Abstract
In the era of coronavirus disease 2019 (COVID‐19), the management of cardiac implantable electronic devices infections with concomitant viral infection has not been completely defined yet. In this explorable context, we report the first experience of a Cardiac resynchronization therapy with defibrillator (CRT‐D) implantation after transvenous lead extraction for endocarditis in a COVID‐19 patient. We describe both the measures and procedures implemented to reduce the cross‐infection in the operating room and our clinical practice to improving procedure effectiveness on patient care.
Keywords: cardiac resynchronization therapy with defibrillator, cardiovascular implantable electronic devices, covid‐19, endocarditis, transvenous lead extraction
1. INTRODUCTION
Transvenous lead/device extraction (TLE) is an integral part of management of patients with cardiovascular implantable electronic devices (CIEDs). Data on frequency of CIED lead extractions are estimated to be 10000 to 15000 annually worldwide. 1 , 2 Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) causing COVID‐19 has reached pandemic levels since March 2020. SARS‐CoV‐2 not only causes viral pneumonia but has major implications for the cardiovascular (CV) system. Patients with CV risk factors including male gender, advanced age, diabetes, hypertension and obesity as well as patients with established CV and cerebrovascular diseases have been identified as particularly vulnerable populations with increased morbidity and mortality when affected by COVID‐19. 3 Although autopsies of patients with COVID‐19 infection and limited clinical experiences have revealed infiltration of the myocardium by interstitial mononuclear inflammatory cells, 3 no clear recommendation were given for SARS‐CoV‐2‐associated myocarditis treatment. The Guidance on electrophysiology (EP) procedure considers TLE for endocarditis an urgent procedure within CIED patients. 4 We herein report our experience with the management of a CRT‐D patient with COVID‐19 who underwent TLE due to infective endocarditis (IE) and describe the measures and procedures implemented to reduce the cross‐infection in the operating room (OP). To the best of our knowledge no Hospital in Europe has performed and reported lead extraction and re‐implantation in the same procedure in COVID‐19 positive patients.
2. CASE REPORT
A 77‐years‐old man was admitted in our hospital in mid‐October 2020 presenting with low grade fever, fatigue, and dry cough in the last 7 days associated with hypotension and other signs of sepsis. He suffered an ischaemic stroke in 2019 and hence underwent percutaneous coronary intervention. He was also affected by aortic stenosis, type II diabetes, chronic obstructive pulmonary disease (COPD) and with a history of atrial fibrillation (AF). In March 2019 he underwent CRT‐D implantation due to complete atrioventricular (AV) lock with ventricular escape rhythm. Upon admission, auscultation revealed fine crackles bilaterally and a loud systolic murmur at the base of the heart with normal blood pressure (60/110 mmHg) and mild oxygen desaturation (96% in room air). Inflammatory markers and troponin were elevated (troponin T 388 ng/L; cut‐off for normal <14 ng/L). ECG showed paced rhythm. His chest radiograph showed both‐side, lower zone patchy consolidation (Figure 1) confirmed also by chest computed tomography (CT) findings that presented the typical signs of COVID‐19 pneumonitis: multifocal consolidation and ground‐glass opacification in both lungs in the lower lobe and a peripheral dominant distribution with total score of 8. Antibody test was positive confirming an immune response ongoing to the virus. The patient tested positive upon polymerase chain reaction (PCR) SARS‐CoV‐2 testing and was started on drug treatment (hydroxychloroquine and antiviral agents) as appropriate for the current indications. The patient was in a life‐threatening or disabling situation and his clinical condition was complicated by COPD and other comorbidities, as above described. His imaging examinations and microbiologic findings yielded positive catheter blood cultures compatible with a diagnosis of Staphylococcus epidermidis caused IE. The bacterial colonization resulted both in adherence to the leads and surface biofilm formation. In order to identify lead vegetations and valvular involvement, transthoracic (TTE) and transoesophageal echocardiography (TEE) were both performed and were consistent with IE (Figure 2). Colour Doppler examination revealed the presence of vegetations on leads with a diameter >10 mm (range, 10–20 mm). At the beginning of November 2020, the patient managed in our routine clinical practice 5 underwent TLE. All parts of the hardware system, the device and all leads were completely removed. Considering the SARS‐CoV‐2 infection and pacemaker dependency, a multidisciplinary team of cardiologists, cardiac surgeons, and anaesthesiologists determined that re‐implantation could not be delayed as suggested by the EHRA consensus paper. 6 After TLE, we contextually decided to approach the contralateral side for definitive CRT‐D re‐implantation on‐stage (Figure 3). The procedure was performed in accordance with ESC Guidance document 3 and “Handbook of COVID‐19 prevention and treatment” 7 in order to assess the level of protection to be adopted by health care providers (HCP) as well as donning/removing of personal protective equipment (PPE), respectively. A high‐level threshold of personal protection was applied with Level III protection: disposable surgical cap, medical protection mask (FFP3), work uniform, gown, disposable surgical gloves, full‐face respiratory protective devices and powered air‐purifying respirator (PAPR) including eyes protection. The patient had a surgical mask in accordance with World Health Organization recommendations. The procedure was completed in the presence of a negative pressure OR (routinely disinfected and sterilized) and by employing a limited number of healthcare operators: one nurse, one operator, one radiologist at the console and one anaesthesiologist outside the OR. At disposal “support staff” ready to intervene in case of haemodynamic instability of the patient was readily available in case of need. As additional precaution, in order to minimize the exposure of the technical team, sterile electrical extension cords were adopted to connect the OR with a clean pre‐operative room. A temporary lead and an external pacemaker as a bridge to a new system were necessary. Intubation threshold was lowered to avoid emergent intubation and aerosol generation in the OR, analgo‐sedation was employed. Among all available tools, the modern 80‐Hz GlideLight laser sheath (Spectranetics, Colorado Springs, CO, USA) as powered sheath and the novel Bridge Occlusion Balloon (Spectranetics, Colorado Springs, CO, USA) as rescue tool were also employed. The total procedure was performed in 2.5 h. No major complications both in terms of operative and postoperative in‐hospital events, were reported, although patients with IE were at a significantly higher risk of major complication during the extraction procedure. 2 No cross‐infection after TLE and re‐implantation was verified. Finally, the patient was transferred to the sub‐intensive care unit (ICU). After 7 days of sub‐ICU his clinical conditions improved. At 1 month following initial diagnosis of IE, a TEE was performed showing absence of IE recurrences.
FIGURE 1.
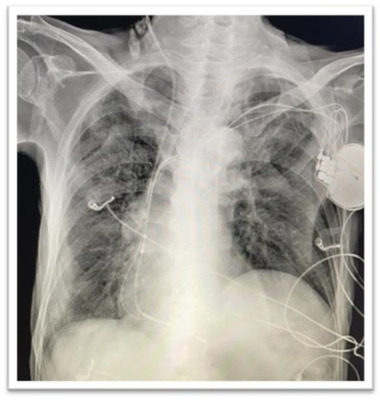
Chest x‐rays in a cardiac resynchronization therapy with defibrillator (CRT‐D) patient with COVID‐19 pneumonia pre‐transvenous lead extraction (TLE) [Colour figure can be viewed at wileyonlinelibrary.com]
FIGURE 2.
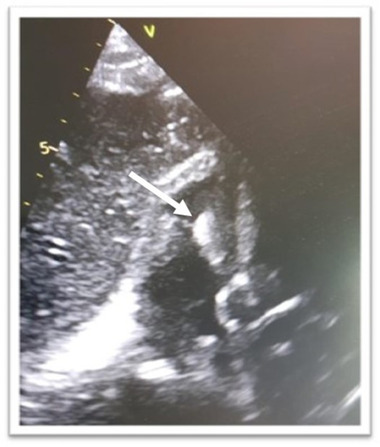
Transesophageal echocardiogram showing leads vegetation [Colour figure can be viewed at wileyonlinelibrary.com]
FIGURE 3.
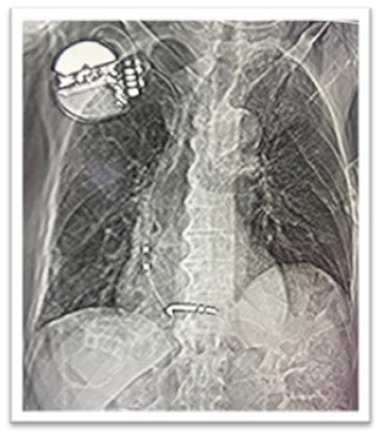
Chest x‐rays of cardiac resynchronization therapy with defibrillator (CRT‐D) contralateral re‐implantation after transvenous lead extraction (TLE) [Colour figure can be viewed at wileyonlinelibrary.com]
3. DISCUSSION
In the era of COVID‐19, the management of cardiac implantable electronic device infections with concomitant viral infection has not been completely defined yet. Several guidelines have already proposed general approaches concerning the management of CIED infection associated with bacteremia or sepsis; however, no real‐world data are currently available in this specific setting. According to Guidance on EP procedures, 4 as urgent elective EP procedure, we decided to perform TLE for endocarditis in order to substantially decrease the risk of clinical decompensation. The choice to intervene immediately (after 3 days the diagnosis of CIED infection) was made so as to decrease hospital mortality and avoid future hospitalizations. 2 During the procedure all protective measures were implemented and more appropriate procedures were set up in order to prevent nosocomial infections both for health care personnel and for the patient. 8 All types of risks were assessed by a multidisciplinary team: intra and inter procedural risks such as the decision to proceed immediately with contralateral CRT‐D re‐implantation on‐stage. We were aware of possible consequences, re‐implantation after TLE could increase the time of exposure of the potential viral contagion for the medical staff but it was necessary to guarantee the optimal treatment for the patient. Indeed, according to our clinical practice, the contextual re‐implantation minimizes the rate of IE recurrences at long term follow‐up; in our experience the immediate antibiotic treatment with daptomycin avoids the formation of biofilm considered as possibly responsible of IE recurrences. In this scenario the use of modern tools such as 80‐Hz powered sheath has enabled both to complete leads removal and to shorten extraction times with a decrease in general procedural risk. In our experience the precautions and related management procedures have played a crucial role in the prevention of nosocomial spread of the virus. We cannot establish if endocarditis was triggered by SARS‐COV‐2 infection, as no long‐term effects of a COVID‐19 infection are known yet, but these effects of a SARS‐coronavirus infection justify surveillance of recovered COVID‐19 infection patients. In our opinion, the adoption of new protocols as the immediate re‐implantation after TLE in addition to modern technology contributes to improving procedure effectiveness on patient care.
4. CONCLUSION
Scientific progress, modern tools and management procedures are essential for the success of procedures, in COVID‐19 patients above all.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The patient provided informed consent. A copy of consent is available for review by the Editor‐in‐Chief of this journal.
CONFLICTS OF INTEREST
None declared.
AVAILABILITY OF DATA AND MATERIALS STATEMENT
The data that support the findings of this case report are available from the corresponding author upon reasonable request.
De Vivo S, Barberio M, Corrado C, et al. CRT implantation after transvenous lead/device extraction (>TLE) in a patient with COVID‐19: Endocarditis triggered by syndrome coronavirus 2 (SARS‐COV‐2) infection? A case report. Pacing Clin Electrophysiol. 2022;45:807–810. 10.1111/pace.14218
Funding informationNone.
REFERENCES
- 1. Wilkoff BL, Love CJ, Byrd CL, et al. Transvenous lead extraction: Heart Rhythm Society expert consensus on facilities, training, indications, and patient management: this document was endorsed by the American Heart Association (AHA). Heart Rhythm. 2009;6(7):1085‐104. [DOI] [PubMed] [Google Scholar]
- 2. Sood N, Martin DT, Lampert R, Curtis JP, Parzynski C, Clancy J. Incidence and predictors of perioperative complications with transvenous lead extractions real‐world experience with National Cardiovascular Data Registry. Circ Arrhythm Electrophysiol. 2018;11(2):e004768. [DOI] [PubMed] [Google Scholar]
- 3. Daniele A, Elena A, Emanuele B, et al. European Society of Cardiology guidance for the diagnosis and management of cardiovascular disease during the COVID‐19 pandemic: part 1—epidemiology. European Heart Journal, 2022 [DOI] [PMC free article] [PubMed]
- 4. Lakkireddy DR, Chung MK, Gopinathannair R, et al. Guidance for cardiac electrophysiology during the COVID‐19 pandemic from the Heart Rhythm Society COVID‐19 task force; electrophysiology section of the American College of Cardiology; and the Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology, American Heart Association. Heart Rhythm. 2020;17(9):e233‐e241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kusumoto FM, Schoenfeld MH, Wilkoff BL, et al. HRS Expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm Soc. 2017;14(12):e503‐e551. [DOI] [PubMed] [Google Scholar]
- 6. Blomström‐Lundqvist C, Traykov V, Erba PA, et al. European Heart Rhythm Association (EHRA) international consensus document on how to prevent, diagnose, and treat cardiac implantable electronic device infections‐endorsed by the Heart Rhythm Society (HRS), the Asia Pacific Heart Rhythm Society (APHRS), the Latin American Heart Rhythm Society (LAHRS), International Society for Cardiovascular Infectious Diseases (ISCVID) and the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) in collaboration with the European Association for Cardio‐Thoracic Surgery (EACTS). Europace. 2020;22(4):515‐549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liang T Handbook of COVID‐19 Prevention and Treatment. 2020
- 8. Anesthetic management of patients with COVID 19 infections during emergency procedures. J Cardiothorac Vasc Anesth. 2020;34(5):1125‐1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this case report are available from the corresponding author upon reasonable request.


