P/F variation; D, Respiratory rate variation
NIV was successful in 38 (48.1%) patients (Table 1). EOT was necessary in 21 patients (26.6%). Death occurred in 20 patients (25.3%). Regarding the duration of NIV, in patients who were successful it continued for 8.7 days, in patients who underwent EOT it continued for 2.9 days and in the deceased continued for 6.3 days. As for the latter, 2/20 were eligible for intubation and 18/20 were not eligible for intubation due to age and numerous and severe comorbidities (arterial hypertension, heart failure, pulmonary embolism, obesity, diabetes, etc.). The significantly different features between the groups of success and failure of NIV were as follows: Charlson comorbidity index (P = 0.02), SOFA score at admission (P = 0.0002), use of β blockers (P = 0.02), pO2/FiO2 ratio at admission (P = 0.02), and the use of tocilizumab (P = 0.02) (Table 2). The univariate Cox regression model on the parameters associated with NIV failure showed that only SOFA score at admission (HR 1.46, 95%CI 1.19‐1.81, P = 0.0003), the use of tocilizumab (HR 0.49, 95%CI 0.26‐0.94, P = 0.03) and the use of β blockers (HR 2.13, 95%CI 1.11‐4.08, P = 0.023) were associated with this outcome. Finally, a multivariate Cox regression model considering SOFA at admission, apO2/FiO2 at admission, age, Charlson Comorbidity Index and days from symptoms onset to NIV application demonstrated that only SOFA score at admission was associated with the risk of failure (HR 1.38, 95%CI 1.07‐1.78, P = 0.013).
TABLE 2.
Multifactorial analysis on the parameters associated with success or failure of NIV
| Demographics | NIV success | NIV failure | P value |
|---|---|---|---|
| No. of patients (%) | 38 (48) | 41 (52) | |
| Age (year), mean ± SD | 65.2 ± 10.5 | 67.7 ± 12.1 | 0.32 |
| Male, n (%) | 28 (73.7) | 28 (68.3) | 0.62 |
| Former smokers, n (%) | 10 (26.3) | 13 (31.7) | 0.63 |
| Non smokers, n (%) | 28 (73.7) | 26 (63.4) | 0.35 |
| BMI, mean ± SD | 29.8 ± 5.6 | 29.6 ± 4.8 | 0.78 |
| Number of comorbidities, mean ± SD | 2.5 ± 2 | 3.3 ± 2.1 | 0.09 |
| Charlson Comorbidity index, mean ± SD | 2.8 ± 1.7 | 4 ± 2.5 | 0.02 |
| SOFA index at admission, mean ± SD | 3.7 ± 0.9 | 4.8 ± 1.4 | 0.0002 |
| Symptoms | |||
| Fever, n (%) | 38 (100) | 40 (97.6) | >0.9 |
| Dyspnea, n (%) | 20 (52.6) | 22 (53.7) | >0.9 |
| Cough, n (%) | 25 (65.8) | 20 (51.2) | 0.25 |
| Fatigue, n (%) | 3 (7.9) | 4 (9.8) | >0.9 |
| Days from symptoms onset to NIV application, mean ± SD | 10.6 ± 4.3 | 9.9 ± 6.2 | 0.3 |
| CT features | |||
| % extent, mean ± SD | 42.3 ± 14.9 | 45.9 ± 18.9 | 0.36 |
| Presence of consolidations, n (%) | 25 (65.8) | 24 (58.5) | 0.6 |
| Concomitant medication | |||
| ACE‐i/ARB, n (%) | 19 (50) | 22 (53.7) | 0.8 |
| Antiaggregants, n (%) | 6 (15.8) | 13 (31.7) | 0.12 |
| β blockers, n (%) | 4 (10.5) | 14 (34.2) | 0.02 |
| NIV settings | |||
| Average duration (days), mean ± SD | 8.7 ± 4 | 4.7 ± 4 | <0.0001 |
| EPAP (cm H2O), mean ± SD | 9.6 ± 2.3 | 9.3 ± 2.4 | 0.58 |
| IPAP (cm H2O), mean ± SD | 17.7 ± 2.5 | 17.7 ± 2 | 0.81 |
| FiO2 (mmHg), mean ± SD | 61.2 ± 7.8 | 64.9 ± 12.9 | 0.18 |
| Arterial blood gases and respiratory rate | |||
| pH at admission | 7.46 ± 0.05 | 7.44 ± 0.05 | 0.18 |
| at 72 h | 7.45 ± 0.03 | 7.41 ± 0.1 | 0.95 |
| at 7 days | 7.44 ± 0.3 | 7.47 ± 0.4 | 0.07 |
| pO2 at admission (mmHg), mean ± SD | 67.1 ± 14.9 | 67.6 ± 24.4 | 0.91 |
| at 72 h | 87.8 ± 19.3 | 82.1 ± 42.4 | 0.01 |
| at 7 days | 108.3 ± 45.2 | 78.3 ± 23.4 | 0.04 |
| pCO2 at admission (mmHg), mean ± SD | 36.2 ± 4.7 | 36.8 ± 7.3 | 0.98 |
| at 72 h | 39.7 ± 5 | 42.5 ± 17 | 0.88 |
| at 7 days | 40.6 ± 5.3 | 38 ± 7.1 | 0.31 |
| pO2/FiO2 at admission (mmHg), mean ± SD | 127.5 ± 35.8 | 113.3 ± 45.8 | 0.02 |
| at 72 h | 166.5 ± 67 | 141.5 ± 102 | 0.006 |
| at 7 days | 207.7 ± 79.6 | 139.6 ± 91.4 | 0.008 |
| RR at admission (breaths/min), mean (mmHg), mean ± SD | 25.1 ± 4.1 | 24.1 ± 5.9 | 0.48 |
| at 72 h | 22.9 ± 4.1 | 30.7 ± 9.5 | 0.04 |
| at 7 days | 19.4 ± 2.6 | 30 ± 9 | 0.04 |
| Treatment | |||
| Tocilizumab | 25 (65.8) | 16 (39) | 0.02 |
| IV, n (%) | 19 (50) | 9 (22) | 0.01 |
| SC, n (%) | 6 (15.8) | 7 (17) | >0.9 |
| Steroids (at least methylprednisolone 0.75‐1 mg/kg/die) | 28 (73.7) | 27 /75.8) | 0.47 |
Abbreviations: ACE‐i, angiotensin‐converting‐enzyme inhibitors; ARB, angiotensin II receptor blockers; BMI, body mass index; DC, Subcutaneous; EPAP, expiratory positive airway pressure; FiO2, fraction of inspired oxygen; IPAP, inspiratory positive airway pressure; IV, intravenous; NIV, noninvasive ventilation; paCO2, partial pressure of carbon dioxide; pO2, partial pressure of oxygen; SD, standard deviation; SOFA, sequential organ failure assessment.
In the group of patients having failed a trial with NIV and then being intubated, compared to those who continued NIV, there was no higher mortality rate (43% vs. 36%, P = 0.61). Intubation was applied only in 21 patients in our population. The other 20 patients were represented by 18 patients who had a do‐not‐intubate indication after a multidisciplinary evaluation (by the pulmonologist and the intensivist) due to the presence of severe comorbidities, for whom NIV was the last salvage therapy. The remaining two patients have not been evaluated, and they died of sudden death in a relatively stable phase of the disease. Between intubated patients, all 21 had both low oxygen saturation and pO2/FiO2 levels and high respiratory rate; in addition, two of them had hemodynamic instability, one of them coma and another one had abundant tracheal secretions.
By evaluating the ICU survival outcome of the subgroup of patients intubated after NIV, 57% of the patients were discharged whilst 43% died.
4. DISCUSSION
Gattinoni and colleagues recently pointed out two major phenotypes for COVID‐19 pneumonia. 4 The first one is called “type L,” due to low lung elastance present at diagnosis. Afterwards, some patients can worsen, showing another phenotype called “type H,” due to high lung elastance, much more similar to classical ARDS. The authors hypothesised that NIV may have a role, especially in L phenotype patients, reducing the risk of progression to H phenotype. As for now, in COVID‐19 ARDS patients, the decision as to whether to subject patients to NIV or to proceed with early EOT is still very controversial and debated. 5
In our hospital, nearly 400 patients with COVID‐19 were admitted between March and May 2020, during the first wave of the pandemic, in the period where our data have been collected. Our data concern the 79 patients treated with NIV admitted to our ward, the pulmonology unit, which was the only division where NIV was performed in the hospital. In other units, CPAP has been also performed as noninvasive respiratory support. Due to the lack of resources, our hospital was organised with a model of escalation of care: the patients were admitted at the Emergency Department and triaged on the severity of respiratory failure in three areas: the first where only conventional oxygen was administered, the second where CPAP and NIV has been performed (the pulmonology unit stand in this second area) and the third area was represented by the ICU, where mechanical ventilation has been carried out. Conventionally, NIV was applied in patient with a worsening of respiratory failure represented by a PaO2/FiO2 ratio of less than 200 and a respiratory rate more or equal of 25 breaths per minute. The patient admitted with a PaO2/FiO2 ratio between 200 and 300 mmHg were admitted to the first area of the hospital and treated with standard oxygen therapy. As we previously stated, the scarce availability of beds during the first wave of the pandemic did not allow us to treat patients earlier in the course of respiratory failure with NIV.
In this retrospective study, NIV strategy demonstrated efficacy in half of patients evaluated, despite a preventilation PaO2/FiO2 ratio similar to cohort of invasively ventilated patients in ICU. 6 In the group of nonresponder patients, NIV was stopped after 2.95 days, therefore, without a particular delay for EOT. NIV failure was correlated with older age (over 70 years) and with the presence of more comorbidities than responders’ group.
Other studies conducted on critically ill patients undergoing invasive mechanical ventilation (IMV) showed a mortality rate between 52.4% and 86.5%. 7 , 8 Mortality rate of our cohort was significantly lower. These data are even more important also because available data on NIV effectiveness in coronavirus ARDS are contradictory, scarce and mainly deriving from studies on Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS). 9 , 10 Moreover, WHO interim guidelines on ARF in COVID‐19 currently recommend NIV only in selected patients with hypoxemic respiratory failure. 11 Patients in our study had severe hypoxemic respiratory failure with normal pCO2 values. NIV use is largely beneficial in the COPD patients who were hypercapnic, and their reported benefits in this study despite relatively normal PaCO2 values suggest that these benefits are results of NIV improving hypoxemia and not on treating hypercapnia.
The multicenter observational study to UNderstand the Global impact of Severe Acute respiratory FailurE (LUNG SAFE) showed that there is no significant difference in the ICU and hospital mortality rates of ARDS patients undergoing NIV or mechanical ventilation, when ARDS severity, demographic characteristics and associated comorbidities of both treatment groups are matched. This study showed that the mortality and failure rate of NIV in the NIV group correlated with the severity of the patient’s respiratory failure. 12 A previous multicenter study showed that a 1 hour early NIV trial in ARDS patients on admission to the ICU may be useful to stratify them clinically and avoid unnecessary EOT in more than half of the patient population. 13 Several studies have reported the use of NIV in severe acute respiratory disease and have shown that it can avoid intubation in up to 70% of patients with hypoxic respiratory failure. In a retrospective study on COVID‐19 patients, 14 the authors demonstrated that mortality was higher in the intubated group (96%) than in the NIV group (92%). A similar study in COVID‐19 patients showed a mortality rate of 86% and 57% in the intubated and NIV groups, respectively. 15 A recent study reported a favourable outcome of NIV in COVID‐19 patients with a nonsevere form of respiratory failure. A low risk of airborne transmission to healthcare professionals (HCP) with a proper interface was also found in this work. 16 The possible generation of aerosols and the transmission of infection to HCP are a concern about the use of NIV during the COVID‐19 pandemic. The efficacy of NIV in 20 patients with SARS coronavirus (SARS‐CoV) infection and its risk of transmission amongst healthcare professionals was evaluated in a Chinese study. 17 The authors demonstrated that NIV was effective in treating SARS‐CoV‐associated acute respiratory failure, and none of the healthcare professionals tested positive for SARS‐CoV at the end of the study. Even a very recent Italian study has confirmed that the application of NIVs outside the ICU is feasible, safe for HCP and associated with favourable outcomes. 18
Previous studies on the field demonstrated that NIV failure is a risk factor for mortality in ARDS patients who undergo EOT. 12 For this reason, NIV has to be performed in highly monitored areas and by expert physicians. Nevertheless, in our study we did not find a higher mortality rate in patients having failed a trial with NIV and then being intubated, compared to those who continued NIV. On the other hand, it is crucial to find parameters able to predict NIV failure, in order to evaluate the best ventilatory strategy; in this view, it has been recently highlightened that the change in oesophageal pressure could be a reliable predictor of NIV outcome in the first 24 hour. 19
An important observation to report is that humidified high flow nasal cannula (HHFNC) was not routinely used in our Hospital because of shortage of devices during the first period of the pandemic. Furthermore, we preferentially use NIV for our better experience on this technique. For these reasons, NIV has been utilised in patients with a moderate to severe disease in our Unit as the first choice. On the other hand, during the outbreak there was also a low ICU bed availability, having some difficulties in treating many patients with mechanical ventilation.
To our knowledge, in literature there are not indications about the optimal titration of NIV settings both in COVD‐19 related respiratory failure and in de novo respiratory failure. In our study population, IPAP was titrated in order to optimise patient comfort, minimise the breathing efforts and achieve a tidal volume of approximately 400‐500 mL, considering the dead space of the face mask and the height and weight of the patient, in order to avoid barotrauma. PEEP was titrated on patients’ comfort and considering the weight. FiO2 was titrated in order to reach a SpO2 between 92% and 97%. The mean values (± standard deviation) of IPAP, PEEP and FiO2 were, respectively, 17.7 (±2.2), 9.5 (±2.4), and 63.1 (±10.8).
In our report there is a high mortality rate compared with other similar reports 18 . This could be explained by some important points: the high proportion of do‐not‐intubate patients, who received NIV as a last rescue therapy for their respiratory failure, the low PaO2/FiO2 ratio at admission in our ward and the high SOFA score at admission.
The principal limitation of our study is that it is a single centre study with a relatively small number of patients, however greater than in other previous studies. Another limitation is that it was conducted during a pandemic, with ICU beds availability varying over time; this aspect may have led to different patient selection criteria regarding the possible EOT.
5. CONCLUSIONS
COVID‐19 ARDS requires a greater understanding of the modalities of respiratory support and a best evaluation of the real effectiveness of NIV.
These preliminary data from our COVID‐19 referral Centre, focused on noninvasively ventilated patients with COVID‐19 ARDS, showed that NIV was effective in almost half of the patients. This also allowed to reduce the pressure on the ICU in a dramatic scenario, avoiding IOT in a large number of patients. A wider‐scale use of NIV could potentially help reduce a progressive and probably inevitable depletion of ICU resources in the event of a very high demand for beds. Nevertheless, multicentre studies on larger cases are needed in the future for more reliable evaluations.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest regarding the publication of this article.
AUTHOR CONTRIBUTIONS
F. Menzella designed the study, wrote and revised the paper. C. Barbieri and M. Fontana collected and analysed the data. P. Ruggiero, C. Castagnetti and G. Ghidoni carried out bibliographic research and contributed to the writing of the paper. F. Livrieri, R. Piro and L. Ghidorsi performed the statistical analysis. NF supervised and reviewed the manuscript.
ETHICAL APPROVAL
This study was approved by the Local Institutional Review Board (n° 2020/0045199). Since not all patients were able to give their informed consent, the Ethics Committee waived this requirement. Informed consent was sought from all surviving patients as soon as they regained their mental competence. All investigations were conducted according to the principles of the Helsinki Declaration.
Menzella F, Barbieri C, Fontana M, et al. Effectiveness of noninvasive ventilation in COVID‐19 related‐acute respiratory distress syndrome. Clin Respir J. 2021;15:779–787. 10.1111/crj.13361
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Piva S, Filippini M, Turla F, et al. Clinical presentation and initial management critically ill patients with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection in Brescia, Italy. J Crit Care. 2020;58:29‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Remuzzi A, Remuzzi G. COVID‐19 and Italy: what next? Lancet. 2020;395(10231):1225‐1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rochwerg B, Brochard L, Elliott MW, et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J. 2017;50(2):1602426. [DOI] [PubMed] [Google Scholar]
- 4. Gattinoni L, Chiumello D, Caironi P, et al. COVID‐19 pneumonia: different respiratory treatments for different phenotypes?. Intensive Care Med. April 14, 2020;46(6):1099–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ñamendys‐Silva SA. Respiratory support for patients with COVID‐19 infection. Lancet Respir Med. 2020;8(4):e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zangrillo A, Beretta L, Scandroglio AM, et al. Characteristics, treatment, outcomes and cause of death of invasively ventilated patients with COVID‐19 ARDS in Milan, Italy. Crit Care Resusc. April 23, 2020;22(3):200–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arentz M, Yim E, Klaff L, et al. Characteristics and outcomes of 21 critically ill patients with COVID‐19 in Washington State. JAMA. 2020. 10.1001/jama.2020.4326. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centred, retrospective, observational study. Lancet Respir Med. 2020. 10.1016/S2213-2600(20)30079-5. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arabi YM, Arifi AA, Balkhy HH, et al. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome infection. Ann Intern Med. 2014;160:389‐397. [DOI] [PubMed] [Google Scholar]
- 10. Cheung TMT, Yam LYC, So LKY, et al. Effectiveness of noninvasive positive pressure ventilation in the treatment of acute respiratory failure in severe acute respiratory syndrome. Chest. 2004;126(3):845‐850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. World Health Organization . Clinical management of severe acute respiratory infection (SARI) when COVID‐19 disease is suspected: interim guidance, 13 March 2020. World Health Organization; 2020. https://apps.who.int/iris/handle/10665/331446. Accessed May 23, 2020. [Google Scholar]
- 12. Bellani G, Laffey JG, Pham T, et al. Noninvasive ventilation of patients with acute respiratory distress syndrome. Insights from the LUNG SAFE study. Am J Respir Crit Care Med. 2017;195:67‐77. [DOI] [PubMed] [Google Scholar]
- 13. Antonelli M, Conti G, Esquinas A, et al. A multiple‐center survey on the use in clinical practice of noninvasive ventilation as a first‐line intervention for acute respiratory distress syndrome. Crit Care Med. 2007;35:18‐25. [DOI] [PubMed] [Google Scholar]
- 14. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centred, retrospective, observational study. Lancet Respir Med. 2020;8:475‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cascella M, Rajnik M, Cuomo A, et al. Features, evaluation and treatment coronavirus (COVID‐19). In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. January 2020. Updated April 6, 2020. Available from https://www.ncbi.nlm.nih.gov/books/NBK554776/ [Google Scholar] [Google Scholar]
- 17. Cheung TMT, Yam LYC, So LKY, et al. Effectiveness of noninvasive positive pressure ventilation in the treatment of acute respiratory failure in severe acute respiratory syndrome. Chest. 2004;126:845‐850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Franco C, Facciolongo N, Tonelli R, et al. Feasibility and clinical impact of out‐of‐ICU non‐invasive respiratory support in patients with COVID‐19 related pneumonia. Eur Respir J. August 3, 2020;56(5):2002130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tonelli R, Fantini R, Tabbì L, et al. Early inspiratory effort assessment by esophageal manometry predicts noninvasive ventilation outcome in De Novo respiratory failure. A pilot study. Am J Respir Crit Care Med. 2020;202(4):558‐567. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


