Abstract
Aim
This study aims to assess rates of antibiotic prescriptions and its determinants in in children with COVID‐19 or Multisystem Inflammatory Syndrome (MIS‐C).
Methods
Children <18 years‐old assessed in five Latin Americas countries with a diagnosis of COVID‐19 or MIS‐C were enrolled. Antibiotic prescriptions and factors associated with their use were assessed.
Results
A total of 990 children were included: 921 (93%) with COVID‐19, 69 (7.0%) with MIS‐C. The prevalence of antibiotic use was 24.5% (n = 243). MIS‐C with (OR = 45.48) or without (OR = 10.35) cardiac involvement, provision of intensive care (OR = 9.60), need for hospital care (OR = 6.87), pneumonia and/or ARDS detected through chest X‐rays (OR = 4.40), administration of systemic corticosteroids (OR = 4.39), oxygen support, mechanical ventilation or CPAP (OR = 2.21), pyrexia (OR = 1.84), and female sex (OR = 1.50) were independently associated with increased use of antibiotics. There was significant variation in antibiotic use across the hospitals.
Conclusion
Our study showed a high rate of antibiotic prescriptions in children with COVID‐19, in particular in those with severe disease or MIS‐C. Prospective studies are needed to provide better evidence on the recognition and management of bacterial infections in COVID‐19 children.
Keywords: COVID‐19, SARS‐COV‐2, antibiotics, stewardship
Abbreviations
- MIS‐C
multisystem inflammatory syndrome
- NICU
neonatal intensive care unit
- PICU
pediatric intensive care unit
- RT‐PCR
real time polymerase chain reaction
- CDC
centers for disease and control
Key Notes.
To date, there are no comprehensive data on antibiotic use in children with COVID‐19 and Multisystem Inflammatory Syndrome.
Our study showed a high rate of antibiotic prescriptions in children with COVID‐19 and in particular in those with severe disease or Multisystem Inflammatory Syndrome
High antibiotic prescriptions may fuel antibiotic resistance, better local guidelines are needed to ensure that antibiotics are prescribed to those with higher risk of bacterial co‐infections
1. INTRODUCTION
Months after the first description of COVID‐19 in China, growing evidence is raising about the impact of SARS‐CoV‐2 infection on the pediatric population. Several studies from China, 1 Europe, 2 , 3 United States 4 and Latin America 5 are clarifying that COVID‐19 in children is typically mild, although patients with medically complex conditions or those of minority race/ethnicity deserve more attention because they may be at risk of more severe disease. 4
The Multisystem Inflammatory Syndrome (MIS‐C), an entity not yet fully clarified related to SARS‐CoV‐2, is a severe complication of the exposition to the virus, which may require Intensive Care Admission, mechanical ventilation and cardio‐respiratory support, rarely leading to death. 6 This clinical syndrom is characterized, by fever, systemic inflammation, and multisystem involvement, most commonly abdominal and cardiac, apparently driven by an uncontrolled immune response activated by the virus, where specific immune cells and autoantibodies can play a role. 7 This scenario overlaps also the toxic shock syndrome related with Staphylococcus aureus and other bacteria, making the clinical differential diagnosis difficult.
Because SARS‐CoV‐2 is a viral infection, and the resulting disease is usually mild in children, it is not expected that a child with COVID‐19 would routinely receive antimicrobials. This is particularly true for the second period of the pandemic, when the non‐utility of azithromycin, initially suggested as a drug with potential anti‐viral properties, 8 has been showed. 9 The MIS‐C can be an exception to this concept, since the severe and acute presentation may be similar to the toxic‐shock syndrome and available consensus documents suggest empiric wide‐spectrum antibiotic therapy until bacterial infections are ruled‐out. 10
Nevertheless, there are growing concerns about the possible negative impact of the pandemic on antimicrobial use. While this is particularly discussed for adults with COVID‐19, 11 Velasco‐Arnaiz et al 12 reported preliminary data suggesting that the pandemic has the potential to have a significant impact on antimicrobial use in the pediatric inpatient population. They did assess antibiotic prescriptions during and before the pandemic, but did not assess directly antibiotic use and its determinants in COVID‐19 children.
Since cases are constantly raising worldwide, it is expected that SARS‐CoV‐2 will circulate still for a long time, therefore the appropriate management of children with COVID‐19 is a priority. While the pandemic only determined a limited direct impact on children, inappropriate prescriptions have the potential of worsening an already dangerous situation, i.e. antimicrobial resistance.
Due to the gap in available literature, we performed a multinational study in Latin America aiming to assess the use of antibiotics in children with COVID‐19 and understand the determinants of its use.
2. MATERIALS AND METHODS
2.1. Study design and participants
This study is part of an ongoing independent project assessing COVID‐19 and MIS‐C in Latin American children, already presented elsewhere 13 and with a previous published paper describing an initial group of 409 children with confirmed COVID‐19. 5 For the current study, we aimed to assess determinants of antibiotic use in children with COVID‐19 or with MIS‐C. We implemented the previously used dataset 2 , 5 including data regarding name of antibiotic used and the reason why the attending clinician decided to administer antibiotics. The remaining variables are those previously used and included age, gender, symptoms, imaging, underlying medical conditions, need for hospital and NICU/PICU admission, respiratory and cardiovascular support, other viral co‐infections, drugs used to treat COVID‐19, development of MIS‐C and type of organ involvement, and outcome.
SARS‐CoV‐2 infection was defined as a positive PCR test on nasopharyngeal swab or, in case of shortage of nasopharyngeal swabs/PCR tests given the context of the study, children with a compatible clinical presentation and clinical history with a positive serological test were included.
MIS‐C due to SARS‐CoV‐2 was defined according to the CDC criteria: An individual aged <21 years (we only included if younger than 18 years) presenting with (i) fever, (ii) laboratory evidence of inflammation, and (iii) evidence of clinically severe illness requiring hospitalization, with multisystem (>2) organ involvement (cardiac, renal, respiratory, hematologic, gastrointestinal, dermatologic or neurological); and (iv) no alternative plausible diagnoses; and (v) positive for current or recent SARS‐CoV‐2 infection by RT‐PCR, serology, or antigen test; or exposure to a suspected or confirmed COVID‐19 case within the 4 weeks prior to the onset of symptoms.
The study was reviewed and approved by the CoviD in sOuth aMerIcaN children—study GrOup core group and approved by the Ethics Committee of the coordinating center and by each participating center (Mexico: COMINVETICA‐30072020‐CEI0100120160207; Colombia: PE‐CEI‐FT‐06; Peru: No. 42‐IETSI‐ESSALUD‐2020; Costa Rica: CEC‐HNN‐243‐2020). The study was conducted in accordance with the Declaration of Helsinki and its amendments. No personal or identifiable data were collected during the conduct of this study.
2.2. Statistical analysis
Summary statistics for the study sample were presented as counts and percentages. The association of relevant demographic and clinical characteristics with antibiotic use was assessed using a multivariable logistic regression model; the effect size of covariates was expressed by odds ratios (ORs) with 95% confidence intervals (CIs). The variables considered in this analysis were age, sex, medical history of immunodeficiency, immunosuppressants or chemotherapy, hospital care, pyrexia, upper and lower respiratory tract infections, gastrointestinal symptoms, headache, chest X‐ray abnormalities, respiratory support, administration of systemic corticosteroids, and diagnosis of MIS‐C, both with and without cardiac involvement. A set of dummy variables for individual hospitals was also included in the model to adjust for the potential bias of confounding by center. All data were analyzed using the Stata 15 software (StataCorp. 2017. Stata Statistical Software: Release 15. StataCorp LLC). The significance level was set at 5% and all tests were 2‐sided.
3. RESULTS
3.1. Study population
A total of 990 children were enrolled: 921 children (93.0%) with COVID‐19 and 69 children (7.0%) with MIS‐C (Peru (n = 383, 38.7%), Costa Rica (n = 299, 30.2%), Argentina (n = 253, 25.6%), Colombia (n = 43, 4.3%) and Mexico (n = 12, 1.2%).
The demographic and clinical characteristics of the 990 study patients are summarized in Table 1. The median age was 3 years (interquartile range: 1–9), ranging from 2 days to 17 years; 484 (48.9%) were female. The most common known source of transmission of the infection was a parent, considered the index case in 281 (28.4%) cases. A total of 303 (30.6%) children were admitted to hospital and 47 (4.7%) required admission to a Pediatric Intensive Care Unit (PICU).
TABLE 1.
Characteristics of the study sample (n = 990)
| Characteristic | n | % |
|---|---|---|
| Female sex | 484 | 48.9 |
| Age group | ||
| 0 year | 202 | 20.4 |
| 1–2 years | 229 | 23.1 |
| 3–5 years | 144 | 14.5 |
| 6–11 years | 247 | 24.9 |
| 12–17 years | 168 | 17.0 |
| COVID‐19 confirmed by real‐time PCR | 639 | 64.5 |
| Positive SARS‐CoV‐2 IgG | 352 | 35.6 |
| Delay between onset and diagnosis | ||
| 0–1 day | 437 | 44.1 |
| 2–7 days | 460 | 46.5 |
| >7 days | 93 | 9.4 |
| Likely index case | ||
| Parent | 281 | 28.4 |
| Sibling | 14 | 1.4 |
| Other | 120 | 12.1 |
| Unknown | 575 | 58.1 |
| Medical history | ||
| Known history of BCG vaccine | 740 | 74.7 |
| Pre‐existing medical conditions | 128 | 12.9 |
| Immunosuppressants at the time of diagnosis | 11 | 1.1 |
| Primary or secondary immunodeficiency | 8 | 0.8 |
| Chemotherapy over the last 6 months | 8 | 0.8 |
| Admitted to the hospital | 303 | 30.6 |
| Intensive care during hospital stay | 47 | 4.7 |
| Symptoms | ||
| Pyrexia (≥38.0/≥100.4°C/°F) | 677 | 68.4 |
| Upper respiratory tract infection | 466 | 47.1 |
| Diarrhea and/or vomiting | 301 | 30.4 |
| Lower respiratory tract infection | 215 | 21.7 |
| Headache | 104 | 10.5 |
| Chest X‐ray | ||
| Not performed | 705 | 71.2 |
| Negative | 193 | 19.5 |
| Positive (abnormal findings a and/or ARDS b ) | 92 | 9.3 |
| Respiratory support | ||
| Oxygen support | 117 | 11.8 |
| Mechanical ventilation | 31 | 3.1 |
| Continuous positive airway pressure (CPAP) | 11 | 1.1 |
| Extracorporeal membrane oxygenation (ECMO) | 0 | 0.0 |
| Administration of inotropes | 29 | 2.9 |
| Co‐infections detected in respiratory samples(s) c | 14 | 1.4 |
| Drug administration | ||
| Systemic corticosteroids | 90 | 9.1 |
| Intravenous immunoglobulin (IVIG) | 60 | 6.1 |
| Hydroxychloroquine | 9 | 0.9 |
| Oseltamivir | 8 | 0.8 |
| Lopinavir or ritonavir | 3 | 0.3 |
| Non‐corticosteroid immunosuppressants | 3 | 0.3 |
| Favipiravir | 2 | 0.2 |
| Remdesivir | 2 | 0.2 |
| Chloroquine, ribavirin or zanamivir | 0 | 0.0 |
| MIS‐C diagnosis | ||
| No | 921 | 93.0 |
| Yes, with no cardiac or joint involvement | 33 | 3.3 |
| Yes, with cardiac involvement d | 23 | 2.3 |
| Yes, with joint involvement | 11 | 1.1 |
| Yes, with cardiac and joint involvement d | 2 | 0.2 |
| Tocilizumab administration to treat MIS‐C | 8 | 0.8 |
| Current status | ||
| All symptoms resolved | 969 | 97.9 |
| Dead e | 8 | 0.8 |
| Still symptomatic | 7 | 0.7 |
| Long‐term sequelae | 6 | 0.6 |
| Center | ||
| Peru | 383 | 38.7 |
| Costa Rica | 299 | 30.2 |
| Argentina | 253 | 25.6 |
| Colombia | 43 | 4.3 |
| Mexico | 12 | 1.2 |
Abbreviations: ARDS, Acute respiratory distress syndrome; BCG, bacillus Calmette–Guérin; COVID‐19, coronavirus disease 2019; IgG, immunoglobulin G; MIS‐C, multisystem inflammatory syndrome; PCR, polymerase chain reaction; SARS‐CoV‐2, Severe acute respiratory syndrome coronavirus 2.
45 cases of interstitial disease, 30 cases of consolidation, 4 cases of pleural effusion and 13 unspecified diagnoses.
3 cases of interstitial disease, 3 cases of consolidation and 10 unspecified diagnoses.
8 mycoplasmas, 3 rhinoviruses, 1 cytomegalovirus, 1 Epstein–Barr virus and 1 unspecified virus.
10 cases of pericardial effusion, 6 cases of coronary dilatation, 5 cases of myocarditis and 4 cases of “other” cardiac involvement.
Mean time from symptom onset to death was 14 ± 8 days, ranging from 3 to 27.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Fever was reported in 677 cases (68.4%); 466 (47.1%) children had symptoms suggestive of upper respiratory tract infection while 215 (21.7%) had lower respiratory tract symptoms; 301 (30.4%) had gastrointestinal symptoms. A chest radiograph was done in 285 (28.8%) patients. Of these, 92 (32.3%) had abnormal X‐ray findings. Respiratory co‐infections (confirmed by PCR) were detected in 14 (1.4%) children. Mean length from symptoms onset and microbiological test was 3.4 days (SD 5.9 days).
A total of 118 individuals required respiratory support. Among them, 31 (3.1%) required mechanical ventilation and 11 (1.1%) continuous positive airway pressure (CPAP), all the others low‐flow oxygen therapy. among these patients, 37 (3.7%) had multiple respiratory support (i.e., oxygen plus CPAP and/or mechanical ventilation)”. A total of 29 (2.9%) patients required inotropic support. Eight children died (0.8%). Further details described in Table 1.
Bacteria were isolated from cultures in 13 cases. Escherichia coli in five cases (three from urine, two from peritoneal fluid), Methicilline‐resistant S. aureus in two cases (from skin pus), S. pyogenes in one case (from pharynx), E. faecalis in one (urine), P. aeroginosas in one (broncoalveolar fluid), K. pneumonia in one (peritoneal fluid), S. hominis in one (blood). None of these patients with culture‐positive infections were diagnosed with MIS‐C.
3.2. Antibiotic use in COVID‐19 and MIS‐C children
The prevalence of antibiotic use was 24.5% (n = 243). As shown in Figure 1, sepsis was the most common reason for administering antibiotics (22.6%), followed by pneumonia (13.6%), surgical causes (11.5%) and upper or mild respiratory infections (9.5%). Information about the classes of antibiotics used was available for 153 (63.0%) patients. Among the 84 patients that received single antibiotic therapies, 32 (13.2%) were prescribed ceftriaxone, 13 (5.3%) azithromycin, 10 (4.1%) cefotaxime, 9 (3.7%) amoxicillin, 6 (2.5%) clindamycin, 2 (0.8%) ampicillin, 2 (0.8%) cefalexine, 2 (0.8%) cefalotin, 2 (0.8%) cefepime, 2 (0.8%) trimethoprim, and the remaining 4 (1.6%) amikacin, ciprofloxacin, clarithromycin or metronidazole. The other 69 patients who were prescribed combination therapies received amikacin plus ampicillin in 16 cases (6.6%), meropenem plus vancomycin in 10 (4.1%), cefotaxime plus metronidazole in 10 (4.1%), amikacin plus ceftazidime in 9 (3.7%), ceftriaxone plus vancomycin in 5 (2.1%), ceftriaxone plus metronidazole in 5 (2.1%), ampicillin plus gentamicin in 4 (1.6%), cefotaxime plus clindamycin in 3 (1.2%), amikacin plus cefotaxime in 1 (0.4%), cefotaxime plus vancomycin in 1 (0.4%), and triple combinations of amikacin, ampicillin, cefotaxime, ceftazidime, clindamycin, meropenem, metronidazole or vancomycin in the remaining 5 (2.1%). The percentage distribution of single and combination antibiotic therapies grouped in classes is illustrated in Figure 2. The length of antibiotic treatment was available in just 94 out of 243 patients (38.7%): mean = 6.9 ± 4.5 days, median [IQR] =7 days [4–7], range = 2–21 days. The rate of antibiotic prescriptions remained stable during the whole study period with an average decrease of −0.6% (95% CI −2.6%, 1.3%) from April 2020 to October 2020 (Figure 3).
FIGURE 1.
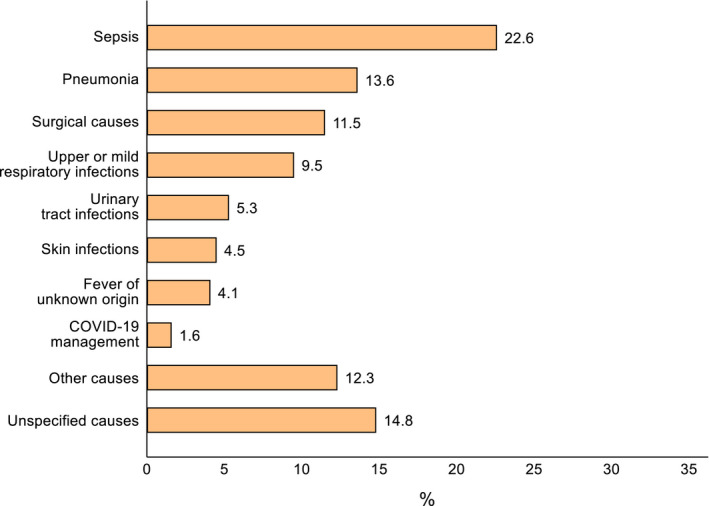
Clinical reasons for antibiotic use (n = 243). Sepsis was defined on a clinical diagnosis
FIGURE 2.
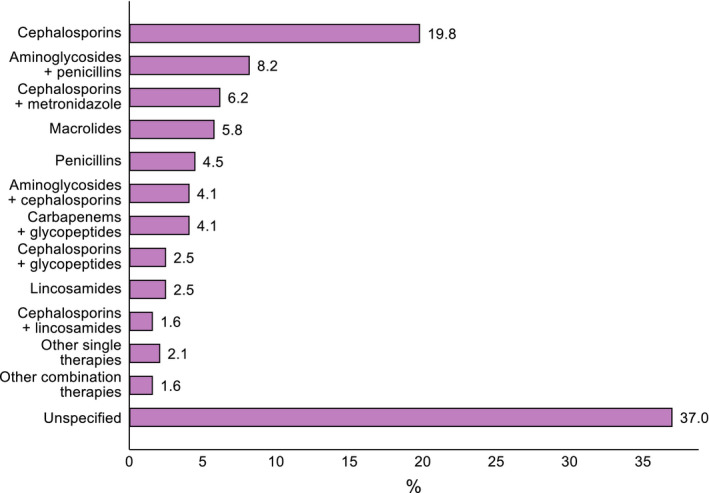
Classes of antibiotics administered to the patients, alone or in combination (n = 243). Notes: Other single therapies include aminoglycosides, fluoroquinolones, mentronidazole and trimethoprim; other combination therapies include multiple prescriptions of aminoglycosides, carbapenems, cephalosporins, glycopeptides, penicillins or metronidazole
FIGURE 3.
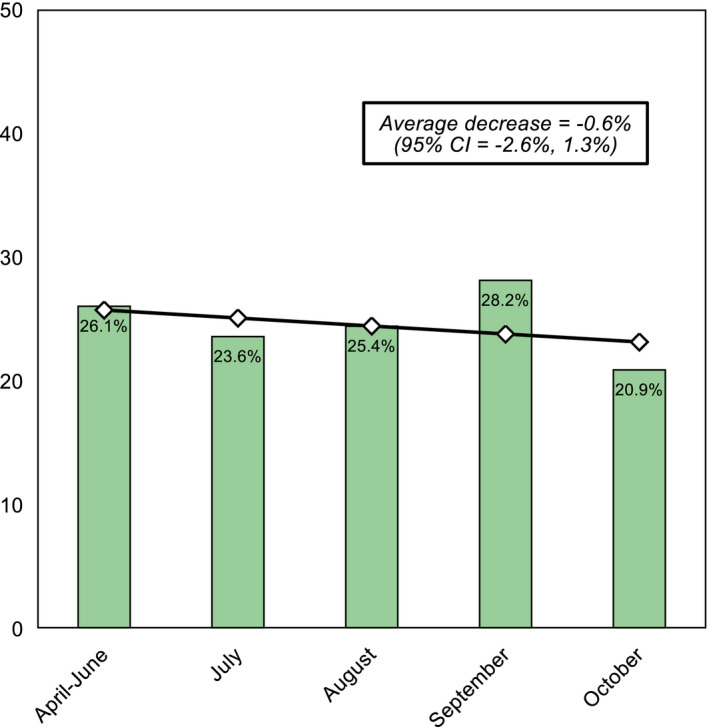
Prevalence of antibiotic use between April and October 2020. Notes: Linear trend was assessed using a linear regression model with variance‐weighted least squares
On multivariable analysis (Table 2), MIS‐C with cardiac involvement (OR = 45.48), MIS‐C with no cardiac involvement (OR = 10.35), provision of intensive care (OR = 9.60), need for hospitalization (OR = 6.87), abnormal X‐ray findings and/or ARDS detected through chest X‐rays (OR = 4.40), administration of systemic corticosteroids (OR = 4.39), oxygen support, mechanical ventilation or CPAP (OR = 2.21), pyrexia (OR = 1.84), and female sex (OR = 1.50) were independently associated with increased use of antibiotics. On the contrary, lower respiratory tract infections not suggestive of pneumonia/ARDS and not requiring respiratory support (OR = 0.34) were independently associated with decreased use of antibiotics. Of note, MIS‐C was associated with more deaths (5.8% vs. 0.4%), as compared to COVID‐19 without MIS‐C (Fisher's exact p‐value <0.001), highlighting the more severe picture of MIS‐C children. We also found large and significant variations in antibiotic use across the hospitals.
TABLE 2.
Multivariable logistic regression analysis of antibiotics use (n = 990).
| Characteristic | Odds ratio | p‐value | 95% confidence interval | |
|---|---|---|---|---|
| Lower bound | Upper bound | |||
| Sex | ||||
| Male | Ref. | |||
| Female | 1.50 | 0.040 | 1.02 | 2.21 |
| Age group | ||||
| 0 year | Ref. | |||
| 1–2 years | 0.63 | 0.118 | 0.35 | 1.13 |
| 3–5 years | 0.82 | 0.556 | 0.43 | 1.58 |
| 6–11 years | 1.13 | 0.670 | 0.65 | 1.98 |
| 12–17 years | 0.92 | 0.821 | 0.47 | 1.82 |
| Immunosuppressants, immunodeficiency or chemo | ||||
| No | Ref. | |||
| Yes | 1.65 | 0.451 | 0.45 | 6.05 |
| Hospitalization | ||||
| No | Ref. | |||
| Yes, without intensive care | 6.87 | <0.001 | 4.34 | 10.89 |
| Yes, with intensive care | 9.60 | <0.001 | 2.77 | 33.27 |
| Pyrexia (≥38.0/≥100.4°C/°F) | ||||
| No | Ref. | |||
| Yes | 1.84 | 0.011 | 1.15 | 2.96 |
| Upper respiratory tract infection | ||||
| No | Ref. | |||
| Yes | 1.08 | 0.730 | 0.71 | 1.65 |
| Diarrhea and/or vomiting | ||||
| No | Ref. | |||
| Yes | 1.05 | 0.822 | 0.67 | 1.64 |
| Lower respiratory tract infection | ||||
| No | Ref. | |||
| Yes | 0.34 | 0.007 | 0.16 | 0.74 |
| Headache | ||||
| No | Ref. | |||
| Yes | 0.88 | 0.746 | 0.42 | 1.87 |
| Chest X‐ray abnormalities | ||||
| No | Ref. | |||
| Yes | 4.40 | <0.001 | 1.99 | 9.71 |
| Oxygen support, mechanical ventilation and/or CPAP | ||||
| No | Ref. | |||
| Yes | 2.21 | 0.050 | 1.002 | 4.88 |
| Administration of systemic corticosteroids | ||||
| No | Ref. | |||
| Yes | 4.39 | <0.001 | 2.01 | 9.58 |
| MIS‐C diagnosis | ||||
| No | Ref. | |||
| Yes, w/o cardiac involvement | 10.35 | 0.050 | 1.005 | >100 |
| Yes, w/ cardiac involvement | 45.48 | 0.011 | 2.44 | >100 |
| Center | ||||
| Hospital San Bartolomé (PE) | Ref. | |||
| Hospital Nacional de Niños (CR) | 0.58 | 0.034 | 0.34 | 0.96 |
| Hospital Isidoro Iriarte (AR) | 0.25 | <0.001 | 0.14 | 0.46 |
| Hospital Edgardo Rebagliati Martins (PE) | 0.04 | 0.014 | 0.00 | 0.53 |
| Hospital Pablo Tobón Uribe (CO) | 0.05 | <0.001 | 0.01 | 0.25 |
| Clínica Jasmédica (PE) | 1.48 | 0.466 | 0.51 | 4.28 |
| Hospital General Regional 200 Tecámac (MX) | 12.24 | 0.045 | 1.06 | >100 |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
4. DISCUSSION
In our study, the prevalence of antibiotic prescribing in children with COVID‐19 and MIS‐C was 24.5%. We found significant variations in classes of antibiotics used and even large differences across the hospitals. The rate of antibiotic prescriptions was significantly higher in children with MIS‐C, those requiring respiratory support, those with radiologic evidence of pneumonia/ARDS and those with fever. Interestingly, younger children and those with symptoms suggestive of lower respiratory tract infections without radiologic evidence of pneumonia/ARDS and not requiring respiratory support were less frequently prescribed with antibiotics. Importantly, also the only need for admission to the hospital was associated with a higher rate of antibiotic prescription. To our knowledge, this is the first multinational study assessing the use of antibiotics in children with COVID‐19 and MIS‐C, therefore pediatric studies to compare our findings are not available.
Velasco‐Arnaiz et al 12 are the only authors that evaluated antibiotic use in a pediatric referral center before and during the pandemic. The use of azithromycin, initially considered as first‐line therapy in severe COVID‐19 patients in combination with hydroxychloroquine, increased, particularly in PICU setting. The use of ceftriaxone and teicoplanin, doubled in the PICU in April 2020 compared with April 2019. In non‐PICU patients, piperacillin‐tazobactam and ciprofloxacin use increased. Other antibiotics for community‐acquired infections were prescribed less than in the same period in 2019, and cefazolin use decreased due to the dramatic drop in the number of surgeries. Also in our cohort, cephalosporins were frequently prescribed, while, interestingly, macrolides represented only 9.2% of all prescriptions. This is probably because the peak of pediatric cases in Latin America was registered when the concept of utility of azythromicine in COVID‐19 was weaker. We were not aware of any issues with antibiotic shortage which may have influenced antibiotic choices.
Confirmed or suspected sepsis was the main reason for antibiotic prescription. This was an expected finding, since the pathogenesis 7 and the more severe clinical presentation of MIS‐C overlap with those of sepsis, and there is general consensus for starting broad‐spectrum antibiotics in these children. 10 However, MIS‐C children represented only 7.0% of the entire cohort, while 24.5% of children received antibiotics. These data suggest a potential overuse of empirical antibiotics in COVID‐19 children. Considering that COVID‐19 is often a milder disease in children compared with adults, 14 the pediatric community is expected to empirically use antibiotics less frequently. However, the rate of prescriptions we detected is not widely different from those reported in adult studies. In fact, in our study, the need for hospital admission was independently associated with a higher probability of receiving antibiotic (OR 6.87, 95% CI 4.34–10.89). Addressing adult studies, Seatone et al reported that 38.3% of COVID‐19 patients were prescribed antibiotics. Antibiotic prevalence was 45.0%, and 73.9% were prescribed for suspected respiratory tract infection. Amoxicillin, doxycycline and co‐amoxiclav accounted for over half of all antibiotics in non‐critical care wards, and meropenem, piperacillin‐tazobactam and co‐amoxiclav accounted for approximately half prescribed in critical care. 15
Although there are no data on bacterial co‐infections in children with COVID‐19 that may inform better policies of pediatric antimicrobial stewardships during the pandemic, even in adults, where COVID‐19 is having a much more severe impact, the burden of bacterial co‐infections seems to be relatively low in most published studies. 11 , 16 , 17 , 18 , 19 , 20 , 21 , 22 Buehrle et al found bacterial infections in 31% (5/16) of COVID‐19 patients, while antibiotics were administered to 56% (9/16) of patients during hospitalization, but 100% (9/9) of patients requiring ICU care. 11 In Spain, Garcia‐Vidal et al 16 found that 31/989 (3%) COVID‐19 adults presented with community‐acquired co‐infections, mainly Streptococcus pneumoniae and S. aureus pneumonia. Hospital‐acquired infection was diagnosed in 43/989 patients (4%), with 25/44 (57%) occurring in critical care (mainly Pseudomonas aeruginosa, E. coli, Klebsiella spp., and S. aureus). Coagulase‐negative staphylococci were the most common organisms causing documented bloodstream infection (7/16; 44%). Low observed rates of bacterial and fungal infection in COVID‐19 patients have also been reported from the UK, where Hughes identified bacterial infection in 51/ 836 COVID‐19 patients (6%). 17 , 18 A review of eighteen full texts showed that 62/806 (8%) COVID‐19 patients experienced bacterial/fungal co‐infection during hospital admission, while on secondary analysis, 1450/2010 (72%) of patients were found to have received antimicrobial therapy. 19 One Italian study even saw a reduction in Clostridioides difficile infections in hospitalized patients. 20 In a rapid review, Fattorini et al found that only 1.3% of 522 COVID‐19 patients in intensive care units, and apparently no COVID19 patients in other units, developed a healthcare‐associated super‐infection with antimicrobial‐resistant bacteria. 21 , 22
In our study, having signs or symptoms suggestive of lower respiratory tract infections, without radiologic evidence of pneumonia/ARDS, was associated with a lower probability of receiving antibiotics. This finding may be explained by the fact that in pediatrics such presentations are usually suggestive of a clinical diagnosis of bronchiolitis, wheezing or asthma, conditions that do not require routine antibiotic administration.
Our study clearly shows a high variability of reasons for antibiotic prescriptions and regimens chosen, as well as a significant variability among different centers. These findings highlight the uncertainties that physicians daily face in the management of COVID‐19 patients. While the World Health Organization currently recommends against the prescribing of antimicrobials in mild to moderate COVID‐19 cases without clear indication of bacterial infection, 23 the difficulty in differentiating COVID‐19 from bacterial infections on initial presentation challenges clinicians and antimicrobial stewardship practices. 24 Almost after one year of the pandemic, there is no evidence to support decision‐making on bacterial infection and antimicrobial stewardship in the context of COVID‐19, 18 particularly in children. This uncertainty is likely to drive unnecessary antimicrobial prescribing in COVID‐19 children who are unlikely, according to adult evidences, to benefit from empiric antibiotic prescriptions. This scenario will potentially increase the selection of drug resistant infections 25 and will make patients more vulnerable to bacterial infections, even during future viral pandemics that may favor bacterial co‐infections from drug resistant bugs. 26
Our study has some limitations to address. We did not collect bacteria isolation and antibiotic sensitivities throughout the pandemic in the participating centers. Blood results, including inflammatory markers, were not collected. In addition, an independent expert did not assess the appropriateness of antibiotic prescription, nor the length of administration. The main reason for this approach was that Latin American clinicians are still struggling in the front‐line, with hospitals having limited human resources to dedicate extra time for clinical research. Last, a large proportion of children have not been tested with PCR test on nasopharyngeal test due to unavailability of them during certain periods of the pandemic, as may have happened in LMICs settings worldwide. The presence of IgG in these patients may be due to the fact that some patients have been evaluated several days from symptoms onset. In any case, it is possible that some cases have been misdiagnosed, although local experts were allowed to include the patients if history and clinical findings, along with tests, were considered suggestive for COVID‐19. In order to allow wider participation of clinicians from LMICs which may have experienced lack of resources, we decided to include these cases. Despite these limitations, this study provides the largest overview of antibiotic use in children with COVID‐19 and MIS‐C to date.
In conclusion, our study showed a high rate of antibiotic prescriptions in children with COVID‐19 and in particular in those with severe disease or MIS‐C. Importantly, we found a significant variation in reasons for prescriptions of antibiotics and type of chosen therapies, as well in hospital practices, highlighting current uncertainties and lack of guidelines for the recognition of bacterial infections in COVID‐19 children. Prospective studies are urgently needed to provide better evidence on the recognition and management of bacterial infections in COVID‐19 children, as well as to develop dedicated antimicrobial stewardship programs.
CONFLICT OF INTEREST
Nothing to declare.
ETHICAL APPROVAL
Approved by each institution (codes provided in methods).
ACKNOWLEDGEMENTS
We are grateful to all collaborators that helped the development of the DOMINGO study group.
Funding information
The corresponding authors had full access to all the data and had the final responsibility for the decision to submit for publication.
DATA AVAILABILITY STATEMENT
The dataset generated for this study is available upon request to the corresponding author.
REFERENCES
- 1. Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID‐19 among children in China. Pediatrics. 2020;145(6):e20200702. 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 2. Götzinger F, Santiago‐García B, Noguera‐Julián A, et al. ptbnet COVID‐19 Study Group. COVID‐19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020;4(9):653‐661. 10.1016/S2352-4642(20)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Parri N, Lenge M, Cantoni B, et al. COVID‐19 in 17 Italian pediatric emergency departments. Pediatrics. 2020;146(6):e20201235. 10.1542/peds.2020-1235 [DOI] [PubMed] [Google Scholar]
- 4. Bailey LC, Razzaghi H, Burrows EK, et al. Assessment of 135 794 pediatric patients tested for severe acute respiratory syndrome coronavirus 2 across the United States. JAMA Pediatr. 2021;175(2):176. 10.1001/jamapediatrics.2020.5052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Antúnez‐Montes OY, Escamilla MI, Figueroa‐Uribe AF, et al. COVID‐19 and multisystem inflammatory syndrome in Latin American children: a multinational study. Pediatr Infect Dis J. 2021;40(1):e1‐e6. 10.1097/INF.0000000000002949 [DOI] [PubMed] [Google Scholar]
- 6. Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS‐CoV‐2. JAMA. 2020;324(3):259‐269. 10.1001/jama.2020.10369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buonsenso D, Riitano F, Valentini P. Pediatric inflammatory multisystem syndrome temporally related with SARS‐CoV‐2: immunological similarities with acute rheumatic fever and toxic shock syndrome. Front Pediatr. 2020;11(8):574. 10.3389/fped.2020.00574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Damle B, Vourvahis M, Wang E, Leaney J, Corrigan B. Clinical pharmacology perspectives on the antiviral activity of azithromycin and use in COVID‐19. Clin Pharmacol Ther. 2020;108(2):201‐211. 10.1002/cpt.1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cavalcanti AB, Zampieri FG, Rosa RG, et al. Hydroxychloroquine with or without Azithromycin in Mild‐to‐Moderate Covid‐19. N Engl J Med. 2020;383(21):2041‐2052. 10.1056/NEJMoa2019014. Erratum in: N Engl J Med. 2020 Nov 19;383(21):e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harwood R, Allin B, Jones CE, et al. A national consensus management pathway for paediatric inflammatory multisystem syndrome temporally associated with COVID‐19 (PIMS‐TS): results of a national Delphi process. The Lancet Child & Adolescent Health. 2021;5(2):133‐141. 10.1016/S2352-4642(20)30304-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Buehrle DJ, Decker BK, Wagener MM, et al. Antibiotic consumption and stewardship at a hospital outside of an early coronavirus disease 2019 epicenter. Antimicrob Agents Chemother. 2020;64(11):e01011‐e1020. 10.1128/AAC.01011-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Velasco‐Arnaiz E, López‐Ramos MG, Simó‐Nebot S, et al. Pediatric antimicrobial stewardship in the COVID‐19 outbreak. Infect Control Hosp Epidemiol. 2020;24:1‐3. 10.1017/ice.2020.312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Antúnez‐Montes OY, Escamilla MI, Figueroa‐Uribe AF, et al. COVID‐19 in South American children: a call for action. Pediatr Infect Dis J. 2020;39(10):e332‐e334. 10.1097/INF.0000000000002851 [DOI] [PubMed] [Google Scholar]
- 14. Viner RM, Mytton OT, Bonell C, et al. Susceptibility to SARS‐CoV‐2 infection among children and adolescents compared with adults: a systematic review and meta‐analysis. JAMA Pediatr. 2021;175(2):143. 10.1001/jamapediatrics.2020.4573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Seaton RA, Gibbons CL, Cooper L, et al. Survey of antibiotic and antifungal prescribing in patients with suspected and confirmed COVID‐19 in Scottish hospitals. J Infect. 2020;81(6):952. 10.1016/j.jinf.2020.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garcia‐Vidal C, Sanjuan G, Moreno‐García E, et al. Incidence of co‐infections and superinfections in hospitalized patients with COVID‐19: a retrospective cohort study. Clin Microbiol Infect. 2021;27(1):83‐88. 10.1016/j.cmi.2020.07.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hughes S, Troise O, Donaldson H, Mughal N, Moore LSP. Bacterial and fungal coinfection among hospitalized patients with COVID‐19: a retrospective cohort study in a UK secondary‐care setting. Clin Microbiol Infect. 2020;26(10):1395‐1399. 10.1016/j.cmi.2020.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rawson TM, Wilson RC, Holmes A. Understanding the role of bacterial and fungal infection in COVID‐19. Clin Microbiol Infect. 2021;27(1):9‐11. 10.1016/j.cmi.2020.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rawson TM, Moore LSP, Zhu N, et al. Bacterial and fungal co‐infection in individuals with coronavirus: A rapid review to support COVID‐19 antimicrobial prescribing. Clin Infect Dis. 2020:71(9):2459‐2468. 10.1093/cid/ciaa530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bentivegna E, Alessio G, Spuntarelli V, et al. Impact of COVID‐19 prevention measures on risk of health care‐associated Clostridium difficile infection. Am J Infect Control. 2020;S0196‐6553(20)30891‐9. Epub ahead of print. 10.1016/j.ajic.2020.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fattorini L, Creti R, Palma C, et al. Bacterial coinfections in COVID‐19: an underestimated adversary. Ann Ist Super Sanita. 2020;56(3):359‐364. 10.4415/ANN_20_03_14 [DOI] [PubMed] [Google Scholar]
- 22. Monnet DL, Harbarth S. Will coronavirus disease (COVID‐19) have an impact on antimicrobial resistance? Euro Surveill. 2020;25(45):2001886. 10.2807/1560-7917.ES.2020.25.45.2001886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. World Health Organization . Clinical management of COVID‐19. 2020. https://www.who.int/publications/i/item/clinical‐management‐of‐covid‐19 [PubMed]
- 24. Huttner BD, Catho G, Pano‐Pardo JR, Pulcini C, Schouten J. COVID‐19: don't neglect antimicrobial stewardship principles! Clin Microbiol Infect. 2020;26:808‐810. 10.1016/j.cmi.2020.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Holmes AH, Moore LSP, Sundsfjord A, et al. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet. 2016;387(10014):176‐187. 10.1016/S0140-6736(15)00473-0 [DOI] [PubMed] [Google Scholar]
- 26. Vaillancourt M, Jorth P. The unrecognized threat of secondary bacterial infections with COVID‐19. MBio. 2020;11(4):e01806‐20. 10.1128/mBio.01806-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset generated for this study is available upon request to the corresponding author.


