Abstract
Solid organ transplant (SOT) recipients run a high risk for adverse outcomes from COVID-19, with reported mortality around 19%. We retrospectively reviewed all known Swedish SOT recipients with RT-PCR confirmed COVID-19 between March 1 and November 20, 2020 and analyzed patient characteristics, management, and outcome. We identified 230 patients with a median age of 54.0 years (13.2), who were predominantly male (64%). Most patients were hospitalized (64%), but 36% remained outpatients. Age >50 and male sex were among predictors of transition from outpatient to inpatient status. National early warning Score 2 (NEWS2) at presentation was higher in non-survivors. Thirty-day all-cause mortality was 9.6% (15.0% for inpatients), increased with age and BMI, and was higher in men. Renal function decreased during COVID-19 but recovered in most patients. SARS-CoV-2 antibodies were identified in 78% of patients at 1–2 months post-infection. Nucleocapsid-specific antibodies decreased to 38% after 6–7 months, while spike-specific antibody responses were more durable. Seroprevalence in 559 asymptomatic patients was 1.4%. Many patients can be managed on an outpatient basis aided by risk stratification with age, sex, and NEWS2 score. Factors associated with adverse outcomes include older age, male sex, greater BMI, and a higher NEWS2 score.
KEYWORDS: clinical research / practice, health services and outcomes research, immunosuppressant, infection and infectious agents - viral, infectious disease, kidney (allograft) function / dysfunction, organ transplantation in general, patient characteristics, patient survival
Abbreviations: BMI, body mass index; CCI, Charlson comorbidity index; CKD, chronic kidney disease; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; HFNC, high-flow nasal cannula; ICU, intensive care unit; LDH, lactate dehydrogenase; LMWH, low molecular weight heparin; MV, mechanical ventilation; NC, nasal cannula; NEWS2, National Early Warning Score 2; NOAC, non-vitamin K oral anticoagulant; RT-PCR, real-time polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SOT, solid organ transplant; WBC, white blood cells
1. INTRODUCTION
Solid organ transplant (SOT) recipients are considered a risk group regarding Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This may be due to, in part, immunosuppressive treatment and frequent comorbidities. A recent meta-analysis reports an overall mortality of 18.6% among SOT recipients.1 However, three relatively large studies from different countries2, 3, 4 have reported a mortality rate of around 10%, which is lower than observed in other extensive studies on SOT recipients with COVID-19.5, 6, 7, 8, 9, 10 This heterogenicity may be due to inherent shortcomings of single-center reports11 or limitations of registry studies.12 The majority of previously reported cohorts included a high proportion of hospitalized patients who are more likely to have severe disease. Outpatients who have less severe symptoms and better prognosis may have been missed, which could partly explain the difference between studies.
This inconsistency of available data confounds interpretation of the impact of COVID-19 on the transplanted patient population. Large and detailed studies of the relationship between patient management and outcomes, preferably in a multicenter setting, are crucial to creating efficient guidelines for managing affected transplant recipients. Furthermore, it is essential to explain how age, sex, body mass index (BMI), immunosuppression, and comorbidities affect the clinical course and outcomes.
COVID-19 is known to have adverse effects on renal function,13 and it is important to understand how this affects transplanted patients, particularly those with a renal graft. Lastly, given the rapid development of vaccines, it is crucial to examine whether patients on immunosuppressants can mount an adequate and lasting serological response.
Sweden has a highly developed, universally free, public healthcare system. While it was strained in the spring and autumn of 2020, it was not overwhelmed. From a policy viewpoint, the country has tackled the pandemic differently from many other countries, with no mandatory lockdowns and fewer obligatory restrictions. Authorities emphasized general recommendations concerning hygienic measures and social distancing to reduce the spread of the infection in the population.
Transplant recipients were initially encouraged to follow the age-stratified general recommendations from the public health agency without any additional self-isolation practices.
This report describes the Swedish national experience with COVID-19 in SOT recipients, accumulated at Sweden’s transplant centers. We present a national perspective on the patient variables, clinical management, outcomes, renal function, and serological response.
2. MATERIALS AND METHODS
2.1. Patients
The present study included all known SOT patients with real-time polymerase chain reaction (RT-PCR) confirmed COVID-19 diagnosed between March 11 and November 20, 2020 (n = 230). Patients were identified through a dedicated COVID-19 transplantation hotline for clinicians, clinical consultations with transplant physicians and patient coordinators, and a reporting system from referral hospitals. The latter forms the basis for reporting to the registry operated by the Scandiatransplant organ exchange organization (Aarhus University Hospital, Skejby, Denmark). Inclusion was checked for completeness against the National Quality Registry for Renal Failure (Jönköping County Council, Jönköping, Sweden), which has different reporting procedures.
The study was conducted in accordance with the Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects and reviewed and approved by the Swedish Ethical Review Authority (#2020-02153), who waived the requirement for informed consent for the retrospective chart review. The study was registered with ClinicalTrials.gov (NCT04407221).
COVID-19 was diagnosed whenever a patient had typical symptoms or known exposure and was positive for SARS-CoV-2 RNA with RT-PCR using a throat or nasal swab. The electronic medical records of all patients were reviewed. Data on patient characteristics, medical history, disease course, laboratory test results within 48 hours of admission, comorbidities, treatment measures, and outcomes were collected and analyzed. The assessment of comorbidities was performed using the non-age-adjusted Charlson comorbidity index (CCI).14 CCI includes 19 different medical conditions, and each comorbid condition is ranked from 1 to 6 points to sum an index score. Patients were allocated to one of the three categories (0, 1–2, and ≥3).
Disease severity was classified as mild, moderate, severe, and critical, as defined by the COVID-19 Treatment Guidelines Panel of the National Institutes of Health.15 Comparisons of laboratory results were made between patients with mild/moderate versus severe/critical.
The National Early Warning Score 2 (NEWS2) was registered upon contact with the healthcare provider.16 NEWS2 is used to score physiological parameters and includes the respiratory rate, oxygen saturation, need for supplemental oxygen, body temperature, blood pressure, heart rate, and consciousness. Patients were allocated to one of the three categories (0–2, 3–5, and ≥6).
The primary endpoint was 30-day all-cause mortality, counted from the date of the first positive RT-PCR test. Mortality data were retrieved until March 10th, 2021. A definitive outcome was defined as death or hospital discharge for inpatients or at least 30 days of follow-up for outpatients. COVID-19-era was defined arbitrarily as before or after August 1st, 2020, coinciding with the end of the pandemic’s first wave in Sweden.
Data on the total number of transplanted individuals alive at the beginning of the study period were retrieved from the National Quality Registry for Renal Failure, the Swedish thoracic transplantation registry (STRAX), and Scandiatransplant.
2.2. Renal function
Kidney function was expressed as estimated glomerular filtration rate (eGFR) using the creatinine-based Modification of Diet in Renal Disease (MDRD) formula. We defined baseline eGFR as the most recent measurement before the debut of COVID-19 symptoms and compared it to the lowest eGFR during COVID-19 and eGFR at the most recent outpatient visit (0.5–8 months). A reduction >35% during COVID-19 and a failure to attain at least 90% of baseline at follow-up were noted.
2.3. Serology
Antibody results were received from accredited virology laboratories using platforms recommended by the Public Health Agency of Sweden (Guidance for antibody detection in Covid19, article no. 20186). Due to heterogeneous platforms and different laboratories, results are solely expressed as positive or negative instead of antibody levels. The results are presented separately for nucleocapsid and spike targeted antibodies. Platform information is shown in Table S4a,b. Screening tests to determine seroprevalence were done on a convenience sample of geographically dispersed asymptomatic SOT recipients between September and November 2020. We received test results from referral centers on 559 patients representing 5.6% of transplanted patients in Sweden.
2.4. Statistical analyses
Statistical analyses were performed using JMP 10 and SAS 9.4 statistical software (SAS Institute). Data are presented as means and standard deviations, medians and ranges, or numbers and percentages as appropriate. Baseline comparisons for laboratory tests, age, BMI, and time since transplantation, between groups based on severity, were performed using Wilcoxon’s rank sum test. The Chi-squared test or Fisher’s exact test was employed for analyses of contingency tables. Risk factors for 30-day all-cause mortality were analyzed with a logistic regression model adjusted for age, sex, BMI, CCI, COVID-19-era, and the use of tacrolimus. A separate logistic regression analysis was performed using the NEWS2 score, which was divided into three categories. Change in log-transformed estimated glomerular filtration rate (eGFR) during and after COVID-19 was analyzed using a mixed model for repeated measurements (MMRM) with an unstructured covariance matrix. The model included transplant type (kidney/non-kidney), age, sex, BMI, and chronic kidney disease (CKD) stage 4/5, and log-transformed GFR at baseline value was used as a continuous covariate. Effects were nested within time points, thereby allowing for different effects across time points. GFR at all time points was further compared between inpatients and outpatients using Wilcoxon’s rank sum test. Fischer’s exact test was used to examine the relationship between immunosuppression with mycophenolate mofetil (MMF) and the symptom diarrhea and decreased eGFR >35%. All statistical tests were two-tailed, and p values <.05 were considered significant.
3. RESULTS
3.1. Patient characteristics
At the time of the present study, there were approximately 10000 solid organ transplant recipients alive in Sweden. The total patient population included recipients for kidney (6119), liver (2222), heart (1003), and lung (505). During the study period, 230 of these patients tested positive for SARS-CoV-2 with an accredited COVID-19 RT-PCR test. Thus, the cumulative incidence of COVID-19 among Swedish SOT recipients was 2.3%. Divided by organ type, the infection affected 162 kidney-, 35 liver-, 17 heart-, and 16 lung-transplant recipients. Of these, 19 patients had multiple transplants (Table S1). Among the 230 included patients, 17 (7.4%) had been re-transplanted.
The mean patient age was 54.0 (13.2) years, 64% were male, and the median BMI was 26.9 (15.2–42) kg/m2. Baseline and clinical characteristics of the study cohort are detailed in Tables 1 and 2, respectively.
TABLE 1.
Baseline characteristics of transplant recipients with COVID-19 stratified according to hospitalization status
| Total | Inpatients | Outpatients | p value | |
|---|---|---|---|---|
| Number of patients | 230 | 147 (63.9%)a | 83 (36.1%) | |
| Transplant type | .095 | |||
| Kidneyb | 162 (70.4%) | 102 (69.4%) | 60 (72.3%) | |
| Liverc | 35 (15.2%) | 26 (17.7%) | 9 (10.8%) | |
| Heartd | 17 (7.4%) | 7 (4.8%) | 10 (12.1%) | |
| Lunge | 16 (7%) | 12 (8.2%) | 4 (4.8%) | |
| Age at diagnosis (yrs, mean (SD)) | 54.0 (13.2) | 58.1 (11.1) | 46.6 (13.6) | <.001 |
| Age at diagnosis (yrs) | <.001f | |||
| <50 | 70 (30.4%) | 29 (19.7%) | 41 (49.4%) | |
| 50–59 | 79 (34.4%) | 52 (35.4%) | 27 (32.5%) | |
| 60–69 | 55 (23.9%) | 42 (28.6%) | 13 (15.7%) | |
| 70–79 | 24 (10.4%) | 23 (15.7%) | 1 (1.2%) | |
| 80+ | 2 (0.9%) | 1 (0.7%) | 1 (1.2%) | |
| Sex | ||||
| Male (%) | 146 (63.5%) | 99 (67.4%) | 47 (56.6%) | .118 h |
| Female (%) | 84 (36.5%) | 48 (32.6%) | 36 (43.4%) | |
| BMI (kg/m2 median, range) | 26.9 (15.2–42) | 27 (15.2–42) | 26 (16.7–41.2) | .094 |
| BMI (kg/m2) | .374 | |||
| ≤18.5 | 6 (2.6%) | 3 (2%) | 3 (3.6%) | |
| 18.5–25 | 81 (35.22%) | 48 (32.7%) | 33 (39.8%) | |
| 25–30 | 88 (38.3%) | 56 (38.1%) | 32 (38.6%) | |
| >30 | 55 (23.9%) | 40 (27.2%) | 15 (18.1%) | |
| Comorbidities | ||||
| Hypertension (%) | 172 (75.1%) | 114 (77.6%) | 58 (70.7%) | .268 h |
| Diabetes (%) | 69 (30%) | 56 (38.1%) | 13 (15.7%) | <.001h |
| Cardiovascular disease (%) | 20 (8.7%) | 17 (11.6%) | 3 (3.6%) | .050h |
| Renal impairment—CKD 4/5 (%) | 37 (16.1%) | 30 (20.4%) | 7 (8.4%) | .024h |
| Malignancy (%) | 6 (2.6%) | 5 (3.4%) | 1 (1.2%) | .422 h |
| Charlson comorbidity index (CCI) non-age adjusted | <.001 | |||
| CCI score 0 | 35 (15.2%) | 13 (8.8%) | 22 (26.5%) | |
| CCI score 1–2 | 130 (56.5%) | 83 (56.5%) | 47 (56.6%) | |
| CCI score ≥3 | 65 (28.3%) | 51 (34.7%) | 14 (16.9%) | |
| Immunosuppressive treatment | ||||
| Tacrolimus (%) | 189 (82.5%) | 117 (80.1%) | 72 (86.7%) | .277 h |
| Cyclosporin A (%) | 30 (13.1%) | 23 (15.8%) | 7 (8.4%) | .154 h |
| Mycophenolate mofetil (%) | 167 (73.2%) | 103 (71%) | 64 (77.1%) | .354 h |
| Steroids (%) | 194 (84.7%) | 121 (82.9%) | 73 (88%) | .345 h |
| Azathioprine (%) | 12 (5.2%) | 8 (5.5%) | 4 (4.8%) | 1 h |
| mTORi (%) | 14 (6.1%) | 9 (6.2%) | 5 (6%) | 1 h |
| Belatacept (%) | 2 (0.9%) | 2 (1.4%) | 0 (0%) | .536 h |
| Combination | .327 | |||
| Tripple (%) | 158 (69%) | 96 (65.8%) | 62 (74.7%) | |
| Double (%) | 60 (26.2%) | 43 (29.5%) | 17 (20.5%) | |
| Mono (%) | 11 (4.8%) | 7 (4.8%) | 4 (4.8%) | |
| Time since transplantation | ||||
| Most recent Tx, months (median, range) | 78 (0.5–360) | 80 (1–360) | 72 (0.5–332) | .148 |
| <3 m since transplantation (%) | 12 (5.2%) | 8 (5.4%) | 4 (4.8%) | 1 h |
| <12 m since transplantation (%) | 29 (12.6%) | 16 (10.9%) | 13 (15.7%) | .307 h |
Abbreviations: BMI, body mass index; CCI, Charlson comorbidity index; CKD, chronic kidney disease.
Two patients still hospitalized for rehabilitation.
Includes 14 combined kidney–pancreas recipients.
Includes three combined liver–kidney recipients.
Includes one combined heart–kidney recipient.
Includes one combined lung–heart–kidney recipient.
Combining 80+ with 70–79.
Fisher’s exact test.
TABLE 2.
Clinical characteristics of transplant recipients with COVID-19 stratified according to hospitalization status. COVID-19 severity according to NIH treatment guidelines
| Total | Inpatients | Outpatients | p value | |
|---|---|---|---|---|
| Number of patients | 230 | 147 (63.9%) a | 83 (36.1%) | |
| COVID–19 severity | <.001 | |||
| Mild | 122 (53%) | 42 (28.6%) | 80 (96.4%) | |
| Moderate | 28 (12.2%) | 25 (17%) | 3 (3.6%) | |
| Severe | 41 (17.8%) | 41 (27.9%) | 0 (0%) | |
| Critical | 39 (17%) | 39 (26.5%) | 0 (0%) | |
| NEWS2 score on admission or outpatient assessment (n = 215) | <.001 | |||
| 0–2 | 118 (54.9%) | 46 (32.9%) | 72 (96%) | |
| 3–5 | 45 (20.9%) | 42 (30%) | 3(4%) | |
| 6–13 | 52 (24.2%) | 52 (37.1%) | 0 (0%) | |
| Symptoms | ||||
| Fever (temperature ≥38˚C) | 164 (71.3%) | 115 (78.2%) | 49 (59%) | .003b |
| Cough | 126 (54.8%) | 93 (63.3%) | 33 (39.8%) | <.001b |
| Diarrhea | 65 (28.3%) | 54 (36.7%) | 11 (13.3%) | <.001b |
| Dyspnea | 60 (26.1%) | 54 (36.7%) | 6 (7.2%) | <.001b |
| Myalgia | 46 (20%) | 29 (19.7%) | 17 (20.5%) | 1b |
| Rhinitis | 32 (13.9%) | 13 (8.8%) | 19 (22.9%) | .005b |
| Fatigue | 30 (13%) | 18 (12.2%) | 12 (14.5%) | .685b |
| Headache | 29 (12.6%) | 15 (10.2%) | 14 (16.9%) | .153b |
| Nausea/Vomiting | 21 (9.1%) | 18 (12.2%) | 3 (3.6%) | .032b |
| Anosmia/Ageusia | 18 (7.8%) | 6 (4.1%) | 12 (14.5%) | .009b |
| Pharyngitis | 14 (6.1%) | 9 (6.1%) | 5 (6%) | 1b |
Abbreviations: NEWS2, National Early Warning Score 2.
One patient still hospitalized for rehabilitation.
Fisher’s exact test.
The median time from the most recent transplantation was 78 months (0.5–360). In all, 29 patients (12.6%) developed the infection within 1 year of transplantation, and 12 patients (5.2%) within 3 months. No cases of donor-derived COVID-19 were identified. All donors were tested, and Scandiatransplant policy stated that positive donors should not be accepted.
Patients commonly had one or more comorbidities, the most frequent being hypertension (75.1%). The most common presenting symptoms were fever, cough, and diarrhea. There was no association between patients using mycophenolate mofetil (MMF) and having diarrhea on presentation (p = .74). However, there was an association between diarrhea and a >35% reduction in eGFR during COVID-19 (p < .001). Results from laboratory tests are stratified by severity and presented in Table 3. Patients with severe or critical COVID-19 had significantly higher levels of WBC, CRP, LDH, creatinine, procalcitonin, D-dimer, and ferritin compared with patients presenting with mild or moderate disease. Radiological examinations of the lungs were performed in 46% of inpatients and most often showed ground-glass opacities or infiltrates.
TABLE 3.
Laboratory results for solid organ transplant recipients with COVID-19 stratified according to COVID-19 severity. Tested within 48 h of admission or during an outpatient assessment
| Covid severity |
p value, Wilcoxon Test (two-sided) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mild/moderate |
Severe/critical |
||||||||
| N | Min | Median | Max | N | Min | Median | Max | ||
| WBC (×109/L) | 78 | 1.40 | 5.62 | 13.10 | 103 | 1.20 | 6.40 | 21.60 | .0398 |
| CRP (mg/L) | 80 | 0.50 | 16.50 | 206.00 | 105 | 0.60 | 74.00 | 330.00 | <.0001 |
| Procalcitonin (ng/ml) | 12 | 0.04 | 0.11 | 0.29 | 58 | 0.03 | 0.33 | 10.00 | .0012 |
| Lymphocytes (×109/L) | 40 | 0.30 | 0.80 | 2.50 | 80 | 0.10 | 0.70 | 2.90 | .0156 |
| D-dimer (mg/L) | 23 | 0.03 | 0.48 | 2.06 | 71 | 0.08 | 0.84 | 12.90 | .0131 |
| Creatinine (µmol/L) | 78 | 50.00 | 109.00 | 1009.00 | 103 | 47.00 | 161.00 | 1705.00 | <.0001 |
| Ferritin (µg/L) | 21 | 21.00 | 473.00 | 2180.00 | 62 | 47.00 | 756.00 | 6905.00 | .0264 |
| LDH (µkat/L) | 23 | 2.20 | 3.80 | 11.00 | 60 | 2.20 | 5.40 | 9.90 | <.0001 |
Abbreviations: CRP, C-reactive protein; LDH, lactate dehydrogenase; WBC, white blood cells.
The severity of disease on presentation was mild in 122 patients (53%), moderate in 28 (12%), severe in 41 (18%), and critical in 39 (17%). Patients with mild or moderate disease on presentation differed from patients with severe or critical disease by lower age (p < .001) and lower BMI (p = .013), but not the time since most recent transplantation (p = .354).
3.2. Outcomes
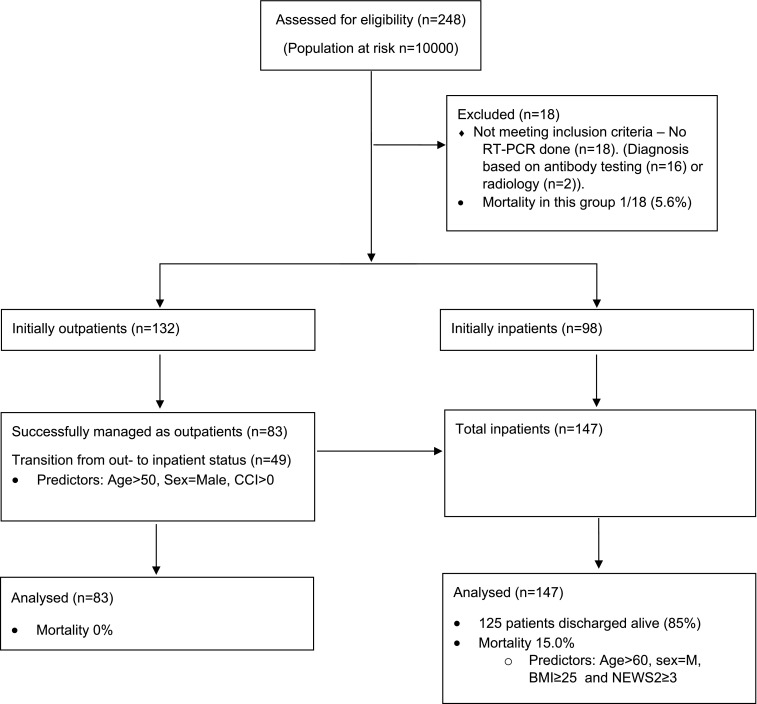
All patients included in this study were evaluated for at least 30 days, and all attained a definitive outcome. Of the 230 patients, 147 (63.9%) were hospitalized, whereas 83 patients (36.1%) were managed entirely on an outpatient basis. Among the whole cohort, the 30-day all-cause mortality was 9.6%. No patients died in the outpatient setting, and the mortality among inpatients was 15.0%. Of the 147 inpatients, a total of 125 patients were discharged alive (85%) ( Figure 1). Survival among patients presenting with mild, moderate, severe, and critical disease was >99%, 93%, 95%, and 56%, respectively. Total 30-day all-cause mortality among liver transplant recipients was 17.1%, kidney transplant recipients, 9.3%, heart transplant recipients, 5.9%, and lung transplant recipients, 0% (Table S1).
FIGURE 1.
Flowchart for the study. BMI, body mass index; CCI, Charlson comorbidity index; NEWS2, National Early Warning Score 2
The associations between different factors and 30-day all-cause mortality are presented in Table 4. Mortality increased along with older age groups, male sex, and increasing BMI categories but not higher CCI scores. There was a tendency toward worse outcomes during the pandemic’s first wave, but this did not reach significance (p = .08). No patients infected within 1 year after organ transplantation died.
TABLE 4.
Odds ratios for the 30-day all-cause mortality endpoint across different subgroups
| 30-day all-cause mortality |
||||
|---|---|---|---|---|
| OR | Lower CI | Upper CI | p value | |
| Parameter | ||||
| age 70+ | 62.06 | 7.97 | 1367.71 | <.001 |
| age 60–69 | 10.95 | 1.58 | 222.25 | .037 |
| age 50–59 | 5.95 | 0.91 | 117.55 | .112 |
| age <50 | 1.00 | |||
| CCI score: ≥3 | 1.64 | 0.21 | 34.39 | .677 |
| CCI score: 1–2 | 1.61 | 0.24 | 32.29 | .673 |
| CCI score: 0 | 1.00 | |||
| Sex: Male | 3.70 | 1.14 | 14.29 | .041 |
| Sex: Female | 1.00 | |||
| BMI: >30 | 5.93 | 1.29 | 35.49 | .031 |
| BMI: 25–30 | 5.38 | 1.37 | 28.70 | .026 |
| BMI: 18.5–25 | 1.00 | |||
| BMI: <18.5 | 7.41 | 0.19 | 212.51 | .246 |
| COVID–19 diagnosis before August 1, 2020 a | 4.47 | 1.03 | 32.32 | .076 |
| COVID–19 diagnosis after August 1, 2020 a | 1.00 | |||
| Not using tacrolimus | 0.81 | 0.21 | 2.63 | .735 |
| Using tacrolimus | 1.00 | |||
Abbreviations: BMI, body mass index; CCI, Charlson comorbidity index.
Before August 1 n = 167, after August 1 n = 63.
In addition, we analyzed the impact of different NEWS2 scores on death using NEWS2 score 0–2 as a reference. The risk for death increased along with a higher NEWS2 score ( Table 5). Although patients with low NEWS2 (≤2) at presentation had a mortality of 3.4%, this proportion progressively increased to 13.3% and 21.2% in patients with NEWS2 between 3–5 and ≥6, respectively.
TABLE 5.
Association between increasing NEWS2 score on presentation and 30-day all-cause mortality
| Parameter | OR | Lower CI | Upper CI | p value |
|---|---|---|---|---|
| NEWS2 score: ≥6 | 7.65 | 2.46 | 28.82 | <.001 |
| NEWS2 score: 3–5 | 4.38 | 1.19 | 17.92 | .028 |
| NEWS2 score: 0–2 | 1.00 |
Abbreviations: NEWS2, National Early Warning Score 2.
Immunosuppression at baseline did not significantly influence 30-day mortality (data not shown). As part of the immunosuppressive regimen, tacrolimus was not shown to be protective in the logistical regression analysis (p = .74). The only two patients receiving belatacept died. Among the 14 patients receiving mTOR inhibitors, two patients (14%) died within 30 days.
An additional eight (3.5%) patients died after 30 days, two during the initial hospital stay on days 32 and 104 (urosepsis and ventilator-associated pneumonia), and six after hospital discharge for reasons not necessarily related to COVID-19 (Table S2). The median follow-up time for this extended mortality assessment was 293 days (range: 110–365).
3.3. Therapeutic management
Oxygen therapy was given to 62.2% of inpatients via nasal cannula (NC) (29%), a high-flow nasal cannula (HFNC) (16.6%), or in connection with mechanical ventilation (MV) (16.6%). Anticoagulation was administered to 77% of inpatients in the form of low molecular weight heparin (LMWH) (64%), novel oral anticoagulants (NOAC) (12%), or warfarin (1%).
Immunosuppressive treatment was modified in 111 patients (48%). In general, immunosuppression was reduced to a greater degree in parallel with increasing COVID-19 disease severity. In patients with mild disease, 27% had their immune suppression reduced, in 46% with moderate disease, 80% with severe disease, and in 92% of patients with critical disease. Steroids constituted an exception since they were usually increased in parallel with the seriousness of the infection ( Table 6). Oral prednisone was increased in 25% of patients already on corticosteroids as maintenance therapy. Dexamethasone was administered to 14 patients and betamethasone to 12, of which three received both. Remdesivir was given to 10 patients (two with mild, three with moderate, three with severe, and two with critical disease severity), who all survived. None of the patients in this series received hydroxychloroquine or lopinavir/ritonavir. Antibiotics were administered to 52 (35.4%) inpatients, mostly as prophylaxis or for suspected superinfection.
TABLE 6.
Treatment and outcome variables of transplant recipients with COVID-19 stratified according to hospitalization status
| Total | Inpatients | Outpatients | p value | |
|---|---|---|---|---|
| Number of patients | 230 | 147 (63.9%) | 83 (36.1%) | |
| No change in immunosuppression | 119 (51.7%) | 52 (35.4%) | 67 (80.7%) | <.001a |
| Decreased immunosuppression | ||||
| Reduction or cessation of antimetabolite | 70/180 (38.9%) | 59/112 (52.7%) | 11/68 (16.2%) | <.001a |
| Reduction or cessation of CNI | 42/219 (19.2%) | 38/140 (27.1%) | 4/79 (5.1%) | <.001a |
| Reduction or cessation of mTORi | 2/14 (14.3%) | 2/9 (22.2%) | 0/5 (0%) | .506a |
| Reduction or cessation of prednisone | 5/194 (2.6%) | 5/121 (4.1%) | 0/73 (0%) | .08 |
| Increased prednisone | 48/194 (24.7%) | 41/121 (33.9%) | 7/73 (9.6%) | .0002 |
| Dexamethasone/Betamethasone | 23 (10.0%) | 23 (15.7%) | 0 (0%) | <.001a |
| Anticoagulation | ||||
| Low molecular weight heparin (LMWH) | 94 (40.9%) | 94 (63.9%) | 0 (0%) | <.001a |
| Non-vitamin K oral anticoagulant (NOAC) | 20 8.7%) | 18 (12.2%) | 2 (2.4%) | .013a |
| Warfarin | 1 (0.4%) | 1 (0.7%) | 0 (0%) | 1a |
| Remdesivir | 10 (4.3%) | 10 (6.8%) | 0 (0%) | .015a |
| Length of stay (median, range) | 8.5 (1–143) | n.a. | ||
| ICU admission | 36 (15.7%) | 36 (24.7%) | n.a. | |
| ICU days | 9.5 (2–61) | n.a. | ||
| Max respiratory support (n = 228) | ||||
| None | 138 (60.5%) | 55 (37.9%) | 83 (100%) | |
| Nasal cannula | 42 (18.3%) | 42 (29%) | 0 (0%) | |
| High flow nasal cannula | 24 (10.5%) | 24 (16.6%) | 0 (0%) | |
| Mechanical ventilation | 24 (10.5%) | 24 (16.6%) | 0 (0%) | |
| Renal function | ||||
| Acute kidney injury (eGFR loss ≥35%) | 46/191 (24.1%) | 46/137 (24.1%) | 0/54 (0%) | <.001a |
| Renal replacement therapy | 21 (9.1%) | |||
| Previously on dialysis | 10 (4.3%) | 9 (6.1%) | 1 (1.2%) | .099a |
| Previously not on dialysis | 11 (5.0%)b | 11 (8.0%)b | 0 (0%) | .008a,b |
| Return to eGFR baseline on follow-up (eGFR loss <10%) | 154/178 (86.4%) | 97/111 (87.4%) | 57/67 (85.1%) | .658a |
| Baseline eGFR n=227 (g-mean (CV%)) | 47.6 (69.5) | 43.1 (71) | 56.7 (61.9) | <.001 |
| COVID–19 eGFR n=191 (g-mean (CV%)) | 32.4 (98.2) | 27.9 (99.4) | 47.3 (75.5) | <.001 |
| Follow-up eGFR n=177 (g-mean (CV%)) | 46.9 (77.6) | 43.5 (82.2) | 53.3 (67.8) | .013 |
| COVID–19/Baseline eGFR ratio n=191 (g-mean (CV%)) | 0.72 (52.4) | 0.65 (59) | 0.92 (12.4) | <.001 |
| Follow-up/Baseline eGFR ratio n=177 (g-mean (CV%)) | 0.99 (17.6) | 1.0 (16.8) | 0.98 (18.7) | .293 |
| Mortality | ||||
| All | 22 (9.6%) | 22 (14.9%) | 0 (0%) | <.001a |
| Transplant recipients <1 yr after transplantation | 0 (0%) | 0 (0%) | 0 (0%) | 1 |
| Inpatients initially treated as outpatients | 5/49 (10.2%) | n.a. |
Abbreviations: CNI, calcineurin inhibitor; eGFR, estimated glomerular filtration rate; mTORi, mammalian target of rapamycin inhibitor.
Fisher’s exact test.
Calculated among patients not previously on dialysis.
For inpatients, the median length of hospital stay was 8.5 days (range: 1–143). Only 15.7% of patients (24.7% of inpatients) were admitted to the intensive care unit (ICU), and the median length of ICU stay was 9.5 days (range: 2–61).
3.4. Patients transitioning from outpatient to inpatient status
Among hospitalized patients, 49 were initially managed as outpatients but deteriorated and required inpatient care within 2 weeks from their initial contact with healthcare providers (Figure 1). Of these 49 patients, 13 presented to the emergency department with mild, 10 moderate, 16 severe, and 10 with critical disease. In all, 31 patients required oxygen therapy (13 NC, 11 HFNC, and 7 MV), 14 were admitted to the ICU, and five patients died. Predictors of transition from outpatient to inpatient status due to clinical deterioration included higher age, higher CCI score, and male sex, but not BMI ( Table 7).
TABLE 7.
Predictors of transition from outpatient to inpatient status
| Parameter | OR | Lower CI | Upper CI | p value |
|---|---|---|---|---|
| age 70+ | 7.32 | 1.10 | 65.23 | .047 |
| age 60–69 | 7.55 | 2.42 | 25.77 | <.001 |
| age 50–59 | 3.54 | 1.36 | 9.68 | .011 |
| age <50 | 1.00 | |||
| CCI score: >=3 | 4.25 | 0.95 | 23.66 | .071 |
| CCI score: 1–2 | 8.30 | 2.36 | 40.11 | .003 |
| CCI score: 0 | 1.00 | |||
| Sex: Female | 0.33 | 0.13 | 0.79 | .015 |
| Sex: Male | 1.00 | 1.00 | 1.00 | |
| BMI: >30 | 1.63 | 0.55 | 4.93 | .377 |
| BMI: 25–30 | 0.61 | 0.23 | 1.56 | .301 |
| BMI: <25 | 1.00 |
Abbreviations: BMI, body mass index; CCI, Charlson comorbidity index.
3.5. Renal function and organ rejections
Estimated GFR was available for 227 patients at baseline, for 191 patients during their course of COVID-19 infection, and for 177 patients at follow-up. The eGFR geometric means for baseline, during COVID-19 and at follow-up were 47.4, 32.1, and 46.6 ml/min, respectively. While CKD stage 4/5 was associated with requiring hospitalization (p = .024), it was not independently associated with mortality in this cohort (data not shown). Geometric mean eGFR declined by 28% for all patients, 35% for inpatients, and 8% for outpatients. A decrease of >35% was observed in 24% of the patients, and 4.8% had a decline necessitating dialysis. High BMI (>30 kg/m2) was associated with a 21% greater relative decrease in eGFR during COVID-19 compared to patients with BMI <25 kg/m2. Younger patients seemed to lose less eGFR during COVID-19. Men had a 20% greater relative decrease in eGFR than women (p = .019). There was no difference between the ability to return to baseline eGFR based on sex or organ type (Table S3). A minority of patients did not return to baseline (14%). The median follow-up time of kidney function was 5 months (range: 0.5–8).
In kidney transplant recipients, two cellular rejections were verified with a biopsy at 3- and 4-months follow-up (both Banff 1B), and one antibody-mediated rejection was identified after 5 months of follow-up. Two kidney recipients were treated empirically for suspected rejection without a biopsy. No kidney grafts were lost in surviving patients. However, a lung graft was found to have acute and chronic rejection at post-mortem examination in a patient who died after hospital discharge during follow-up.
3.6. Serology
The serologic response to SARS-CoV-2 nucleocapsid (N) and spike (S) antigens was assessed in 117 patients during follow-up using several different antibody assays (Table S4a). The proportion of patients with SARS-CoV-2-specific antibodies decreased from 78% after 1–2 months to 62% after 6–7 months, though there was a clear difference between anti-N and anti-S responses. While 62% of patients had anti-N IgG at 1–2 months after COVID-19, the proportion decreased to 54% at 3–4 months and 38% at 5–7 months after the primary infection. Anti-S IgG responses were more durable: 92% at 1–2 months, 84% at 3–4 months, and 76% at 6–7 months.
Screening tests were performed on 559 asymptomatic SOT patients and demonstrated a seroprevalence of 1.4%. The positivity rate was highest for a combined N- and S-platform (Table S4b).
4. DISCUSSION
The present report is the first in-depth, long-term, nationwide analysis of COVID-19 in solid organ recipients from a country with a high incidence of infections. The total 30-day all-cause mortality in this study (9.6%) was lower than that observed in most previous reports on SOT recipients1 but higher than the general Swedish population during the same period (3.1%).17 However, the cumulative incidence of PCR confirmed SARS-CoV-2 infections among Swedish SOT recipients during the study period were comparable to that of the general Swedish population, 2.3% vs. 2.0%, respectively. Therefore, any differences in the case-fatality rate between these two populations should be explained primarily by differences in age, gender distribution, comorbidities, and concurrent medications rather than inclusion bias. While this study shows a lower mortality rate for SOT recipients than most previously published reports, there is no reason to assume that COVID-19 is less threatening to SOT recipients in Sweden than in other countries. Our results confirm the findings in an earlier Swedish study.2 We believe the lower mortality rate found in this study mostly stems from a higher rate of outpatient case identification than patient management differences.
SOT recipients hospitalized for COVID-19 have similar 30-day all-cause mortality as the general Swedish population, 15.0% (n = 22/147) vs. 14.5% (n = 3567/24617), respectively.18 However, the median age was 13 years higher in the latter group18 (55 vs. 68 years), suggesting that transplanted patients should self-isolate more carefully than the general population.
As in previous studies, both in SOT recipients7 , 19 and the general population,20 higher age was a significant risk factor for death. While we found increased odds ratios for all age intervals over 50 years, this only reached statistical significance for patients 60 years of age or older. In line with other reports, obesity21 and male sex20 were associated with increased mortality. No patients with COVID-19 within 1 year of organ transplantation (n = 29) died.
The present study found that the NEWS2 score on admission was a significant predictor of outcome. Studies on the general population with COVID-19 have previously reported similar findings.22 , 23 However, these reports identified and analyzed a cutoff ≥5, which we believe may be too high for transplant recipients. We find a significant increase in the odds for a poor outcome with a NEWS2 score ≥3 in transplanted patients. This finding has important practical implications as it sets a threshold for assessing patients on an outpatient basis and may help guide admission practices.
Many patients were successfully managed in an outpatient setting, and transplant recipients with COVID-19 do not necessarily require hospitalization by default. Outpatient management can be successful if supported by frequent telemedicine contacts and patient self-assessment.4 , 24 However, the finding that over one-third of the patients initially managed as outpatients ultimately were hospitalized underscores the need for continued close outpatient follow-up for detection of clinical deterioration in the weeks following the initial symptoms. Patient characteristics found to predict progression from outpatient to inpatient status were age over 50, male sex, and CCI score over 0, but not BMI.
Sweden has been restrictive with the use of repurposed antiviral treatments in COVID-19 patients. Remdesivir was used sparingly, whereas hydroxychloroquine and antiretrovirals have hardly been used at all. Throughout the pandemic, supportive measures such as oxygen therapy, anticoagulants, and following the RECOVERY Trial,25 corticosteroid therapy, have formed the backbone of COVID-19 treatment. In severe and critical patients, there has been a preference to give oxygen therapy in the form of HFNC rather than with MV, which would necessitate ICU admission. This policy may also have contributed to the lower mortality rate.26
Although reducing immunosuppressants has become an essential part of transplanted patients’ management, this was applied to a lesser degree in Sweden than in other countries.3 , 5 Over half of the patients continued with their regular immunosuppressive regimen, and reductions were uncommon in outpatients. In part, this may be explained by the high frequency of mild disease. It could also reflect an attitude of watchful waiting facilitated by frequent patient contact and iterative reassessments of outpatients.
Antimetabolites were the first drugs to be reduced or withdrawn, followed by CNI. The degree of reduction occurred in parallel with the gravity of the COVID-19 infection, except steroids, which were usually increased along with increasing disease severity. This approach seems to have yielded good overall outcomes without increasing the risk for rejections during the hospital stay and resulting in mostly mild and infrequent rejection episodes during follow-up. Although a recent analysis has suggested a protective effect of tacrolimus among transplanted patients,27 this finding could not be confirmed in the present cohort.
Our analysis confirms that a high proportion of transplanted patients mount an antibody response to COVID-19.28 , 29 While N-specific antibodies decrease rapidly, S-specific antibodies are more durable, showing similar longevity as the general population.30 , 31 Hence, this finding is promising when utilizing S-antigen-based vaccines in the SOT population.
In the present study, we did not identify any cases of RT-PCR confirmed reinfection during follow-up. However, we did find one patient whose antibody levels suddenly increased following typical but mild symptoms 6 months after the primary infection, despite negative RT-PCR (data not shown).
Seroprevalence in asymptomatic individuals was 1.4%. Compared to a cumulative incidence of symptomatic COVID-19 of 2.3%, this may indicate that a considerable proportion of transplant recipients undergo an asymptomatic infection. The loss of N-antibodies in RT-PCR-positive patients suggests that screening of asymptomatic individuals, especially with N-targeted platforms, may underestimate the real incidence of COVID-19 in these patients.
The study’s extended follow-up time also allowed us to study renal function, which declined during the illness but had returned to baseline values in the majority of patients during follow-up. However, 14% of patients did not return to baseline levels suggesting a minority of patients may suffer from long-lasting renal impairment as a complication after COVID-19. Somewhat surprisingly, kidney graft recipients were not overrepresented in this group (Table S3). We identified five episodes of rejection in kidney recipients during the follow-up period, but many patients with renal grafts and eGFR below baseline values were not assessed for rejection. Furthermore, no negative biopsies were identified during follow-up.
This study has several strengths, including a broad, national coverage, completeness of variable data, and extended follow-up. Furthermore, a similar treatment strategy was applied throughout Sweden without the use of controversial interventions such as hydroxychloroquine or antivirals. The dataset was obtained after individually extracting and reviewing patient files following the cross-investigation of each transplant center’s local databases and two different patient registries, allowing for identification and inclusion of virtually all the cases within the study timeframe. The study’s limitations include its retrospective nature and the end date early during the second wave of COVID-19 infections. The retrospective design meant antibody testing was performed on multiple platforms and that antibody data were only available in a subset of patients and at limited time points. Also, S-antibody assays were not available for all patients, which may have caused an underestimation of the total number of patients seroconverting or having antibodies at later time points. In addition, this study was neither designed nor powered to evaluate the effect of specific therapies against COVID-19 and does not allow for conclusions concerning medical interventions. The inclusion of only PCR-confirmed cases can be regarded both as a strength and as a weakness. The strength comprises a high specificity of diagnosis, but at the cost of a lower sensitivity since the limited access to diagnostic tests early in the pandemic may have led to a degree of underdiagnosing and underreporting, particularly of mild outpatient cases.
5. CONCLUSION
Many patients can be managed on an outpatient basis aided by risk stratification with age, sex, and NEWS2 score. Factors associated with adverse outcomes include older age, male sex, greater BMI, and a higher NEWS2 score.
ACKNOWLEDGMENTS
The study was financed by grants from The Kidney Foundation (SWE—Njurstiftelsen), The Healthcare Board, Region Västra Götaland (Hälso-och sjukvårdsstyrelsen) grant number 941182, and a private donation from Paul Frankenius. Gothia Forum provided assistance with project conception, grant applications, and applying for ethical approval. Maria Stendahl and Helena Rydell at the “National Quality Registry for Renal Failure” assisted with crosschecking our databases.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Funding information The Healthcare Board, Region Västra Götaland (Hälso- och sjukvårdsstyrelsen), Grant/Award Number: 941182; The Kidney Foundation (SWE-Njurstiftelsen); Paul Frankenius
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
Table S1-S4
REFERENCES
- 1.Raja MA, Mendoza MA, Villavicencio A, et al. COVID-19 in solid organ transplant recipients: a systematic review and meta-analysis of current literature. Transplant Rev (Orlando). 2020;35(1):100588. doi: 10.1016/j.trre.2020.100588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Felldin M, Søfteland JM, Magnusson J, et al. Initial report from a Swedish high-volume transplant center after the first wave of the COVID-19 pandemic. Transplantation. 2021;105(1):108–114. doi: 10.1097/TP.0000000000003436. [DOI] [PubMed] [Google Scholar]
- 3.Kute VB, Bhalla AK, Guleria S, et al. Clinical profile and outcome of COVID-19 in 250 kidney transplant recipients: a multicenter cohort study from India. Transplantation. 2021;105(4):851–860. doi: 10.1097/TP.0000000000003593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lubetzky M, Aull MJ, Craig-Schapiro R, et al. Kidney allograft recipients, immunosuppression, and coronavirus disease-2019: a report of consecutive cases from a New York City transplant center. Nephrol Dial Transplant. 2020;35(7):1250–1261. doi: 10.1093/ndt/gfaa154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jager KJ, Kramer A, Chesnaye NC, et al. Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int. 2020;98(6):1540–1548. doi: 10.1016/j.kint.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kates OS, Haydel BM, Florman SS, et al. COVID-19 in solid organ transplant: A multi-center cohort study. Clin Infect Dis. 2020.
- 7.Caillard S, Anglicheau D, Matignon M, et al. An initial report from the French SOT COVID Registry suggests high mortality due to COVID-19 in recipients of kidney transplants. Kidney Int. 2020;98(6):1549–1558. doi: 10.1016/j.kint.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cravedi P, Mothi SS, Azzi Y, et al. COVID-19 and kidney transplantation: results from the TANGO international transplant consortium. Am J Transplant. 2020;20(11):3140–3148. doi: 10.1111/ajt.16185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ravanan R, Callaghan CJ, Mumford L, et al. SARS-CoV-2 infection and early mortality of waitlisted and solid organ transplant recipients in England: a national cohort study. Am J Transplant. 2020;20(11):3008–3018. doi: 10.1111/ajt.16247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernández-Ruiz M, Andrés A, Loinaz C, et al. COVID-19 in solid organ transplant recipients: a single-center case series from Spain. Am J Transplant. 2020;20(7):1849–1858. doi: 10.1111/ajt.15929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bellomo R, Warrillow SJ, Reade MC. Why we should be wary of single-center trials. Crit Care Med. 2009;37(12):3114–3119. doi: 10.1097/CCM.0b013e3181bc7bd5. [DOI] [PubMed] [Google Scholar]
- 12.Yazici H. Beware of registries for their biases. Bull NYU Hosp Jt Dis. 2012;70(2):95–98. [PubMed] [Google Scholar]
- 13.Fisher M, Neugarten J, Bellin E, et al. AKI in hospitalized patients with and without COVID-19: a comparison study. J Am Soc Nephrol. 2020;31(9):2145–2157. doi: 10.1681/ASN.2020040509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 15.National Institutes of Health. COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. https://www.covid19treatmentguidelines.nih.gov/. Accessed December 20, 2020. [PubMed]
- 16.Royal College of Physicians . RCP; London: 2017. National Early Warning Score (NEWS) 2: Standardising the assessment of acute-illness severity in the NHS. Updated report of a working party. [Google Scholar]
- 17.Roser M, Ritchie H, Ortiz-Ospina E, Hasell J. (2020) “Coronavirus Pandemic (COVID-19)”. Published online at OurWorldInData.org. https://ourworldindata.org/coronavirus. Accessed December 20, 2020.
- 18.Statistics on COVID-19. The Swedish National Board of Health and Welfare (Swedish). https://www.socialstyrelsen.se/globalassets/1-globalt/covid-19-statistik/statistik-om-slutenvard-av-patienter-med-covid-19/statistik-covid19-vardforlopp.xlsx. Accessed January 12, 2021.
- 19.Oltean M, Søfteland JM, Bagge J, et al. Covid-19 in kidney transplant recipients: a systematic review of the case series available three months into the pandemic. Infect Dis (Lond). 2020;52(11):830–837. doi: 10.1080/23744235.2020.1792977. [DOI] [PubMed] [Google Scholar]
- 20.Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020;81(2):e16–e25. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwok S, Adam S, Ho JH, et al. Obesity: a critical risk factor in the COVID-19 pandemic. Clin Obes. 2020;10(6) doi: 10.1111/cob.12403. e12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gidari A, De Socio GV, Sabbatini S, Francisci D. Predictive value of national early warning score 2 (NEWS2) for intensive care unit admission in patients with SARS-CoV-2 infection. Infect Dis (Lond). 2020;52(10):698–704. doi: 10.1080/23744235.2020.1784457. [DOI] [PubMed] [Google Scholar]
- 23.Myrstad M, Ihle-Hansen H, Tveita AA, et al. National early warning score 2 (NEWS2) on admission predicts severe disease and in-hospital mortality from Covid-19 - a prospective cohort study. Scand J Trauma Resusc Emerg Med. 2020;28(1):66. doi: 10.1186/s13049-020-00764-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Husain SA, Dube G, Morris H, et al. Early outcomes of outpatient management of kidney transplant recipients with coronavirus disease 2019. Clin J Am Soc Nephrol. 2020;15(8):1174–1178. doi: 10.2215/CJN.05170420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Group RC, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. 2020.
- 26.Gershengorn HB, Hu Y, Chen J-T, et al. The impact of high-flow nasal cannula use on patient mortality and the availability of mechanical ventilators in COVID-19. Ann Am Thorac Soc. 2021;18(4):623–631. doi: 10.1513/AnnalsATS.202007-803OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Belli LS, Fondevila C, Cortesi PA, et al. Protective role of tacrolimus, deleterious role of age and comorbidities in liver transplant recipients with Covid-19: results from the ELITA/ELTR multi-center European study. Gastroenterology. 2021;160(4) doi: 10.1053/j.gastro.2020.11.045. 1151-1163.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benotmane I, Gautier Vargas G, Velay A, et al. Persistence of SARS-CoV-2 antibodies in kidney transplant recipients. Am J Transplant. 2020. [DOI] [PMC free article] [PubMed]
- 29.Hartzell S, Bin S, Benedetti C, et al. Evidence of potent humoral immune activity in COVID-19-infected kidney transplant recipients. Am J Transplant. 2020;20(11):3149–3161. doi: 10.1111/ajt.16261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marklund E, Leach S, Axelsson H, et al. Serum-IgG responses to SARS-CoV-2 after mild and severe COVID-19 infection and analysis of IgG non-responders. PLoS One. 2020;15(10) doi: 10.1371/journal.pone.0241104. e0241104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gudbjartsson DF, Norddahl GL, Melsted P, et al. Humoral Immune Response to SARS-CoV-2 in Iceland. N Engl J Med. 2020;383(18):1724–1734. doi: 10.1056/NEJMoa2026116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1-S4
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.