Summary
The frequent association between coronavirus disease 2019 (COVID‐19) and olfactory dysfunction is creating an unprecedented demand for a treatment of the olfactory loss. Systemic corticosteroids have been considered as a therapeutic option. However, based on current literature, we call for caution using these treatments in early COVID‐19–related olfactory dysfunction because: (1) evidence supporting their usefulness is weak; (2) the rate of spontaneous recovery of COVID‐19–related olfactory dysfunction is high; and (3) corticosteroids have well‐known potential adverse effects. We encourage randomized placebo‐controlled trials investigating the efficacy of systemic steroids in this indication and strongly emphasize to initially consider smell training, which is supported by a robust evidence base and has no known side effects.
Keywords: corticosteroids, COVID‐19, olfaction disorder, SARS‐CoV‐2, smell
INTRODUCTION
The high rate of patients experiencing coronavirus disease 2019 (COVID‐19)‐related olfactory dysfunction (C19OD) and mental and physical burden induced by losing the sense of smell is creating an unprecedented demand for a treatment of the olfactory loss.
In contrast to previously known, non‐COVID‐19–associated postinfectious olfactory dysfunction (OD), C19OD patients are being seen earlier in the course of the disease at specialized smell and taste centers. The heightened attention to smell disorders and the pronounced fear that of COVID‐19 associations, paired with the worries about recovery, may bias our approach to very early changes after postinfectious smell disorders. This presents a unique opportunity for an early intervention, but also raises the question whether such intervention is needed to increase the likelihood for olfactory recovery.
Systemic corticosteroids (CS) are part of the ear, nose, and throat (ENT) armamentarium in several inflammatory (i.e., chronic rhinosinusitis [CRS]) and sensorineural conditions (i.e., sudden sensorineural hearing loss [SSNHL] and idiopathic facial palsy). Because C19OD likely results from an inflammatory and a neurosensory process, 1 , 2 systemic CS therapy has been considered as an option to treat C19OD. 3 However, it is important to consider the balance of risks and benefits of systemic CS. In the absence of data supporting the lack of major side effects of systemic CS in COVID‐19 patients, several professional groups such as the European Academy of Allergy and Clinical Immunology (EAACI) 4 have called for caution and recommended against the use of systemic CS in CRS during COVID‐19. Moreover, the efficacy of CS in their classical neurological indications, such as SSNHL and acute spinal cord injury, remains questionable, 5 , 6 and it is well know that their use is associated with potentially serious side effects. 6 Therefore, when considering whether such treatment should be used for C19OD, natural history and potential added benefit versus treatment risk must be carefully considered.
As an expert group in clinical olfaction, we aim to briefly review and summarize evidence for and against CS treatment in C19OD based on current literature on COVID‐19 and postinfectious olfactory loss in general. Additionally, a Delphi process was performed to collect individual opinions.
RATE OF COVID‐19–RELATED OD AND SPONTANEOUS RECOVERY
Recent meta‐analyses have determined that the pooled frequency of OD in COVID‐19 patients (either based on questionnaire or smell psychophysical tests) was 56% 7 to 61%. 8 However, there is extremely high variability in the reported prevalence of this symptom, ranging from 5% 9 to 98%. 10
Similarly, documented rates of olfactory recovery amongst COVID‐19 patients vary, most likely due to methodological discrepancies and sampling bias both between and within studies. However, in general there appears to be a high rate of recovery. At 1 month, authors found resolution rates of OD in 33% to 96% of patients. 11 , 12 , 13 , 14 , 15 At 2 months, normal olfactory function was reported by 54% of patients 16 (for more details, see Figure 1). 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 When assessing olfactory function based on psychophysical testing (Figure 2), follow‐up studies found a 63% rate of normosmia at 5 weeks, 20 54% to 79% at 2 months, 21 , 22 , 23 86% at 3 months, 24 and 95% at 6 months. 19 Of note, the rate of hyposmia in the general population is ∼20%. 25 , 26 Although it is probable that a majority of patients reporting C19OD had normal olfaction at baseline, it cannot be ruled out that some were hyposmic at baseline and became alerted to it through COVID‐related public attention.
FIGURE 1.
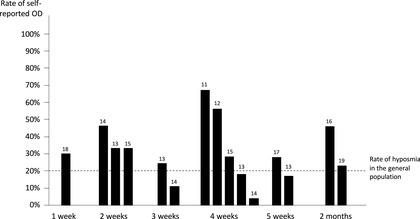
Rates of self‐reported olfactory loss (absent or incomplete recovery) among COVID‐19 patients over time, based on available literature. References are mentioned at the top of the bars. Time is expressed as the average delay between the onset of the COVID‐19 and rating of olfactory function. The dashed horizontal line represents the rate of hyposmia among the general population. 25 COVID‐19, coronavirus disease 2019.
FIGURE 2.
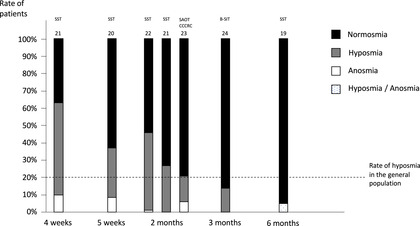
Distribution of the rates of normosmia, hyposmia, and anosmia among patients infected by SARS‐CoV‐2, at 4 weeks, 5 weeks, 2 months, 3 months, and 6 months after the onset of the disease. Olfactory function was assessed using diverse psychophysical methods (SST, SAOT, CCCRC, and B‐SIT). References are reported at the top of the bar. The dashed horizontal line represents the rate of hyposmia among the general population. 25 B‐SIT, Brief Smell Identification Test; CCCRC, Connecticut Chemosensory Clinical Research Center; SAOT, self‐administrated olfactory test; SARS‐CoV‐2, severe acute respiratory syndrome‐coronavirus‐2; SST, Sniffin’ Sticks Test.
Consequently, it appears from the current literature that C19OD is largely reversible for most affected patients in the short‐term to medium‐term, whereas a significant minority progress to a persistent olfactory dysfunction characterized by hyposmia and, in many cases, parosmia, typical of a neural postinfectious OD. 27
USEFULNESS OF SYSTEMIC CS IN POSTINFECTIOUS OD
Although several studies have investigated the usefulness of CS administered through different formulations, routes, and doses in patients with postinfectious olfactory dysfunction (PIOD), their general efficacy remains controversial. 28 , 29
A recent systematic evidence‐based review investigated the use of CS in nonsinonasal OD, 29 and reported very weak evidence to support the use of systemic CS therapy (level 4). Another recent evidence‐based review of treatment options for PIOD concluded that olfactory training is a recommendation for the treatment of PIOD, whereas systemic or topical steroids remain “optional” due to the lack of high‐quality studies. 28
It has been suggested that the favorable effect of CS in PIOD could be attributed to their effects on any underlying sinonasal inflammation, 29 , 30 theoretically resulting from mucosal effects of an upper respiratory tract infection. Thus, standard rhinological examination remains mandatory in patients with persistent olfactory loss, and rhinosinusitis must be treated appropriately. 30 , 31
Importantly, although CS are often reported as having the potential to improve olfactory function, an animal study found that they may impair the neuronal regeneration at the level of the olfactory epithelium. 32
Therefore, there is low level evidence supporting the usefulness of CS in PIOD. Although level 4 studies suggest that systemic CS could improve olfactory loss in PIOD, there is a lack of high‐quality studies, and no consensus can be reached at this time. On the other hand, there is also low evidence that systemic CS are not effective or would impair olfactory function. Currently, systemic CS are thus considered as a therapeutic option in selected patients and after personalized consideration of the potential risk. 3 , 28
In contrast, a currently accepted recommendation for the treatment of non‐COVID‐19 PIOD is smell training, 28 , 30 which has been shown with solid data 28 , 30 to improve the recovery of PIOD.
SYSTEMIC CS IN COVID‐19 PATIENTS
At the onset of the pandemic, caution was recommended regarding the use of systemic CS due to the uncertainty regarding their immunosuppressive effect in COVID‐19. However, they have turned out to constitute an important weapon against COVID‐19, 33 and are recommended by the World Health Organization (WHO) in patients with severe and critical COVID‐19 because they appear to reduce 28‐day mortality. 34 Conversely, their use is not recommended in patients with mild COVID‐19 because it may increase the risk of death when administrated in non‐severe COVID‐19 patients, 33 , 34 although this was a conditional recommendation based on low‐certainty evidence. Of note, it has been suggested that OD mainly affects patients with mild COVID‐19, 35 although this is still debated.
A recent case report found that a patient with C19OD receiving oral prednisolone, after failure of topical steroids, improved 6 days later. 36 However, because of the rate of spontaneous recovery of C19OD, it cannot be ruled out that this is the natural evolution of the disease. Recently, a prospective study aimed to compare the efficacy of systemic CS associated with olfactory training (nine patients) to olfactory training alone (OT‐only) (18 patients). 37 The study found that only patients with combined therapy significantly improved olfactory function at 10 weeks follow‐up. However, this has to be tempered by the fact that groups were not exactly similar. There was a higher number, and hence a higher variance, of patients in the OT‐only group. Also, patients receiving systemic CS were mainly anosmic and had therefore a higher chance to spontaneously improve their olfactory function. 38 Moreover, there were no data regarding endoscopic examination and signs of nasal inflammation. Another prospective study evaluating the rate of recovery of OD among COVID‐19 patients found that neither topical (administrated in 71 patients) nor systemic CS (administrated in 58 patients) influenced the prognosis of olfactory recovery. 39 Therefore, there is currently no robust evidence supporting a potential effect of systemic CS in COVID‐19 patients.
DELPHI PROCESS
To collect individual opinion of experts from the Clinical Olfaction Working Group, a Delphi process was performed. Members were asked to answer five questions (Q1–Q5), by answering whether they fully/partly agreed (FPA) or fully/partly disagreed (FPD).
Q1. Systemic CS should be prescribed within the first 3 weeks after the onset of C19OD: 11% FPA; 89% FPD.
Q2. Systemic CS should be considered as a first‐line treatment in C19OD: 16% FPA; 84% FPD.
Q3. There is no place for systemic CS in the treatment of C19OD: 21% FPA; 79 % FPD.
Q4. In the lack of evidence, caution should prevail, and systemic CS should not be considered as a standard treatment in patients with C19OD: 95% FPA; 5% FPD.
Q5. Olfactory training should be prescribed as soon as possible in the course of C19OD: 89% FPA; 11% FPD.
DISCUSSION
Current evidence supporting the usefulness of systemic CS in PIOD, and in particular for C19OD, is weak, whereas the rate of spontaneous recovery of C19OD is high. Therefore, a majority of the experts think that caution must prevail (Q4), and systemic CS should not be considered as standard‐of‐care treatment intended for all patients having C19OD (Q2), in the early phase of the disease (Q1).
Conversely, it also appears that a majority of the experts find that systemic CS have a potential place in the treatment of C19OD (Q3). Indeed, if signs of nasal inflammation are present at the time of clinical examination, systemic CS could constitute a treatment option, in selected cases.
None of the questions reach a 100% consensus, reflecting a lack of knowledge regarding the balance of risk and benefits for systemic CS in C19OD. Therefore, randomized placebo‐controlled trials should be considered, and the question of the dose and duration of the treatment must be investigated. Moreover, it is notable, from our cumulative experience, that many C19OD patients complain of parosmia, frequently appearing several months after the acute infectious event. 27 Therefore, long‐term follow‐up studies are needed to evaluate whether CS treatment has the potential to decrease the risk of developing qualitative ODs. Given that many patients appear to recover without intervention, it will likely take large numbers of patients in such trials to demonstrate enhanced recovery with corticosteroid treatment, although, sadly, given the recent increases in COVID‐19, recruitment may not be difficult.
Considering the full spectrum of the route of administration of CS, an alternative is the use of topical CS. The option of nasal lavage with CS should also be investigated because it has been suggested that it could constitute an efficient and potentially less harmful alternative compared to systemic CS. 40 However, their efficacy has not been specifically studied in PIOD patients and more data are needed to confirm their usefulness. Although topical CS are considered as not useful to treat PIOD, 29 this may be related to the fact that classical nasal spray administration does not reach the olfactory cleft. It has been suggested that the use of specific nasal cannulas for administration of sprays, 41 exhalation delivery systems, 42 or application of nasal drops in the Kaiteki position 43 could be effective for delivering medications into the olfactory cleft. Therefore, studies considering the effectiveness of different routes of administration of CS will be useful to define the most efficient option, if there is one.
Finally, experts largely agree that olfactory training should be prescribed as soon as possible in the course of C19OD (Q5).
CONCLUSION
Currently there is no evidence that any kind of CS treatment may substantially change the outcome of C19OD. In contrast, there is sufficient evidence that even limited unjustified systemic CS treatment has harmful side effects such as long‐term increased risk for hip fractures or decompensating glaucoma. 44 In light of the huge number of patients possibly receiving steroids for C19OD, based on poor evidence, we call for caution using these treatments. At the same time, we encourage controlled studies investigating this issue. As an expert group we strongly emphasize the initial consideration of smell training. Smell training has no known side effects and is low cost. Moreover, it is the only available treatment for PIOD supported by a robust evidence base. 28 , 30
CONFLICT OF INTEREST
None provided.
Huart C, Philpott CM, Altundag A, et al. Systemic corticosteroids in coronavirus disease 2019 (COVID‐19)‐related smell dysfunction: an international view. Int Forum Allergy Rhinol. 2021;11:1041–1046. 10.1002/alr.22788
REFERENCES
- 1. Le Guennec L, Devianne J, Jalin L, et al. Orbitofrontal involvement in a neuroCOVID‐19 patient. Epilepsia. 2020;61(8):e90‐e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yamagishi M, Fujiwara M, Nakamura H. Olfactory mucosal findings and clinical course in patients with olfactory disorders following upper respiratory viral infection. Rhinology. 1994;32:113‐8. [PubMed] [Google Scholar]
- 3. Hopkins C, Alanin M, Philpott C, et al. Management of new onset loss of sense of smell during the COVID‐19 pandemic ‐ BRS Consensus Guidelines. Clin Otolaryngol. 2021;46(1):16‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Klimek L, Jutel M, Bousquet J, et al. Management of patients with chronic rhinosinusitis during the COVID‐19 pandemic ‐ an EAACI position paper. Allergy. In press. Epub 2020 Oct 19. 10.1111/all.14629. Accessed February 23, 2021. [DOI] [PubMed] [Google Scholar]
- 5. Wei BP, Stathopoulos D, O'Leary S. Steroids for idiopathic sudden sensorineural hearing loss. Cochrane Database Syst Rev. 2013;2013(7):CD003998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu Z, Yang Y, He L, et al. High‐dose methylprednisolone for acute traumatic spinal cord injury: a meta‐analysis. Neurology. 2019;93:e841‐e850. [DOI] [PubMed] [Google Scholar]
- 7. Pang KW, Chee J, Subramaniam S, Ng CL. Frequency and clinical utility of olfactory dysfunction in COVID‐19: a systematic review and meta‐analysis. Curr Allergy Asthma Rep. 2020;20:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hajikhani B, Calcagno T, Nasiri MJ, et al. Olfactory and gustatory dysfunction in COVID‐19 patients: a meta‐analysis study. Physiol Rep. 2020;8:e14578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mao L, Wang M, Chen S, et al. Neurological manifestations of hospitalized patients with COVID‐19 in Wuhan, China: a retrospective case series study. medRxiv. Epub February 25, 2020. 10.1101/2020.02.22.20026500. Accessed February 23, 2021. [DOI] [Google Scholar]
- 10. Moein ST, Hashemian SM, Mansourafshar B, Khorram‐Tousi A, Tabarsi P, Doty RL. Smell dysfunction: a biomarker for COVID‐19. Int Forum Allergy Rhinol. 2020;10:944‐950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Amer MA, Elsherif HS, Abdel‐Hamid AS, Elzayat S. Early recovery patterns of olfactory disorders in COVID‐19 patients: a clinical cohort study. Am J Otolaryngol. 2020;41:102725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fjaeldstad AW. Prolonged complaints of chemosensory loss after COVID‐19. Dan Med J. 2020;67(8):A05200340. [PubMed] [Google Scholar]
- 13. Paderno A, Mattavelli D, Rampinelli V, et al. Olfactory and gustatory outcomes in COVID‐19: a prospective evaluation in nonhospitalized subjects. Otolaryngol Head Neck Surg. 2020;163(6):1144‐1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Panda S, Mohamed A, Sikka K, et al. Otolaryngologic manifestation and long‐term outcome in mild COVID‐19: experience from a tertiary care centre in India. Indian J Otolaryngol Head Neck Surg. 2021;73:72‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reiter ER, Coelho DH, Kons ZA, Costanzo RM. Subjective smell and taste changes during the COVID‐19 pandemic: short term recovery. Am J Otolaryngol. 2020;41:102639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brandão Neto D, Fornazieri MA, Dib C, et al. Chemosensory dysfunction in COVID‐19: prevalences, recovery rates, and clinical associations on a large Brazilian sample. Otolaryngol Head Neck Surg. In press. Epub September 1, 2020. 10.1177/0194599820954825. Accessed February 23, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cho RHW, To ZWH, Yeung ZWC, et al. COVID‐19 viral load in the severity of and recovery from olfactory and gustatory dysfunction. Laryngoscope. 2020;130:2680‐2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ramasamy K, Saniasiaya J, Abdul Gani N. Olfactory and gustatory dysfunctions as a clinical manifestation of coronavirus disease 2019 in a Malaysian tertiary center. Ann Otol Rhinol Laryngol. In press. Epub October 7, 2020. 10.1177/0003489420963165. Accessed February 23, 2021. [DOI] [PubMed] [Google Scholar]
- 19. Lechien JR, Chiesa‐Estomba CM, Beckers E, et al. Prevalence and 6‐month recovery of olfactory dysfunction: a multicentre study of 1363 COVID‐19 patients. J Intern Med. In press. Epub January 5, 2021. 10.1111/joim.13209. Accessed February 23, 2021. [DOI] [PubMed] [Google Scholar]
- 20. Le Bon SD, Pisarski N, Verbeke J, et al. Psychophysical evaluation of chemosensory functions 5 weeks after olfactory loss due to COVID‐19: a prospective cohort study on 72 patients. Eur Arch Otorhinolaryngol. 2021;278(1):101‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Iannuzzi L, Salzo AE, Angarano G, et al. Gaining back what is lost: recovering the sense of smell in mild to moderate patients after COVID‐19. Chem Senses. 2020;45:875‐881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Otte MS, Eckel HNC, Poluschkin L, Klussmann JP, Luers JC. Olfactory dysfunction in patients after recovering from COVID‐19. Acta Otolaryngol. 2020;140(12):1032‐1035. [DOI] [PubMed] [Google Scholar]
- 23. Vaira LA, Hopkins C, Petrocelli M, et al. Smell and taste recovery in coronavirus disease 2019 patients: a 60‐day objective and prospective study. J Laryngol Otol. 2020;134:703‐709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ugurlu BN, Akdogan O, Yilmaz YA, et al. Quantitative evaluation and progress of olfactory dysfunction in COVID‐19. Eur Arch Otorhinolaryngol. In press. Epub January 1, 2021. 10.1007/s00405-020-06516-4. Accessed February 23, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Landis BN, Konnerth CG, Hummel T. A study on the frequency of olfactory dysfunction. Laryngoscope. 2004;114:1764‐1769. [DOI] [PubMed] [Google Scholar]
- 26. Oleszkiewicz A, Kunkel F, Larsson M, Hummel T. Consequences of undetected olfactory loss for human chemosensory communication and well‐being. Philos Trans R Soc Lond B Biol Sci. 2020;375(1800):20190265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hong S‐C, Holbrook EH, Leopold DA, Hummel T. Distorted olfactory perception: a systematic review. Acta Otolaryngol. 2012;132(Suppl 1):S27‐S31. [DOI] [PubMed] [Google Scholar]
- 28. Hura N, Xie DX, Choby GW, et al. Treatment of post‐viral olfactory dysfunction: an evidence‐based review with recommendations. Int Forum Allergy Rhinol. 2020;10:1065‐1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yan CH, Overdevest JB, Patel ZM. Therapeutic use of steroids in non‐chronic rhinosinusitis olfactory dysfunction: a systematic evidence‐based review with recommendations. Int Forum Allergy Rhinol. 2019;9:165‐176. [DOI] [PubMed] [Google Scholar]
- 30. Hummel T, Whitcroft KL, Andrews P, et al. Position paper on olfactory dysfunction. Rhinol Suppl. 2017;54:1‐30. [DOI] [PubMed] [Google Scholar]
- 31. Fokkens WJ, Lund VJ, Hopkins C, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology. 2020;58(Suppl S29):1‐464. [DOI] [PubMed] [Google Scholar]
- 32. Chang SY, Glezer I. The balance between efficient anti‐inflammatory treatment and neuronal regeneration in the olfactory epithelium. Neural Regen Res. 2018;13:1711‐1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. RECOVERY Collaborative Group ; Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid‐19 ‐ preliminary report. N Engl J Med. In press. Epub July 17, 2020. 10.1056/nejmoa2021436. Accessed February 23, 2021. [DOI] [Google Scholar]
- 34. World Health Organization (WHO) . Corticosteroids for COVID‐19: Living Guidance. Geneva, Switzerland: WHO; 2020. https://www.who.int/publications/i/item/WHO-2019-nCoV-Corticosteroids-2020.1. Accessed February 23, 2021. [Google Scholar]
- 35. Yan CH, Faraji F, Prajapati DP, Ostrander BT, Deconde AS. Self‐reported olfactory loss associates with outpatient clinical course in COVID‐19. Int Forum Allergy Rhinol. 2020;10:821‐831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Touisserkani SK, Ayatollahi A. Oral corticosteroid relieves post‐COVID‐19 anosmia in a 35‐year‐old patient. Case Rep Otolaryngol. 2020;2020:5892047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Le Bon S‐D, Konopnicki D, Pisarski N, Prunier L, Lechien JR, Horoi M. Efficacy and safety of oral corticosteroids and olfactory training in the management of COVID‐19‐related loss of smell. Eur Arch Otorhinolaryngol. In press. Epub January 9, 2021. 10.1007/s00405-020-06520-8. Accessed February 23, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cavazzana A, Larsson M, Münch M, Hähner A, Hummel T. Postinfectious olfactory loss: a retrospective study on 791 patients. Laryngoscope. 2018;128:10‐15. [DOI] [PubMed] [Google Scholar]
- 39. Chiesa‐Estomba CM, Lechien JR, Radulesco T, Michel J, Sowerby LJ, Hopkins C, Saussez S. Patterns of smell recovery in 751 patients affected by the COVID‐19 outbreak. Eur J Neurol. 2020;27:2318‐2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nguyen TP, Patel ZM. Budesonide irrigation with olfactory training improves outcomes compared with olfactory training alone in patients with olfactory loss. Int Forum Allergy Rhinol. 2018;8:977‐981. [DOI] [PubMed] [Google Scholar]
- 41. Shu C‐H, Lee Po‐L, Shiao An‐S, Chen K‐T, Lan M‐Y. Topical corticosteroids applied with a squirt system are more effective than a nasal spray for steroid‐dependent olfactory impairment. Laryngoscope. 2012;122:747‐750. [DOI] [PubMed] [Google Scholar]
- 42. Senior BA, Schlosser RJ, Bosso J, Soler ZM. Efficacy of the exhalation delivery system with fluticasone in patients who remain symptomatic on standard nasal steroid sprays. Int Forum Allergy Rhinol. In press. Epub September 24, 2020. 10.1002/alr.22693. Accessed February 23, 2021. [DOI] [PubMed] [Google Scholar]
- 43. Mori E, Merkonidis C, Cuevas M, Gudziol V, Matsuwaki Y, Hummel T. The administration of nasal drops in the “Kaiteki” position allows for delivery of the drug to the olfactory cleft: a pilot study in healthy subjects. Eur Arch Otorhinolaryngol. 2016;273:939‐943. [DOI] [PubMed] [Google Scholar]
- 44. Yasir M, Goyal A, Bansal P, Sonthalia S. Corticosteroid adverse effects . Treasure Island, FL: StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK531462/. Accessed February 23, 2021. [PubMed] [Google Scholar]


