Dear Editor,
Numerous skin manifestations associated with COVID‐19 infection have been reported so far. 1 , 2 , 3 They include vesicular or maculo‐papular skin rashes, livedoid/necrotic lesions, urticaria, chilblains‐like lesions and drug induced eruptions. 1
Clinical trial results for BNT162b2 mRNA Covid‐19 vaccine reported mild‐to‐moderate pain at the injection site within 7 days after administration, with severe pain in <1% of participants and redness or swelling in a lower percentage. Local reactions incidence did not increase after the second dose and were mostly mild‐to‐moderate and resolved within 1–2 days. 4
Since vaccines have been approved by regulatory authorities and administered on large scale, cases of severe allergic reaction, including anaphylaxis after receipt of the first dose, have been described for both Moderna and Pfizer vaccine. 5 , 6 Among the cases reported after Pfizer‐BioNTech COVID‐19 Vaccine, 21 patients manifested anaphylaxis with a rate of 11.1 per million doses administered: 17 of them had a documented history of allergies or allergic reactions, while seven patients had a history of anaphylaxis. 6 The onset of symptoms was reported to occur within few minutes after vaccine receipt.
Cutaneous manifestations after vaccination have not yet been described in the literature, except a recent overview on cutaneous reactions in clinical trials, with a set of consideration for counselling, prevention and management of possible cutaneous adverse reactions. 7 These included injection site pain and swelling and, for Moderna vaccine, injection site urticaria, maculo‐papular rash and reactions to dermal filler following vaccination. 7 Based on our experience, 3170 healthcare providers were vaccinated with Pfizer‐BioNTech COVID‐19 Vaccine, and 0.91% (29 cases) developed mild adverse effects. Among these cases, 38% (11 patients), reported in Table 1, developed cutaneous symptoms, such as erythemato‐oedematous reaction at injection site, diffuse morbilliform rash, mild erythema and positive dermographism (Fig. 1). One patient experienced, apart from a mild urticarial rash, a flare up of his previously well‐controlled atopic dermatitis under treatment with dupilumab (Fig. 1d). In four patients (36.3%) extracutaneous manifestations occurred such as laryngospasm, periorbital oedema, and angioedema; these data are consistent with CDC report. 6 All manifestations resolved spontaneously within 2–3 days without treatment, except in the patients with extracutaneous symptoms. In addition, the patient who manifested a relapse of atopic dermatitis underwent a short oral steroids course prescribed by his general practitioner. Although the majority of patients (72.7%, eight cases) had a previous history of allergy or allergic diathesis, the skin reactions were very mild.
Table 1.
Demographics, history and clinical features in 11 patients with cutaneous manifestations after vaccine receipt
| N | Sex | Age | Vaccine dose | Onset | Clinical features | Extracutaneous manifestations | Allergy‐related history |
|---|---|---|---|---|---|---|---|
| 1 | F | 67 | 1° | 1 day | Itchy erythemato‐oedematous plaque at injection site | N | N |
| 2 | F | 61 | 2° | 2 days | Erythema & swelling of left foot dorsum | N | N |
| 3 | F | 55 | 1° | 8 days | Erythema and itch of face | Y | Y |
| 4 | F | 59 | 2° | 3 days | Diffuse erythematous rash | Y | Y |
| 5 | F | 62 | 1° | 1 h | Itchy erythemato‐oedematous plaque at injection site | Y | Y |
| 6 | F | 38 | 1° | 1 h | Erythema of both legs | Y | Y |
| 7 | M | 56 | 1° | 1 h | Urticaria at injection site | N | Y |
| 8 | F | 56 | 2° | 5 h | Diffuse erythematous rash of trunk | N | Y |
| 9 | M† | 29 | 1° | 7 days | Erythema and swelling of left chest | N | Y |
| 10 | M | 36 | 2° | 48 h | Diffuse erythematous rash of trunk | N | N |
| 11 | M | 32 | 1° | 2 days | Urticarial rash, flare‐up of atopic dermatitis | N | Y |
F, female; M, male; N, No; Y, yes.
Previous SARS‐CoV‐2 infection.
Figure 1.
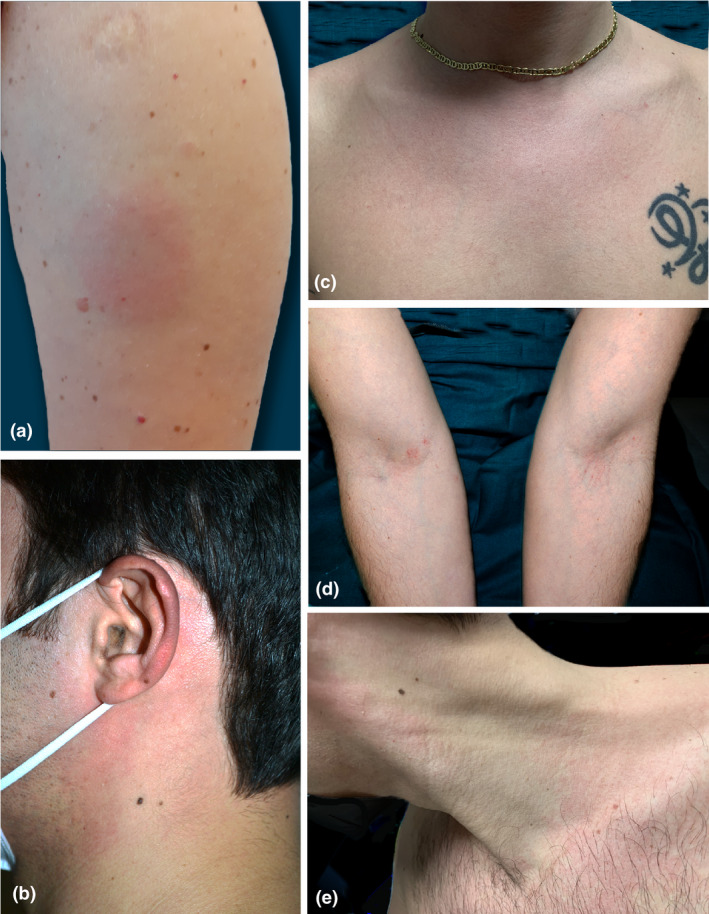
Spectrum of cutaneous manifestations after administration of Pfizer‐BioNTech COVID‐19 Vaccine. (a) Erythemato‐oedematous plaque at injection site; (b) Erythematous rash of neck and ear, (c) and on the chest; (d) Erythemato‐squamous and xerotic plaques of the antecubital fossae, at left excoriated erythematous linear lesions from scratching; and (e) erythema on the left lateral region of the neck and chest.
Media spread alarmism regarding severe anaphylactic reactions and a hypothetical exclusion of people with an allergic diathesis from vaccination. In our experience, cutaneous adverse reactions from COVID‐19 vaccine were very rare, all mild and characterized by rapid, and generally spontaneous resolution. Flares of pre‐existent dermatitis could alarm patients and physicians and doubts arise regarding the management of patient in therapy with biologic agents. Altogether cutaneous reactions observed in our series do not constitute a contraindication to a second dose of vaccine. The dermatologist, in collaboration with the colleagues of occupational medicine service, and immunologists should reassure patients for both recurrence of previously diagnosed cutaneous diseases and onset of new skin lesions.
Funding sources and conflict of interest
The article is original, never submitted elsewhere, no conflicts of interest are to be declared, and all authors contributed significantly to the manuscript. Two of the authors (MEH and AD) of this publication are members of the European Reference Network ERN‐SKIN and of vascular anomalies working group (VASCA WG) of the ERN for rare multisystemic vascular diseases (VASCERN).
Acknowledgements
We thank the patients for giving their written informed consent to publication to their clinical details and photos. We thank Mr. Gabriele Bacile for iconography preparation.
References
- 1. Andina D, Belloni‐Fortina A, Bodemer C et al. Skin manifestations of COVID‐19 in children: part 1. Clin Exp Dermatol 2021; 46: 444–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marzano AV, Genovese G, Moltrasio C et al. Italian skincovid‐19 network of the italian society of dermatology and sexually transmitted diseases (SIDeMaST). The clinical spectrum of COVID‐19‐associated cutaneous manifestations: an Italian multicentre study of 200 adult patients. J Am Acad Dermatol 2021; 84: 1356–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. El Hachem M, Diociaiuti A, Concato C et al. A clinical, histopathological and laboratory study of 19 consecutive Italian paediatric patients with chilblain‐like lesions: lights and shadows on the relationship with COVID‐19 infection. J Eur Acad Dermatol Venereol 2020; 34: 2620–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Polack FP, Thomas SJ, Kitchin N et al. safety and efficacy of the BNT162b2 mRNA Covid‐19 Vaccine. N Engl J Med 2020; 383: 2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. CDC COVID‐19 Response Team; Food and Drug Administration . Allergic reactions including anaphylaxis after receipt of the first dose of moderna COVID‐19 vaccine ‐ United States. December 21, 2020‐January 10, 2021. MMWR Morb Mortal Wkly Rep 2021; 70: 125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. CDC COVID‐19 Response Team; Food and Drug Administration . Allergic Reactions including anaphylaxis after receipt of the first dose of Pfizer‐BioNTech COVID‐19 vaccine ‐ United States. December 14‐23, 2020. MMWR Morb Mortal Wkly Rep 2021; 70: 46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rice SM, Ferree SD, Mesinkovska NA, Kourosh AS. The art of prevention: COVID‐19 vaccine preparedness for the dermatologist. Int J Womens Dermatol 2021; 7: 209–212. 10.1016/j.ijwd.2021.01.007. PMID: 33457487; PMCID: PMC7802521 [DOI] [PMC free article] [PubMed] [Google Scholar]


